[18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Findings in Anti–Gamma-Aminobutyric Acid B Receptor Encephalitis: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Mar;27(1):e46-50 | Epub 7 March 2024
[18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Findings in Anti–Gamma-Aminobutyric Acid B Receptor Encephalitis: A Case Report
KL Chiu, WH Ma, KM Ma
Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr KL Chiu, Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China. Email: klchiu@ha.org.hk
Submitted: 4 July 2022; Accepted: 5 September 2022.
Contributors: KLC designed the study, acquired and analysed the data, and drafted the manuscript. WHM and KMM critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2023-175-1). The patient was treated in accordance with the Declaration of Helsinki. The requirement for patient consent was waived by the Board due to the use of anonymised patient data.
INTRODUCTION
Encephalitis is a severe inflammatory disorder of
the brain that develops as a rapidly progressive
encephalopathy and may affect patients of all ages.[1]
Autoimmune encephalitis, which is the most common
cause of non-infectious encephalitis[2] characterised
by the presence of autoantibodies against different
neuronal targets, may be associated with various
cancers. Anti–gamma-aminobutyric acid B (anti-GABAB) receptor encephalitis is a relatively uncommon
entity. Very few have been reported in findings on
[18F]fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG PET/CT) and its role
in diagnosis and management.[3] We present a patient with
anti-GABAB receptor encephalitis who underwent [18F]
FDG PET/CT.
CASE PRESENTATION
A 79-year-old man with a history of hypertension,
diabetes mellitus, hyperlipidaemia and gout was
admitted for status epilepticus. He had no known history
of epilepsy or malignancy and presented with a history of gradual onset slurring of speech and generalised
weakness over a few days, followed by repeated
seizures and status epilepticus on the day of admission.
Cerebrospinal fluid findings following lumbar puncture
were not suggestive of infection. Electroencephalogram
revealed mild slowing background with excessive slow
wave; epileptiform discharge was not detected. CT of the
brain revealed no acute intracranial haemorrhage but a
hyperdense lesion at the left frontal lobe, suspicious of
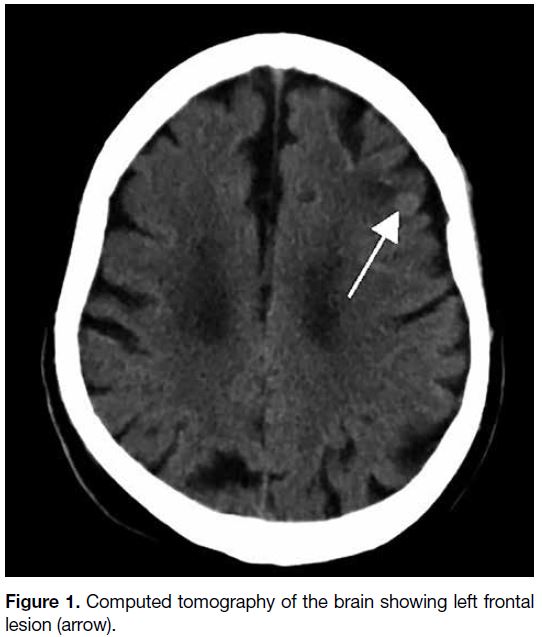
brain tumour (Figure 1).
Figure 1. Computed tomography of the brain showing left frontal lesion (arrow).
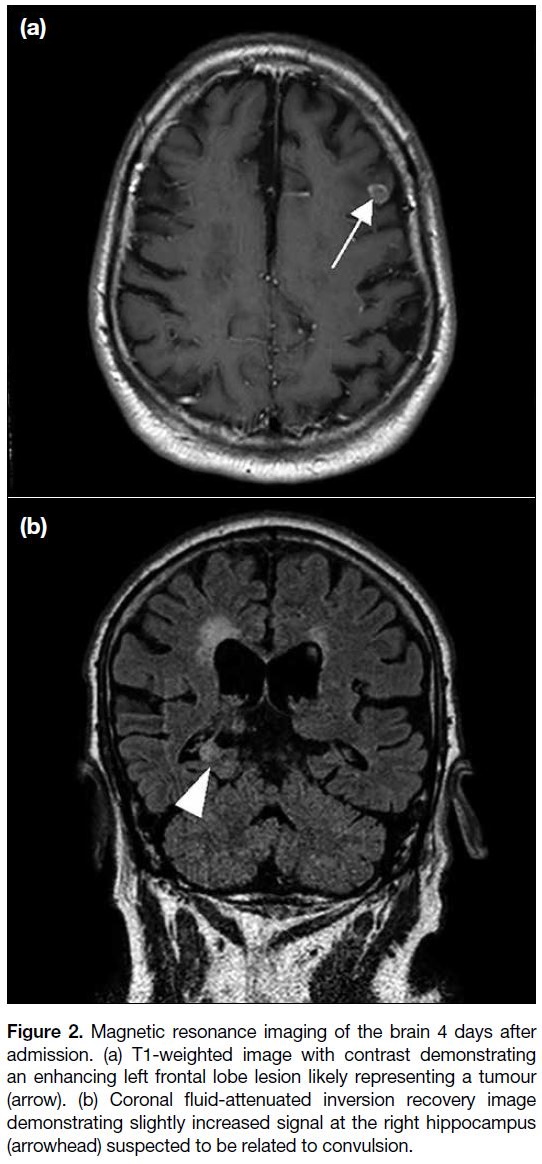
Magnetic resonance imaging (MRI) of the brain
performed 4 days after admission revealed a small
enhancing intra-axial lesion in the left frontal lobe
with restricted diffusion and perilesional oedema,
likely representing a tumour. Slightly increased fluid-attenuated
inversion recovery (FLAIR) signal was also
evident in the right hippocampus, suspected to be related
to convulsion (Figure 2).
Figure 2. Magnetic resonance imaging of the brain 4 days after
admission. (a) T1-weighted image with contrast demonstrating
an enhancing left frontal lobe lesion likely representing a tumour
(arrow). (b) Coronal fluid-attenuated inversion recovery image
demonstrating slightly increased signal at the right hippocampus
(arrowhead) suspected to be related to convulsion.
Tumour marker testing revealed elevated
carcinoembryonic antigen level at 14.4 ug/L (reference interval, ≤ 5.0). Paraneoplastic markers
(anti-Hu/Yo/Ri) were negative. Autoimmune markers
(anti–NMDA [N-methyl-D-aspartate] receptor,
anti-CASPR2 [contactin-associated protein-like
2], anti-AMPA1/2 [alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid 1/2], anti-LGI1
[leucine-rich-glioma-inactivated 1] and anti-DPPX
[dipeptidyl-peptidase-like protein 6] antibodies) were
negative except anti-GABAB1/ anti-GABAB2 receptors
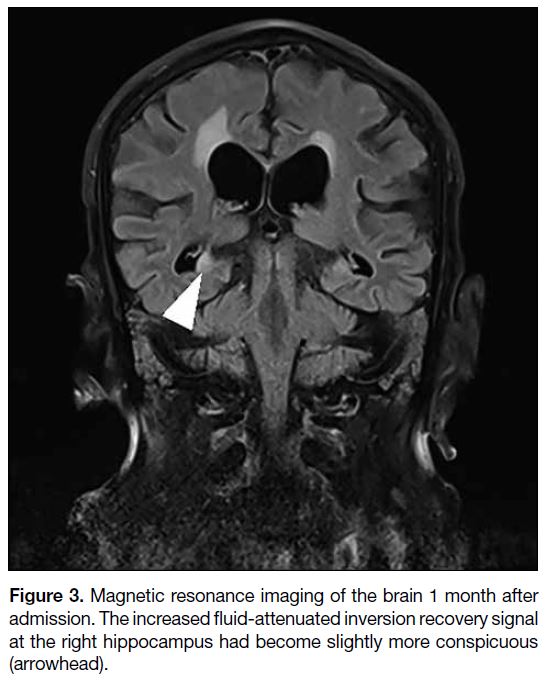
that were positive. MRI of the brain was repeated 1
month after admission and revealed the increased FLAIR
signal at the right hippocampus to have become slightly
more conspicuous (Figure 3).
Figure 3. Magnetic resonance imaging of the brain 1 month after
admission. The increased fluid-attenuated inversion recovery signal
at the right hippocampus had become slightly more conspicuous
(arrowhead).
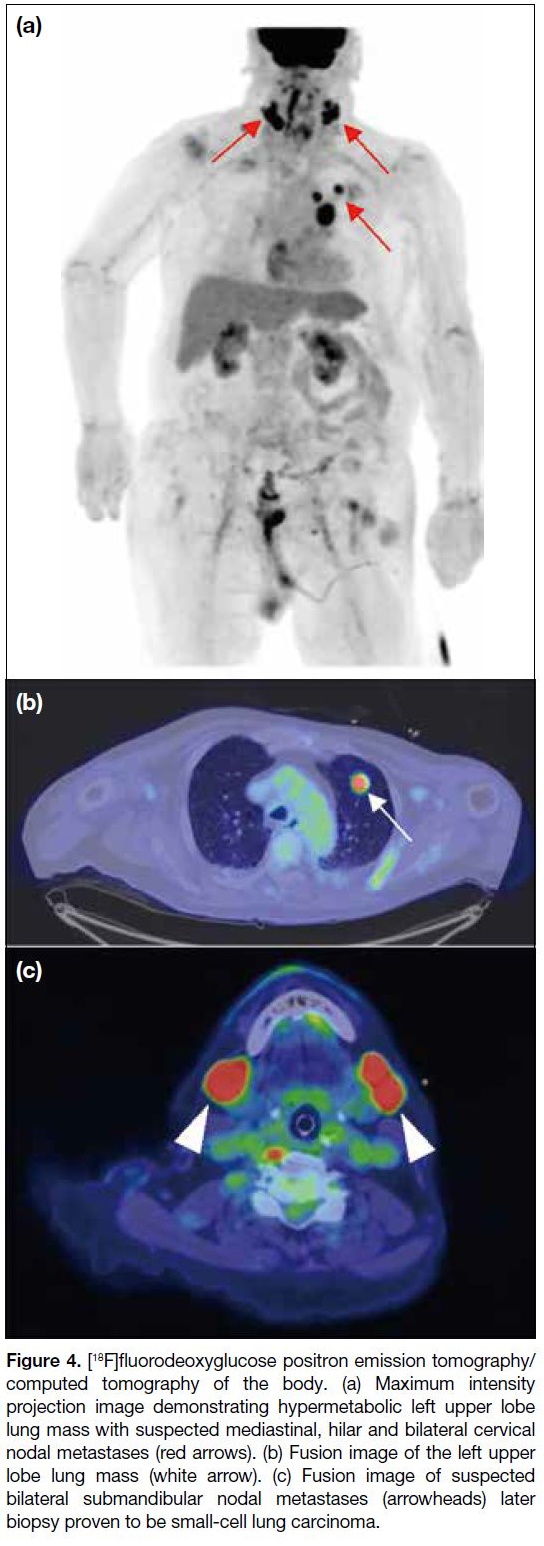
[18F]FDG PET/CT was performed the day after the
second MRI of the brain. A hypermetabolic lung
mass was found in the apicoposterior segment of the
left upper lobe as well as multiple hypermetabolic
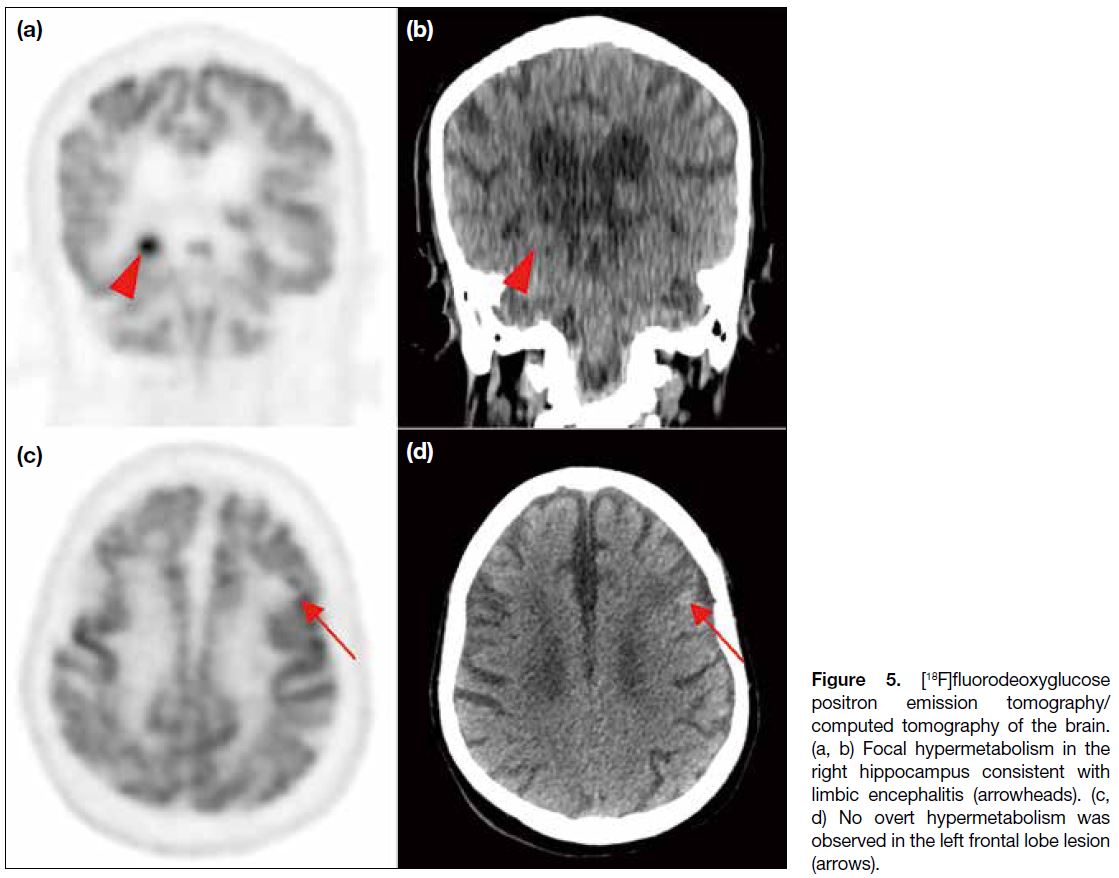
mediastinal, hilar and cervical lymph nodes (Figure 4). Findings raised suspicion of an underlying primary
lung tumour with multiple nodal metastases. Focal
hypermetabolism observed in the right hippocampus
was consistent with limbic encephalitis. Nonetheless no
overt hypermetabolism was observed in the left frontal
lobe brain lesion (Figure 5).
Figure 4. [18F]fluorodeoxyglucose positron emission tomography/computed tomography of the body. (a) Maximum intensity
projection image demonstrating hypermetabolic left upper lobe
lung mass with suspected mediastinal, hilar and bilateral cervical
nodal metastases (red arrows). (b) Fusion image of the left upper
lobe lung mass (white arrow). (c) Fusion image of suspected
bilateral submandibular nodal metastases (arrowheads) later
biopsy proven to be small-cell lung carcinoma.
Figure 5. [18F]fluorodeoxyglucose
positron emission tomography/computed tomography of the brain.
(a, b) Focal hypermetabolism in
the right hippocampus consistent
with limbic encephalitis (arrowheads).
(c, d) No overt hypermetabolism
was observed in the left frontal lobe
lesion (arrows).
The patient underwent plasmapheresis with substantial improvement in neurological symptoms. Incisional
biopsy of the right submandibular cervical lymph node
confirmed small-cell lung carcinoma. Chemotherapy and
radiotherapy were planned, but the patient developed a
severe hospital-acquired chest infection and sepsis with
rapid deterioration of his condition despite antibiotics.
Oncological treatment was suspended and the patient
eventually succumbed.
DISCUSSION
According to the diagnostic criteria of autoimmune
encephalitis proposed by Graus et al,[1] the diagnosis of
anti-GABAB receptor encephalitis could be established
in the patient presented here. Autoimmune encephalitis
is an emerging neurological disease associated with
neuronal autoantibodies against various neuronal
targets. Encephalitis with autoantibodies against GABAB
receptors is an uncommon entity with an estimated
relative frequency of 5%.[4] GABA receptors play an
important role in neuronal activity associated with
learning, memory and cognitive functions[5] and have
been found to cause limbic encephalitis.[6]
Limbic encephalitis refers to inflammation of the
limbic system and is considered a classic paraneoplastic
syndrome. Common malignancies associated with
limbic encephalitis include lung tumours (especially
small-cell lung cancer), seminoma, thymoma, breast
cancer, and lymphoma. Patients with limbic encephalitis
typically present with memory loss, confusion,
hallucinations, personality change, and seizures. Prompt
diagnosis and management are essential for neurological
recovery. Nevertheless the initial diagnostic tests
currently utilised are mainly cerebrospinal fluid analysis, electroencephalogram, and MRI of the brain.[1] The
role of [18F]FDG PET/CT remains unclear, despite
being a sensitive functional brain imaging technique.
Baumgartner et al[7] reported a higher sensitivity of
[18F]FDG PET/CT in detecting limbic encephalitis—associated pathological findings than MRI. [18F]FDG
PET/CT offers information on the neuronal metabolic
activity that increases in the presence of brain
inflammation. In the case we present, the FLAIR signal
abnormality was very subtle on MRI of the brain.
According to a case series by Höftberger et al,[8] 50%
of patients had small-cell lung carcinoma. They also
demonstrated that a patient may have neurological
improvement with oncological treatment alone.
Therefore, the clinical outcome for patients with anti-GABAB receptor encephalitis and underlying small-cell
lung carcinoma is dictated by successful treatment of the tumour. [18F]FDG PET/CT is also a sensitive oncological
diagnostic tool in addition to functional brain imaging.
Our patient underwent [18F]FDG PET/CT following
equivocal results of other investigations and consequent
diagnosis weeks after initial presentation. Unfortunately,
our patient succumbed and was not able to receive timely
oncological treatment. We suggest that incorporation of
[18F]FDG PET/CT in the initial assessment may benefit
the diagnosis and subsequent initiation of oncological
treatment in patients with underlying tumour as well as
their clinical outcome.
REFERENCES
1. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. Crossref
2. Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835-44. Crossref
3. Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L, et al. Brain 18F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:3847-58. Crossref
4. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179-89. Crossref
5. Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952-62. Crossref
6. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the
antigen. Lancet Neurol. 2010;9:67-76. Crossref
7. Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744-53. Crossref
8. Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology.
2013;81:1500-6. Crossref