Distribution of Urate Crystal Deposition in the Hands and Wrists of Patients with Chronic Gout
ORIGINAL ARTICLE CME
Distribution of Urate Crystal Deposition in the Hands and Wrists of Patients with Chronic Gout
Y Leng1, DLY Chow1, SK Chui1, NSK Ip1, SWC Chan1, KY Choi2, AOC Li1
1 Department of Radiology, Tuen Mun Hospital, Hong Kong
2 Department of Orthopaedics and Traumatology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr Y Leng, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: ly108@ha.org.hk
Submitted: 27 Nov 2019; Accepted: 21 Jan 2020.
Contributors: All authors designed the study. YL, AOCL, SKC and KYC acquired the data. YL analysed the data and drafted the manuscript.
All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by the New Territories West Cluster Clinical Ethics Committee (Ref NTWC/REC/19082).
Abstract
Objectives
We sought to examine the frequency and patterns of monosodium urate (MSU) crystal deposition in the hand/wrist in non-acute gout patients using dual-energy computed tomography (DECT).
Methods
All hand/wrist DECT imaging data of patients with chronic tophaceous gout undergoing their first
examination before dissolution therapy from March 2015 to March 2019 were identified. Cases without positive
MSU crystal deposition were excluded. The reports and images of the positive cases were retrospectively analysed,
and the anatomical locations of all urate crystal depositions were recorded.
Results
A total of 48 cases were identified with positive findings. Thirty of the cases had undergone DECT of both
hands, and 18 had undergone DECT of a single hand. In total, 60 hands/wrists had flexor tendon involvement. The
carpal joints were the most commonly involved site (78.2%). The carpal tunnel was the most commonly involved soft
tissue site in the hand and wrist (71.8%), followed by the fourth (55.1%) and fifth (53.8%) extensor compartments.
The second digit extensor digitorum (47.4%) was the most commonly involved soft tissue site in the hand while the
fourth digit flexor digitorum was the most commonly (46.2%) involved flexor tendon in the hand. In the hand and
wrist soft tissue sites, extensor pollicis brevis (11.5%), flexor carpi ulnaris (11.5%) and extensor compartment I
(11.5%) were involved least commonly. We found Zones II (75%) and IV (78.3%) to be the most commonly involved
flexor tendon zones in the hand.
Conclusion
In this observational study, we have provided a detailed analysis of hand and wrist urate distribution in gout.
Key Words: Gout; Hand; Tomography, X-ray computed; Uric acid
中文摘要
慢性痛風患者手腕尿酸鈉結晶的分佈
冷泳美、周朗妍、徐碩君、葉筱筠、陳煥章、蔡啟堯、李安慈
目的
透過雙能電腦斷層掃描(DECT)檢查非急性痛風患者手腕中尿酸鈉(MSU)結晶沉積發生率和分佈模式。
方法
收集2015年3月至2019年3月期間,慢性痛風石患者於結晶溶出治療前接受檢查的所有手腕DECT影像。排除沒有陽性MSU結晶沉積的病例。回顧分析陽性病例的報告和影像,並記錄所有MSU結晶沉積的解剖位置。
結果
陽性結果共48例,單手及雙手DECT分別佔30例及18例。共60隻手或手腕有屈肌腱受累。腕關節是最常受累的部位(78.2%)。腕管是手部及腕部最常見的受累軟組織部位(71.8%),其次是第四側伸肌腔(55.1%)和第五側伸肌腔(53.8%)。食指伸肌是手部最常受累的軟組織部位(47.4%),而無名指屈指肌是手部最常受累的屈刪1腱部位(46.2%)。在手和手腕軟組織部位,拇短伸肌(11.5%)、尺側腕屈肌(11.5%)和伸肌隔腔第1區(11.5%)最不常見。手部屈肌腱受累的最常見區域包括第2區(75%)和第4區(78.3%)。
結論
這項觀察性研究為痛風患者手腕MSU分佈提供了詳細分析。
INTRODUCTION
Gout is a disease associated with deposition of
monosodium urate (MSU) crystals. Acute gouty arthritis
is characterised by the rapid onset of severe pain,
swelling, warmth, erythema, and decreased range of
motion in the affected joint.[1] Chronic tophaceous gout is
associated with progressive joint damage, chronic pain,
and disability.
The diagnosis of gout has been based on clinical
presentation, laboratory results, joint aspiration, and
imaging. Patients typically present with mono-articular
arthritis, often affecting the first metatarsophalangeal
(MTP) joint.[2] Hyperuricaemia is an inconsistent finding.
Patients may have ‘normal’ serum urate levels during an
acute gout attack. Some patients may have ‘asymptomatic
hyperuricaemia’ without clinical manifestations of gout or
urate crystal deposition.[3] The gold standard for diagnosis
of gout is presence of birefringent MSU crystals from
the joint aspirate. However, joint aspiration is a painful
invasive procedure and may be false-negative for MSU
crystals even when acute gouty arthritis is present.
Various imaging modalities have been used for the
diagnosis of gout, such as radiography, ultrasound,
computed tomography (CT), and magnetic resonance
imaging (MRI). On plain X-ray, ‘punched-out’ erosions
with overhanging edges are a typical manifestation
of chronic gout. Ultrasound features of gout include joint effusion, synovitis, erosions, tophi, crystalline
aggregates and the ‘double contour sign’ which is
existence of a hyperechoic band over anechoic cartilage.[4]
MRI features of gouty arthropathy are variable and
nonspecific including tophi with variable intensity on
T1-weighted or T2-weighted sequences and variable
enhancement pattern.[5] These imaging modalities are
not highly sensitive or specific for identifying MSU
crystals.
Dual-energy CT (DECT) is advanced technology that
enables excellent visualisation of soft tissue structures,
such as tendons, ligaments, and bursae. It has been used
for the non-invasive diagnosis of established gout with
high sensitivity and specificity.[6] DECT depends on an
accumulation of MSU crystals and is not particularly
accurate for determining acute, early gout. DECT is
particularly helpful in accurate quantification of MSU
deposits and for follow-up. The hand and wrist are
common sites of gouty crystal deposition. However, MSU
involvement of bone/joint and soft tissue in the hands
and wrists has not been systematically characterised.
The aim of this study was to examine the frequency and
patterns of bone/joint and soft tissue involvement in the
hand and wrist of patients with gout using DECT. We
hope to improve understanding of the pathogenesis,
prompt diagnosis, and management of gout with more
detailed knowledge of urate deposition in this disease.
METHODS
This was a retrospective, observational study. In our
hospital, patients with chronic tophaceous gout are
referred for DECT from the Department of Orthopaedics
for pretreatment assessment. Referred patients
undergoing their first DECT of the hand and wrist
between March 2015 and March 2019 were identified
via the electronic health records system. Cases with
positive MSU crystal deposition in the hand and wrist
were included.
Scans were performed using our dual-source DECT
scanner (Siemens SOMATOM Definition Flash).
Parameters were 140 kV for one tube and 80 kV for
the other. A two-material decomposition algorithm
was performed on a multi-technique CT workspace.
The material-specific difference in attenuation of urate
between the two energy levels at 80 kV and 140 kV allowed accurate detection of MSU, which was then
colour coded as green and fused onto the standard
greyscale CT image. These were reviewed as both cross-sectional
and three-dimensional images.
We analysed the reports and images of the positive
cases, with locations of all urate deposition recorded
and classified by anatomical location. MSU crystal
deposition in DECT scans were scored at the tendon
sites, joints, carpal tunnel, and flexor tendon anatomic
zones according to the classification of Kleinert et al[7] and
Verdan.[8] Zone V (from the musculotendinous junction
to the proximal aspect of the carpal tunnel) was not
included in the analysis because it was not completely
included in the scan range in some of the cases.
RESULTS
Among 73 referred patients undergoing first DECT of the
hand and wrist during the study period, 48 patients were
identified with positive findings who met the inclusion
criteria. Patients with positive findings consisted of
47 men and one woman with median age 61 years
(range, 31-89 years). Among them, 30 patients underwent
DECT of both hands and 18 patients underwent DECT
of a single hand. Therefore, a total of 78 wrists and hands
were affected.
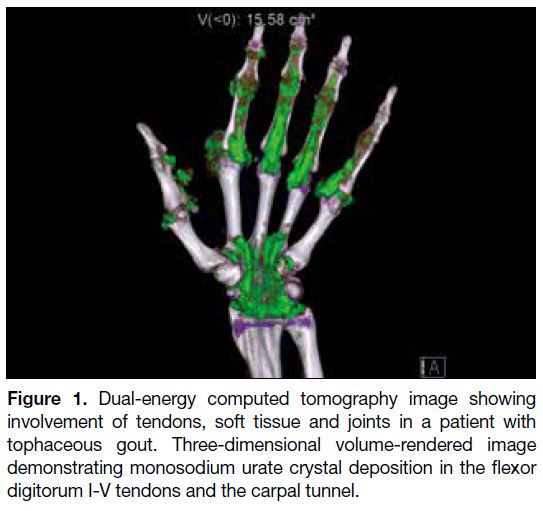
As shown in Table 1, the carpal tunnel was the most
commonly involved soft tissue site at the wrist (71.8%)
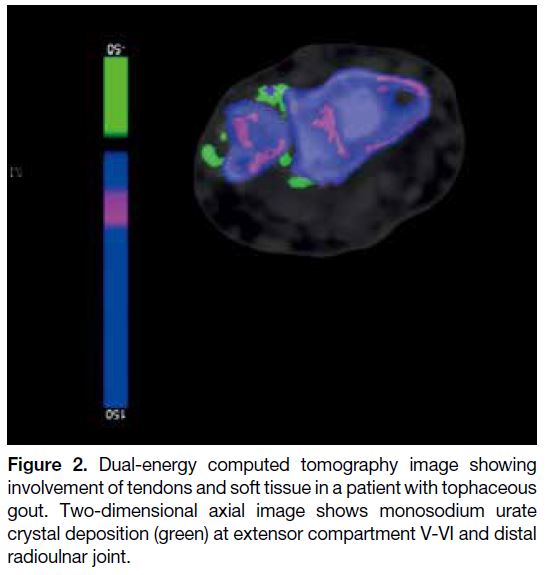
[Figure 1], followed by the fourth (55.1%) and fifth
(53.8%) extensor compartments (Figure 2). In the hands, the second digit extensor digitorum tendon was
the most commonly (47.4%) involved soft tissue site
in the hand, while the fourth digit flexor digitorum was
the most commonly (46.2%) involved flexor tendon
in the hand (Table 2). The intercarpal joints were the
most commonly involved site (78.2%). A total of 60
(77%) of the 78 hands/wrists studied had flexor tendon
involvement. Zones II (75%) and IV (78.3%) were the
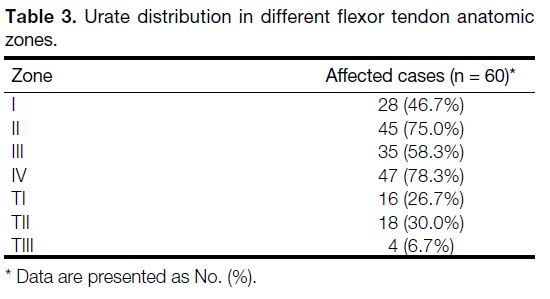
most commonly involved flexor tendon zones (Table 3).
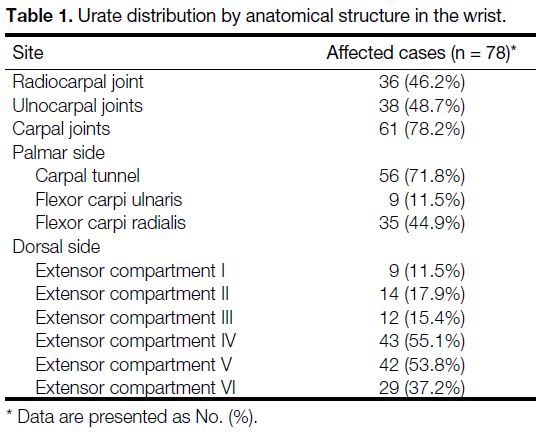
Table 1. Urate distribution by anatomical structure in the wrist.
Figure 1. Dual-energy computed tomography image showing
involvement of tendons, soft tissue and joints in a patient with
tophaceous gout. Three-dimensional volume-rendered image
demonstrating monosodium urate crystal deposition in the flexor
digitorum I-V tendons and the carpal tunnel.
Figure 2. Dual-energy computed tomography image showing
involvement of tendons and soft tissue in a patient with tophaceous
gout. Two-dimensional axial image shows monosodium urate
crystal deposition (green) at extensor compartment V-VI and distal
radioulnar joint.
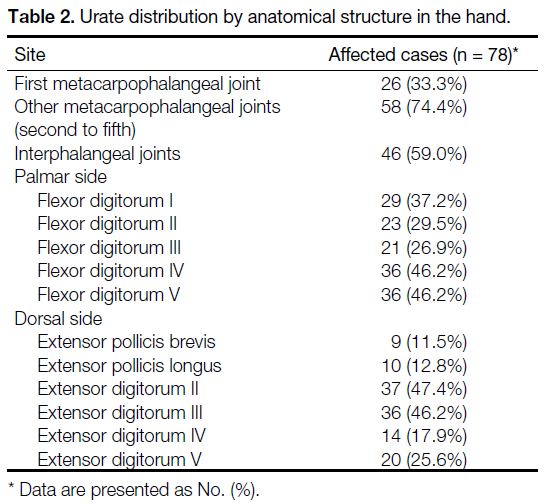
Table 2. Urate distribution by anatomical structure in the hand.
Table 3. Urate distribution in different flexor tendon anatomic zones.
DISCUSSION
The reported prevalence of gout in Hong Kong has risen
continuously over the past decade. In 2016, the crude
prevalence of gout in Hong Kong was 2.9%, which is
similar to rates reported in Western countries.[9]
A few studies about the distribution of gout have shown
that the lower extremity is more often affected than
the upper extremity.[10] [11] [12] Gout in the first MTP joint is
accepted as the most common site of involvement in
clinical and radiographic studies.[13] [14] A DECT study of
148 newly diagnosed gout patients showed the first MTP joint to be the most common site of urate deposition
(44.6%) and much more common than other MTP joints
(17.6%). We found that gout affecting the first MCP
joint is less common than the other MCP joints.
Our study shows that MSU crystal deposition in the hand
and wrist is most common in the carpal joints (78.2%),
followed by the MCP joints (75.6%), and interphalangeal
joints (59%). This is comparable to previous studies.
Research done by Mallinson et al[10] reviewing 148 DECT
cases for the distribution of urate deposition showed that
carpus (12.5%) also had higher urate deposition than
interphalangeal joints (6.4%) and MCP joints (7.4%).
Our study has a much higher prevalence of positive
findings than Mallinson et al,[10] which included DECT
images of hands/wrists, feet/ankles, elbows, and knees,
whereas our study focused only on the hands and wrists.
Another study on DECT of 97 patients with gout also
showed that the carpal joints (56.7%) had a higher rate
of urate deposition than the metacarpophalangeal joints
(42.3%).[15]
Our results show that MSU crystal deposition in the
tendons of the hands/wrists is very common in patients
with gout. A previous DECT study of tendon involvement
in the feet of 92 patients with gout also found common
tendon/ligament involvement in about 65% of feet.[16] The
exact mechanism on why there is crystal deposition on
tendons is not known. Biomechanical strain as a result
of the pressure burden on these tendons may contribute
to crystal deposition.[17] Spontaneous rupture of tendons
secondary to gouty tophaceous deposits can occur.[18] [19]
MSU crystal deposition in hands/wrists can affect
function. In a study of 20 patients with gout, tophaceous
joint disease strongly predicted impaired hand function.[20]
The number of joints in the hand with tophi was the
strongest single predictor of the Sollerman score, and
also predicted other measures of hand mobility and
function. Kleinert et al[7] and Verdan[8] have classified
tendon injuries into five anatomic zones which can have
impact on flexor tendon injury treatment and prognosis.
Our study analysed MSU crystal deposition in four of the
five different flexor tendon anatomic zones. We found
zones II (75%) and IV (78.3%) were the most commonly
involved flexor tendon zones in the hand.
We found that 48.7% of the cases had MSU crystal
deposition in the ulnocarpal joints. Another DECT study
of 97 patients with gout showed 54.6% of patients had
urate deposition in the triangular fibrocartilage complex/distal radioulnar joint.[19] This is similar to our study. A study on arthroscopic findings of seven patients with
wrist gout found focal crystalline precipitates on the
scapholunate and lunotriquetral ligaments but not on the
triangular fibrocartilage complex.[21]
We found that the carpal tunnel was the most commonly
involved soft tissue site in the hand and wrist (71.8%).
Urate deposition in the carpal tunnel can cause secondary
carpal tunnel syndrome.[22] [23]
DECT has relatively high sensitivity and specificity
for the diagnosis of gout. Sensitivity and specificity
for DECT was 100% and 79% to 89%, respectively in
a study of 31 patients who underwent both DECT and
joint aspiration.[24] A meta-analysis of seven studies found
DECT to have a sensitivity of 88% and specificity of
90% for gout.[25]
Our study has some limitations. It was retrospective with
a small number of cases and all DECT examinations
were evaluated by a single radiologist. Patients
were recruited from orthopaedic clinics, where their
gout may have been partially treated. All included
patients had chronic tophaceous gout, which is usually
associated with a number of co-morbidities, including
hypertension, cardiovascular disease, renal impairment,
diabetes, obesity, and hyperlipidaemia. So, this may
contribute to selection bias. Smaller concentrations of
MSU may not be accurately seen on DECT,[26] so it is
possible that small MSU crystal deposits may not have
been detected his method. False-negative results can also
occur in tophi with lower crystal concentrations. In short,
the population of the study is heterogeneous and small.
Further multicentre studies with more patients would be
helpful to confirm our findings.
CONCLUSION
In this study, we have provided a detailed analysis of hand
and wrist urate distribution in gout. Our study supports
that gout affects different locations within the soft tissue
and joints with predilection for particular areas.
REFERENCES
1. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-52. Crossref
2. Roddy E. Revisiting the pathogenesis of podagra: why does gout target the foot? J Foot Ankle Res. 2011;4:13. Crossref
3. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223. Crossref
4. Thiele RG, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford). 2007;46:1116-21. Crossref
5. Girish G, Glazebrook KN, Jacobson JA. Advanced imaging in gout. AJR Am J Roentgenol. 2013;201:515-25. Crossref
6. Nicolaou S, Yong-Hing CJ, Galea-Soler S, Hou DJ, Louis L, Munk P. Dual-energy CT as a potential new diagnostic tool in the
management of gout in the acute setting. AJR Am J Roentgenol.
2010;194:1072-8. Crossref
7. Kleinert H, Kutz J, Ashbell TS, Martinez E. Primary repair of
lacerated flexor tendons in “no man’s land”. J Bone Joint Surg.
1967;49A:577.
8. Verdan CE. Half a century of flexor-tendon surgery. Current status and changing philosophies. J Bone Joint Surg Am. 1972;54:472-91. Crossref
9. Centre for Health Protection, Department of Health, Hong Kong SAR Government. Gout: No longer the disease of kings. Available
from: https://www.chp.gov.hk/files/pdf/ncd_watch_april_2019.pdf.
Accessed 12 Nov 2020.
10. Mallinson PI, Reagan AC, Coupal T, Munk PL, Ouellette H,
Nicolaou S. The distribution of urate deposition within the
extremities in gout: a review of 148 dual-energy CT cases. Skeletal
Radiol. 2014;43:277-81. Crossref
11. Dhanda S, Jagmohan P, Quek ST. A re-look at an old disease: a
multimodality review on gout. Clin Radiol. 2011;66:984-92. Crossref
12. Roddy E, Zhang W, Doherty M. Are joints affected by gout also
affected by osteoarthritis? Ann Rheum Dis. 2007;66:1374-7. Crossref
13. Monu JU, Pope TL Jr. Gout: a clinical and radiologic review. Radiol
Clin North Am. 2004;42:169-84. Crossref
14. Stewart S, Dalbeth N, Vandal AC, Rome K. The first
metatarsophalangeal joint in gout: a systematic review and meta-analysis.
BMC Musculoskeletal Disord. 2016;17:69. Crossref
15. Klauser AS, Halpern EJ, Strobl S, Abd Ellah MM, Gruber J,
Bellmann-Weiler R, et al. Gout of hand and wrist: the value of US
as compared with DECT. Eur Radiol. 2018;28:4174-81. Crossref
16. Dalbeth N, Kalluru R, Aati O, Horne A, Doyle AJ, McQueen FM.
Tendon involvement in the feet of patients with gout: a dual-energy
CT study. Ann Rheum Dis. 2013;72:1545-8. Crossref
17. Sun Y, Ma L, Zhou Y, Chen H, Ding Y, Zhou J, et al. Features of
urate deposition in patients with gouty arthritis of the foot using dual-energy
computed tomography. Int J Rheum Dis. 2015;18:560-7. Crossref
18. Mahoney PG, James PD, Howell CJ, Swannell AJ. Spontaneous
rupture of the Achilles tendon in a patient with gout. Ann Rheum
Dis. 1981;40:416-8. Crossref
19. Jerome JT, Varghese M, Sankaran B, Thomas S, Thirumagal SK.
Tibialis anterior tendon rupture in gout — Case report and literature
review. Foot Ankle Surg. 2008;14:166-9. Crossref
20. Dalbeth N, Collis J, Gregory K, Clark B, Robinson E, McQueen FM.
Tophaceous joint disease strongly predicts hand function in patients
with gout. Rheumatology (Oxford). 2007;46:1804-7. Crossref
21. Wilczynski MC, Gelberman RH, Adams A, Goldfarb CA.
Arthroscopic findings in gout of the wrist. J Hand Surg Am.
2009;34:244-50. Crossref
22. Ge Y, Li F, Chen J, Tian J. Severe carpal tunnel syndrome caused
by gouty tophi diagnosed by dual energy computed tomography:
case report. Arch Rheumatol. 2016;31:284-6. Crossref
23. Lu H, Chen Q, Shen H. A repeated carpal tunnel syndrome due
to tophaceous gout in flexor tendon: a case report. Medicine
(Baltimore). 2017;96:e6245. Crossref
24. Glazebrook KN, Guimar es LS, Murthy NS, Black DF, Bongartz T,
Manek NJ, et al. Identification of intraarticular and periarticular uric
acid crystals with dual-energy CT: initial evaluation. Radiology.
2011;261:516-24. Crossref
25. Yu Z, Mao T, Xu Y, Li T, Wang Y, Gao F, et al. Diagnostic accuracy
of dual-energy CT in gout: a systematic review and meta-analysis.
Skeletal Radiol. 2018;47:1587-93. Crossref
26. Primak AN, Fletcher JG, Vrtiska TJ, Dzyubak OP, Lieske JC,
Jackson ME, et al. Noninvasive differentiation of uric acid versus
non–uric acid kidney stones using dual-energy CT. Acad Radiol.
2007;14:1441-7. Crossref