Tumour-Induced Osteomalacia: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Mar;27(1):e43-5 | Epub 3 January 2024
Tumour-Induced Osteomalacia: A Case Report
TK Chow, KW Chan, YH Hui, WY Ho
Nuclear Medicine Unit, Department of Radiology, Queen Mary Hospital, Hong Kong SAR, China
Correspondence: Dr TK Chow, Nuclear Medicine Unit, Department of Radiology, Queen Mary Hospital, Hong Kong SAR, China. Email: ctk594@ha.org.hk
Submitted: 12 Oct 2022; Accepted: 13 Dec 2022.
Contributors: TKC designed the study, acquired and analysed the data, and drafted the manuscript. KWC, YHH and WYH critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki and provided informed consent for all procedures, and for publication of this case report.
INTRODUCTION
Tumour-induced osteomalacia (TIO), also known as
oncogenic osteomalacia, is a rare acquired paraneoplastic
syndrome. Since the culprit tumour is difficult to localise
using conventional anatomical imaging, functional
imaging plays an important role in localisation. We
present the case of a middle-aged male with TIO who
underwent various imaging investigations with failure
to localise the culprit tumour, where finally a 111indiumpentetreotide
scintigraphy (octreotide scan) located
the tumour over his left foot. This case highlights the
importance of covering the entire body when performing
an octreotide scan in patients with suspected TIO so as
not to miss any hidden tumour in the extremities.
CASE PRESENTATION
A 35-year-old man initially presented with right ankle
pain. Magnetic resonance imaging revealed a non-traumatic
stress fracture of the right distal tibia. One year
later, the patient complained of increasing bone and joint
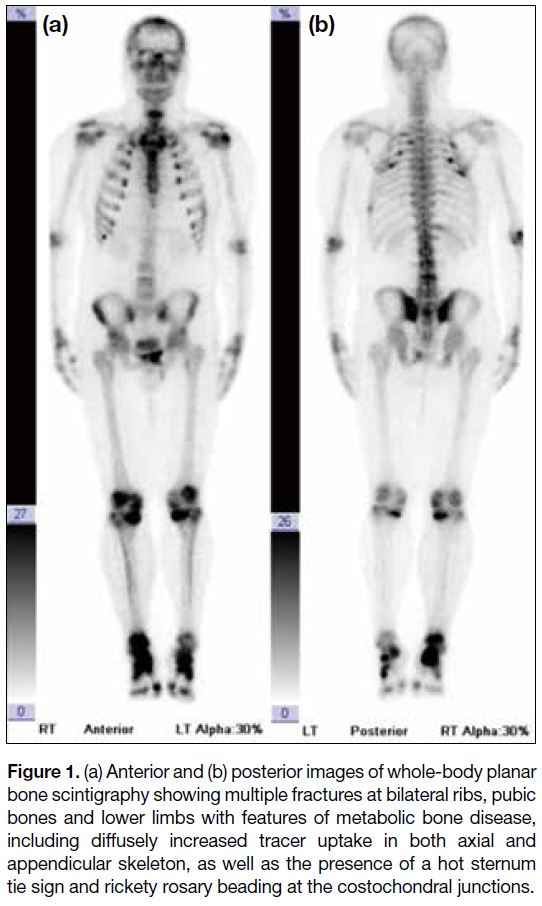
pain. Bone scintigraphy, performed at an external centre,
revealed multiple fractures of the ribs, bony pelvis
and extremities, along with features of metabolic bone
disease (Figure 1).
Figure 1. (a) Anterior and (b) posterior images of whole-body planar
bone scintigraphy showing multiple fractures at bilateral ribs, pubic
bones and lower limbs with features of metabolic bone disease,
including diffusely increased tracer uptake in both axial and
appendicular skeleton, as well as the presence of a hot sternum
tie sign and rickety rosary beading at the costochondral junctions.
Biochemically, the patient was found to have a
hypophosphataemia level of 0.48 mmol/L (normal range:
0.75-1.3) and an elevated alkaline phosphatase level of
852 U/L (normal range: 50-136). Further workup revealed
normal calcium level of 2.28 mmol/L (normal range:
2.11-2.55) and parathyroid hormone level of 4.4 pmol/L
(normal range: 1.6-6.9), low 1,25-dihydroxyvitamin
D level of 4.9 pg/mL (normal range: 19.6-54.3), and
elevated fibroblast growth factor 23 (FGF23) level of
252 RU/mL (normal range: <180). A presumptive
diagnosis of TIO was made in view of his typical
symptoms and biochemical profile. An extensive search
for the culprit tumour by various imaging modalities
was performed. Whole-body magnetic resonance
imaging, octreotide scan (from vertex to mid-thigh) and
18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) [from vertex to thigh]
performed at external centres showed no suspicious
lesion that could indicate TIO.
Two years later, the patient self-detected a lump over the
first web space of his left foot. Contrast CT revealed a
well-circumscribed lobulated isodense mass with avid
heterogenous contrast enhancement at the first web space. The nature of the lesion was non-specific but it
was suspected to be the cause of TIO. The patient was
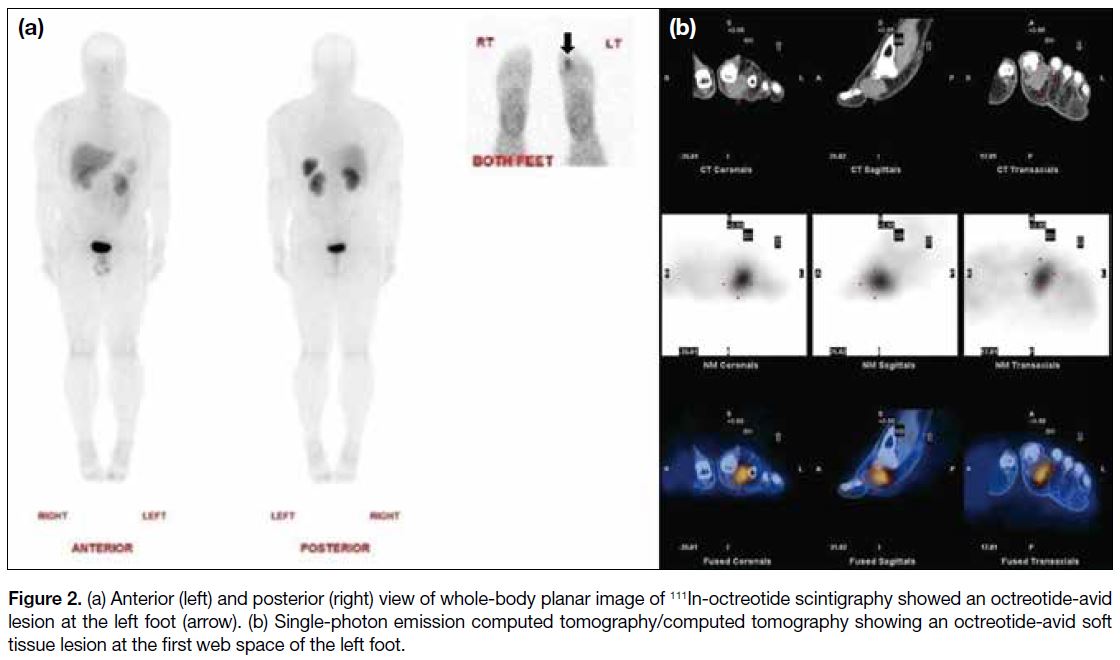
then referred to our centre where whole-body octreotide
scan revealed an octreotide-avid soft tissue mass at the
first web space of the left foot. In the absence of any
other suspicious lesion (Figure 2), this confirmed it to be
the culprit tumour.
Figure 2. (a) Anterior (left) and posterior (right) view of whole-body planar image of 111In-octreotide scintigraphy showed an octreotide-avid
lesion at the left foot (arrow). (b) Single-photon emission computed tomography/computed tomography showing an octreotide-avid soft
tissue lesion at the first web space of the left foot.
Subsequently, surgical resection of the tumour in the
left foot was performed. Histological diagnosis revealed
a phosphaturic mesenchymal tumour composed of
spindle to short oval cells arranged in irregular bundles,
characterised by a rich vasculature and mildly irregular
or twisted outline with indistinct nucleoli. The phosphate
level normalised 3 weeks after the surgery.
DISCUSSION
TIO is caused by a small benign tumour, most commonly classified histopathologically as phosphaturic
mesenchymal tumour, mixed connective tissue type.[1]
The tumour will secrete FGF23, a phosphaturic
hormone that leads to inhibition of renal phosphate
reabsorption. FGF23 also reduces the production of
1,25-dihydroxyvitamin D.[2] Eventually, these will result
in hypophosphataemia.
Patients with TIO present with muscle weakness,
bone pain, and multiple fractures.[3] Characteristic
biochemical features are hypophosphataemia and
hyperphosphaturia. Other common biochemical features
include normal calcium, parathyroid hormone and low-to-normal 1,25-dihydroxyvitamin D level, and elevated
alkaline phosphatase and FGF23 level.[3] Nonetheless
the non-specific nature of the symptoms often leads to
underdiagnosis with a consequent delay in management,
often years after initial presentation.[1]
With a consistent clinical history and typical
biochemical features establishing the diagnosis of TIO,
localisation of the culprit tumour is the next important
step in management. Nonetheless this often presents
another major challenge that delays curative treatment.
The culprit tumour is typically small and can present
anywhere in the body but more commonly in the lower
extremities or craniofacial region.[4] [5] [6] There is no specific
pattern or pathognomonic feature of the culprit tumour
on conventional anatomical imaging,[1] rendering tumour
localisation difficult.
Culprit tumours of TIO frequently express somatostatin
receptors (SSTR), evidenced by avid uptake on
somatostatin analogue imaging.[3] Therefore, 111In-octreotide
scintigraphy and 68Ga-DOTA–conjugated
SSTR-targeting peptide PET/CT (SSTR PET/CT) are
useful for tumour localisation. Due to possibly obscure
locations of culprit tumours, they are easily missed if a
whole-body scan is not performed. Whenever a patient is
suspected to have TIO, it is vital to ensure that the entire
body is included in the scan range so as not to miss any
hidden tumour in the extremities or craniofacial region.
In recent years, SSTR PET/CT imaging has gained
popularity due to its lower radiation exposure, shorter
acquisition time, and improved spatial resolution
compared with 111In-octreotide scintigraphy.[7] [8] Previous
studies have shown that SSTR PET/CT imaging exhibits
the highest sensitivity and specificity among different
functional imaging studies for localising the culprit
tumour of TIO.[8] [9] A recent systematic review and meta-analysis showed a pooled detection rate of 87.6%
for culprit tumour localisation using SSTR PET/CT imaging.[10]
Definitive treatment for TIO is surgical resection of the
culprit tumour. This usually results in rapid resolution of
hypophosphataemia.[1]
CONCLUSION
TIO is a rare but devastating disease. One of the major
challenges is localisation of the culprit tumour and
subsequent curative surgical resection. Somatostatin
analogue imaging, including 111In-octreotide scintigraphy
and 68Ga-DOTA-SSTR PET/CT, is useful in localising
the culprit tumour. It is worth noting that coverage of
the entire body in the scan range is mandatory to avoid
missing any hidden tumour in the extremities.
REFERENCES
1. Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005;294:1260-7. Crossref
2. Hendry DS, Wissman R. Case 165: oncogenic osteomalacia. Radiology. 2011;258:320-2. Crossref
3. Dahir K, Zanchetta MB, Stanciu I, Robinson C, Lee JY, Dhaliwal R, et al. Diagnosis and management of tumor-induced
osteomalacia: perspectives from clinical experience. J Endocr Soc.
2021;5:bvab099. Crossref
4. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53-77. Crossref
5. Zhang J, Zhu Z, Zhong D, Dang Y, Xing H, Du Y, et al. 68Ga DOTATATE PET/CT is an accurate imaging modality in the
detection of culprit tumors causing osteomalacia. Clin Nucl Med.
2015;40:642-6. Crossref
6. Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M, et al. Tumor localization and biochemical response to cure
in tumor-induced osteomalacia. J Bone Miner Res. 2013;28:1386-
98. Crossref
7. Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500-
16. Crossref
8. El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, et al. 68Ga-DOTATATE for tumor
localization in tumor-induced osteomalacia. J Clin Endocrinol
Metab. 2016;101:3575-81. Crossref
9. Breer S, Brunkhorst T, Beil FT, Peldschus K, Heiland M, Klutmann S, et al. 68Ga DOTA-TATE PET/CT allows tumor
localization in patients with tumor-induced osteomalacia but
negative 111In-octreotide SPECT/CT. Bone. 2014;64:222-7. Crossref
10. Meyer M, Nicod Lalonde M, Testart N, Jreige M, Kamani C,
Boughdad S, et al. Detection rate of culprit tumors causing
osteomalacia using somatostatin receptor PET/CT: systematic
review and meta-analysis. Diagnostics (Basel). 2020;10:2. Crossref