Intraventricular H3K27-Altered Diffuse Midline Glioma in Lateral Ventricle: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Mar;27(1):e39-42 | Epub 12 March 2024
Intraventricular H3K27-Altered Diffuse Midline Glioma in Lateral Ventricle: A Case Report
PL Kwok1 YH Lui2, HK Chin1, LY Ho3, SK Chan3, CY Chu1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
2 Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
3 Department of Neurosurgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr PL Kwok, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: kpl995@ha.org.hk
Submitted: 9 January 2023; Accepted: 23 May 2023.
Contributors: PLK and CYC designed the study. All authors acquired the data. PLK and YHL analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: HKECREC-2022-057). The patient was treated in accordance with the Declaration of Helsinki and patient consent was waived by the
Committee due to no disclosure of patient’s identity.
Acknowledgement: The authors thank Prof HK Ng, Department of Anatomical and Cellular Pathology of Prince of Wales Hospital, for advising on the pathological diagnosis of the study.
CASE PRESENTATION
A 41-year-old man with glucose-6-phosphate
dehydrogenase deficiency who was a light smoker
of 5 pack years and social drinker first presented in
2022 in Hong Kong with left hemiparesis, unsteady
gait and headache, with Glasgow Coma Scale score of
13. Non-contrast axial computed tomography of the
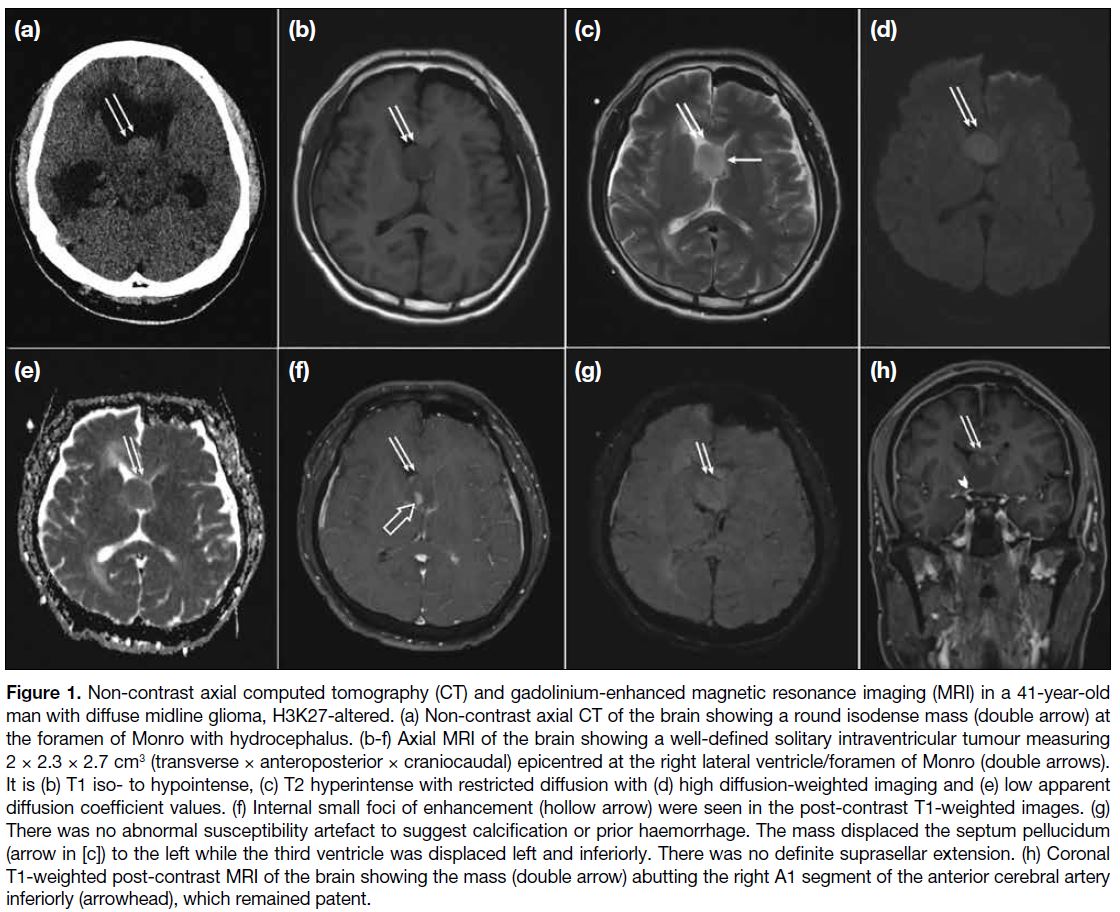
brain revealed a tumour at the foramen of Monro with
hydrocephalus (Figure 1a). Mannitol was administered
and urgent external ventricular shunting was performed
to relieve the hydrocephalus. A well-defined solitary
intraventricular tumour (Figure 1b-h) measuring 2 × 2.3 × 2.7 cm3 was seen on subsequent magnetic resonance
imaging (MRI), epicentred at the right lateral ventricle/foramen of Monro. It was T1 iso- to hypointense, and T2
hyperintense with restricted diffusion and internal small
foci of enhancement. There was no abnormal blooming
artefact. The mass displaced the septum pellucidum
to the left while the third ventricle was displaced to the left and inferiorly. Owing to its intraventricular
location, it was first suspected to be a subependymoma,
ependymoma, or central neurocytoma. Frozen section of
a limited endoscopic biopsy was consistent with a glial
tumour. Given the clinical history, an ependymoma or
subependymoma remained possible but no definitive
diagnosis was reached.
Figure 1. Non-contrast axial computed tomography (CT) and gadolinium-enhanced magnetic resonance imaging (MRI) in a 41-year-old
man with diffuse midline glioma, H3K27-altered. (a) Non-contrast axial CT of the brain showing a round isodense mass (double arrow) at
the foramen of Monro with hydrocephalus. (b-f) Axial MRI of the brain showing a well-defined solitary intraventricular tumour measuring
2 × 2.3 × 2.7 cm3 (transverse × anteroposterior × craniocaudal) epicentred at the right lateral ventricle/foramen of Monro (double arrows).
It is (b) T1 iso- to hypointense, (c) T2 hyperintense with restricted diffusion with (d) high diffusion-weighted imaging and (e) low apparent
diffusion coefficient values. (f) Internal small foci of enhancement (hollow arrow) were seen in the post-contrast T1-weighted images. (g)
There was no abnormal susceptibility artefact to suggest calcification or prior haemorrhage. The mass displaced the septum pellucidum
(arrow in [c]) to the left while the third ventricle was displaced left and inferiorly. There was no definite suprasellar extension. (h) Coronal
T1-weighted post-contrast MRI of the brain showing the mass (double arrow) abutting the right A1 segment of the anterior cerebral artery
inferiorly (arrowhead), which remained patent.
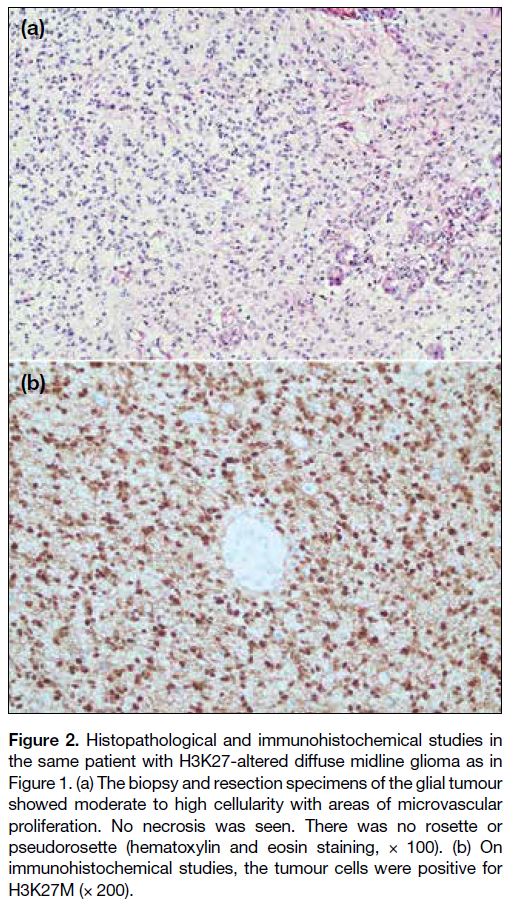
More formalin-fixed paraffin-embedded tissue was
subsequently examined. The glial tumour (Figure 2a) showed moderate cellularity, moderate nuclear
pleomorphism, enlarged hyperchromatic nuclei,
and fibrillary eosinophilic cytoplasm. Mitotic count
was up to 4 mitotic figures per 10 high-power fields.
There was microvascular proliferation but no necrosis
was seen. There was no rosette or pseudorosette.
Immunohistochemical studies revealed that the tumour
cells were positive for GFAP (glial fibrillary acidic
protein), Olig2 (oligodendrocyte transcription factor 2) and H3K27M (Figure 2b), with retained ATRX
(alpha-thalassemia/mental retardation, X-linked).
Staining for p53 showed a wild-type pattern, while
staining for H3K27me3 was lost in tumour cell nuclei.
Stainings for epithelial membrane antigen and IDH1
(isocitrate dehydrogenase 1) R132H were negative.
The Ki-67 proliferative index was up to 30%. Histone
H3 Sanger sequencing detected a mutation of c.83A>T
(p.Lys28Met) [K27M] in the H3F3A gene. The overall
findings were consistent with diffuse midline glioma
(DMG), H3K27-altered (grade 4 of the World Health
Organization [WHO] Classification of Tumours of the
Central Nervous System).
Figure 2. Histopathological and immunohistochemical studies in
the same patient with H3K27-altered diffuse midline glioma as in
Figure 1. (a) The biopsy and resection specimens of the glial tumour
showed moderate to high cellularity with areas of microvascular
proliferation. No necrosis was seen. There was no rosette or
pseudorosette (hematoxylin and eosin staining, × 100). (b) On
immunohistochemical studies, the tumour cells were positive for
H3K27M (× 200).
Following discussion with local colleagues, a final
diagnosis was made of H3K27-altered DMG. Subsequent
workup including MRI of the whole spine (not shown) revealed no spinal cord involvement.
There were limited randomised data and no consensus
on treatment for H3K27-altered DMG for the
patient. Based on available evidence, resection and
chemoradiotherapy with temozolomide was scheduled
followed by adjuvant temozolomide if grade 4 disease
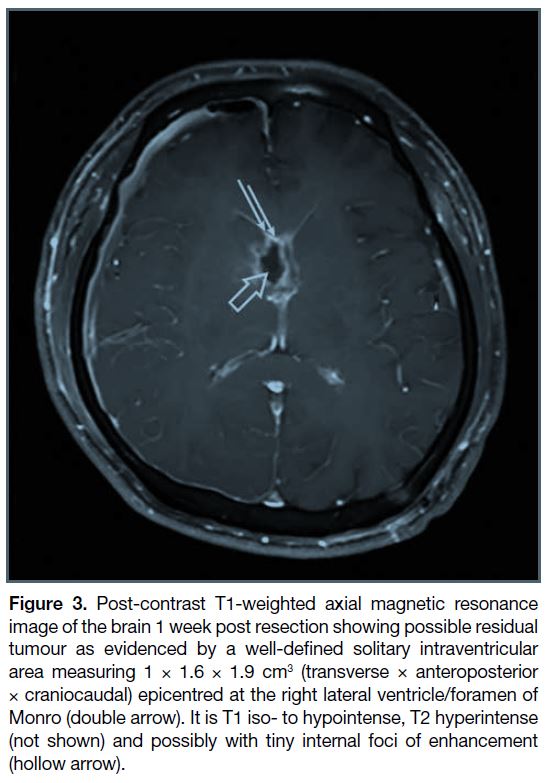
was confirmed. As expected and considering the
presence of infiltration of the basal part, post-resection
MRI showed partial resection (Figure 3). Final
histology of the resected specimen was similar to the
previous biopsy, confirming the diagnosis of a grade-4
H3K27-altered DMG (Figure 2a). Further molecular
studies showed no IDH1 or IDH2 gene mutation on
sequencing and no MGMT (O6-methylguanine-DNA
methyltransferase) gene promoter methylation evident
on polymerase chain reaction.
Figure 3. Post-contrast T1-weighted axial magnetic resonance
image of the brain 1 week post resection showing possible residual
tumour as evidenced by a well-defined solitary intraventricular
area measuring 1 × 1.6 × 1.9 cm3 (transverse × anteroposterior
× craniocaudal) epicentred at the right lateral ventricle/foramen of
Monro (double arrow). It is T1 iso- to hypointense, T2 hyperintense
(not shown) and possibly with tiny internal foci of enhancement
(hollow arrow).
Chemoradiotherapy was completed and the patient was
scheduled for review with possible subsequent adjuvant
chemotherapy.
DISCUSSION
Intraventricular tumours are rare and represent only
0.8% to 1.6% of all intracranial tumours.[1] Most
intraventricular tumours are benign and are more
common in children than in adults, comprising about
16% of childhood and adolescent intracranial tumours.[1]
Common intraventricular tumours include: (1) neoplasm
of the choroid plexus, e.g., choroid plexus papillomas,
choroid plexus carcinomas, meningioma, and metastases;
(2) neoplasm of the ventricular wall and septum
pellucidum, e.g., ependymoma, subependymoma,
subependymal giant cell astrocytoma, and central neurocytoma; (3) secondary intraventricular tumours,
e.g., glioblastoma multiforme; and (4) non-neoplastic
lesions, e.g., colloid cysts, arachnoid cysts, ependymal
cysts, and choroid plexus cysts.[1] The most common
clinical presentations of intraventricular tumours are
secondary to hydrocephalus and subsequent increase in
intracranial pressure, including headache and vomiting
with papilledema in adults.[1]
Patient age, tumour location and imaging features will
narrow the list of differential diagnoses. Ependymomas
and choroid plexus tumours are more often found in
children, and meningiomas and central neurocytoma
are more usually seen in adults. Tumours such as
central neurocytomas and subependymal giant cell
astrocytomas are predominantly found in the anterior
aspect of the lateral ventricles, whilst ependymomas
and subependymomas are more commonly found in the
fourth ventricle.[1]
H3K27M-mutant DMG was included in the 2016
WHO Classification of Tumours of the Central Nervous System, according to its histological and molecular
characteristics. It is usually located in the midline
structures. In the 2021 updated guideline, it was
renamed ‘H3K27-altered’.[2] It is a diffusely infiltrative
WHO grade 4 tumour with very poor prognosis and a
5-year survival of < 1%. Patients with H3-mutant DMGs
have a significantly shorter overall survival than those
with the H3 wild-type.[3] The most common locations
at presentation are the brainstem, thalamus, and spinal
cord. A study has reported better prognosis for patients
with this type of DMG in unusual anatomical locations
(e.g., lateral ventricles) compared with those at the
typical location (i.e., brainstem).[3] It is a unique entity
affecting children and very rarely adults.[4] The average
age at presentation of H3K27M-altered DMG is 7 to
11 years.[5] Its presentation in the lateral ventricle is
extremely rare with only two reported adult cases.[3] [5] It
is therefore uncommon to have H3K27-altered DMG in
the intraventricular region and occurrence is also rare in
adults.
The radiological appearances of H3K27-altered DMG
are highly heterogeneous and lack specificity.[4] On MRI,
the tumours are typically T1 iso- or hypointense and T2
hyperintense, and signal intensities are homogeneous on
fluid-attenuated inversion recovery images. Restricted
diffusion can be seen with invasive growth of the tumour.
Intratumoural haemorrhage and necrosis are common and
ring enhancement of the tumour can be seen although
enhancement is usually not significant.[2] A connection
between imaging findings and the H3K27-altered histone
changes is not known due to the lack of relevant research
making the diagnosis by imaging alone difficult.[2]
Due to the intraventricular location of this high-grade tumour, surgical resection is difficult.[2] The management
plan for our case was maximal safe surgical resection
plus adjuvant radiotherapy. Complete excision was not
possible as the disease was quite infiltrative at the basal
part.
In summary, we report an extremely rare case of primary
lateral ventricle H3K27-altered DMG in a middle-aged
man. Its intraventricular location made diagnosis
based on imaging findings difficult, with our initial
differential diagnoses of subependymoma, ependymoma
and central neurocytoma incorrect. Further endoscopic
biopsy helped confirm the final diagnosis and the patient
underwent resection followed by chemoradiotherapy,
and likely subsequent adjuvant chemotherapy. Early
biopsy and molecular characterisation are the key to
accurate diagnosis and prompt treatment. A high index
of suspicion is needed to avoid missing the diagnosis.
New clinical, imaging and histopathological information
remain to be established but will aid in the diagnosis of
this rare disease.
REFERENCES
1. Agarwal A, Kanekar S. Intraventricular tumors. Semin Ultrasound CT MR. 2016;37:150-8. Crossref
2. Zhao B, Sun K, Zhang Z, Xu T, Zhao L, Liu C, et al. A rare presentation of primary lateral ventricle H3 K27-altered diffuse midline glioma in a 14-year-old girl: a case description. Quant
Imaging Med Surg. 2022;12:5288-95. Crossref
3. Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89-96. Crossref
4. Yekula A, Gupta M, Coley N, U HS. Adult H3K27M-mutant diffuse midline glioma with gliomatosis cerebri growth pattern: case report and review of the literature. Int J Surg Case Rep. 2020;68:124-8. Crossref
5. Luo Y, Zeng L, Xie XQ, Wang F, Liu YZ, Kang JB, et al. H3K27M mutant diffuse midline glioma: a case report. Eur Rev Med Pharmacol Sci. 2020;24:2579-84. Crossref