Contrast-Enhanced Spectral Mammography Versus Magnetic Resonance Imaging: Intra- and Inter-Observer Agreements in Tumour Size Assessment
ORIGINAL ARTICLE
Hong Kong J Radiol 2024 Mar;27(1):e28-38 | Epub 21 March 2024
Contrast-Enhanced Spectral Mammography Versus Magnetic Resonance Imaging: Intra- and Inter-Observer Agreements in Tumour Size Assessment
TY Ko1, AYT Lai1, BST Leung2, MKK Law1, AHC Wong1, KH Chin1, WWC Wong1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
2 Department of Radiology, CUHK Medical Centre, Hong Kong SAR, China
Correspondence: Dr TY Ko, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: kty164@ha.org.hk
Submitted: 6 July 2022; Accepted: 22 January 2023.
Contributors: TYK, AYTL and BSTL designed the study. All authors acquired the data. TYK and AYTL analysed the data and drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: HKECREC-2022-046). The requirement for informed patient consent was waived by the Committee due to the retrospective nature of the
study.
Acknowledgement: The authors thank the following individuals for their contribution in patient selection and clinical assessment: Dr Yeuk-hei Ling (Ruttonjee Hospital), Dr Bonnie Pui-ling Chau (Ruttonjee Hospital), Dr Jennifer Suet-ying Lee (Pamela Youde Nethersole Eastern Hospital), and Dr Lorraine Wai-yan Ma (Pamela Youde Nethersole Eastern Hospital).
Abstract
Introduction
In patients with locally advanced breast carcinomas undergoing neoadjuvant chemotherapy (NAC), imaging monitoring is important for guiding clinical management. Contrast-enhanced spectral mammography (CESM) is a recently introduced modality that may serve this purpose as an alternative to magnetic resonance imaging (MRI). We aimed to investigate intra- and inter-observer agreements in CESM and MRI in assessment of tumour size.
Methods
Imaging studies performed between December 2019 and March 2022 for breast cancer patients undergoing NAC were retrospectively reviewed. Two radiologists measured the largest lesion sizes, intra- and inter-observer agreements were measured using the intraclass correlation coefficient. To assess the agreement between CESM and MRI findings, Lin’s concordance correlation coefficient (CCC) and Bland–Altman plots were used. Scanning time and reading time were recorded and compared.
Results
12 cases of patients who had undergone a total of 20 CESM studies and 18 MRI studies were assessed. The intra-observer agreement for the two radiologists on CESM was 0.983 and 0.996. The inter-observer agreement on CESM was 0.995. For MRI, the intra-observer agreement was 0.975 and 0.984, while the inter-observer agreement was 0.982. The agreement between the 10 baseline CESM and MRI studies was high (CCC = 0.972). Both the scanning time and reading time were significantly shorter for CESM than MRI (both p < 0.001)
Conclusion
Our results provide further evidence of CESM measurement reproducibility before, during, and after NAC. CESM can be considered an alternative assessment modality for monitoring NAC response.
Key Words: Breast neoplasms; Magnetic resonance imaging; Mammography; Neoadjuvant therapy
中文摘要
對比增強波譜乳房造影與磁力共振:腫瘤大小評估中觀察者內和觀察者間的一致性
高子恩、黎爾德、梁肇庭、羅嘉麒、黃可澄、錢凱、黃慧中
引言
對於接受術前輔助化療的局部晚期乳腺癌患者,影像監測在指導臨床治療方面非常重要。對比增強波譜乳房造影(CESM)是最近推出的一種模式,可用於此目的並作為磁力共振的替代方案。本文研究CESM和磁力共振中觀察者內和觀察者間評估腫瘤大小的一致性。
方法
我們對2019年12月至2022年3月期間接受術前輔助化療的乳腺癌患者的影像學檢查進行回顧性分析。最大的病灶尺寸由兩位放射科醫生測量,並使用組內相關系數評估觀察者內和觀察者間的一致性。我們使用Lin氏一致性相關系數和Bland-Altman圖評估CESM和磁力共振結果之間的一致性,以及記錄並比較掃描時間和閱片時間。
結果
12例患者接受了共20次CESM檢查和18次磁力共振檢查。兩位放射科醫生的CESM觀察者內一致性為0.983和0.996,觀察者間一致性為0.995。磁力共振觀察者內一致性為0.975和0.984,觀察者間一致性為0.982。十次基線CESM和磁力共振檢查間的一致性很高(一致性相關系數 = 0.972)。CESM的掃描時間和閱片時間均顯著短於磁力共振(兩者p值均為 < 0.001)。
結論
我們的結果進一步證明了術前輔助化療之前、期間和之後CESM測量的可重複性。CESM可視為術前輔助化療療效監測的替代方式。
INTRODUCTION
Neoadjuvant chemotherapy (NAC) is currently the
treatment of choice for patients presenting with
unresectable disease, locally advanced disease, or
inflammatory breast cancer, all of which NAC may
render resectable.[1] [2] After NAC, an improved tumour-breast
ratio may allow breast-conserving surgery, which
leads to a better cosmetic outcome. In patients with
human epidermal growth factor receptor 2–positive and
triple-negative breast cancer (TNBC) subtypes, findings
of residual disease after NAC provide prognostic
information and can guide further adjuvant therapy.[3] [4]
Accurate assessment of NAC response is therefore
important to guide subsequent management. Initially,
response to NAC was assessed by a combination of
clinical examination, mammography, and ultrasound.
Subsequently, contrast-enhanced magnetic resonance
imaging (MRI) was proven to be a superior imaging
modality, with better assessment of tumour extent,
visualisation of additional tumour foci, and identification
of residual disease after NAC.[5] [6] One of the major
advantages of MRI is the fact that it allows contrast-enhanced
imaging. However, the relatively high cost,
low availability, and long image acquisition time of MRI
may restrict patient access. Recently, contrast-enhanced spectral mammography (CESM) has been developed as
an imaging tool which utilises a dual-energy technique
to combine the advantages of digital mammography
with contrast-enhanced imaging. When evaluating
tumour extent, CESM had better correlation with the
size measured in histopathology specimens when
compared with standard mammography and ultrasound.[7]
In the setting of NAC response monitoring, early data
have shown CESM to be promising when compared to
MRI.[8] [9] [10] One study concluded that CESM has better
agreement with histological assessment of surgical
specimen in demonstrating complete pathological
response as compared to MRI.[8] Initial results[8] [9] [10] have suggested CESM to be a viable alternative to MRI in the setting of NAC response monitoring.
The aim of this study was to assess the intra-observer and inter-observer agreements in CESM and MRI to further
study the validity of CESM as an alternative option for
tumour size evaluation before and after NAC.
METHODS
Patients
From December 2019 to March 2022, a total of 12
patients were referred to Pamela Youde Nethersole Eastern Hospital for imaging monitoring of NAC
response. All patients had confirmed locally invasive
breast carcinoma through tissue sampling and underwent
CESM and/or MRI prior to the commencement of NAC
as a baseline study, with 10 patients undergoing both
CESM and MRI within 3 days of each other. Follow-up
CESM and/or MRI was performed at mid-cycle and/or
end of treatment.
Contrast-Enhanced Spectral Mammography
CESM was performed using the Selenia Dimensions
Mammography system (Hologic, Marlborough [MA],
US). Iohexol (Omnipaque; GE Healthcare, Milwaukee
[WI], US) was used as the CESM contrast agent. The
amount administered was calculated at 1.5 mL/kg,[11]
and administered at a rate of 3 mL/s through a
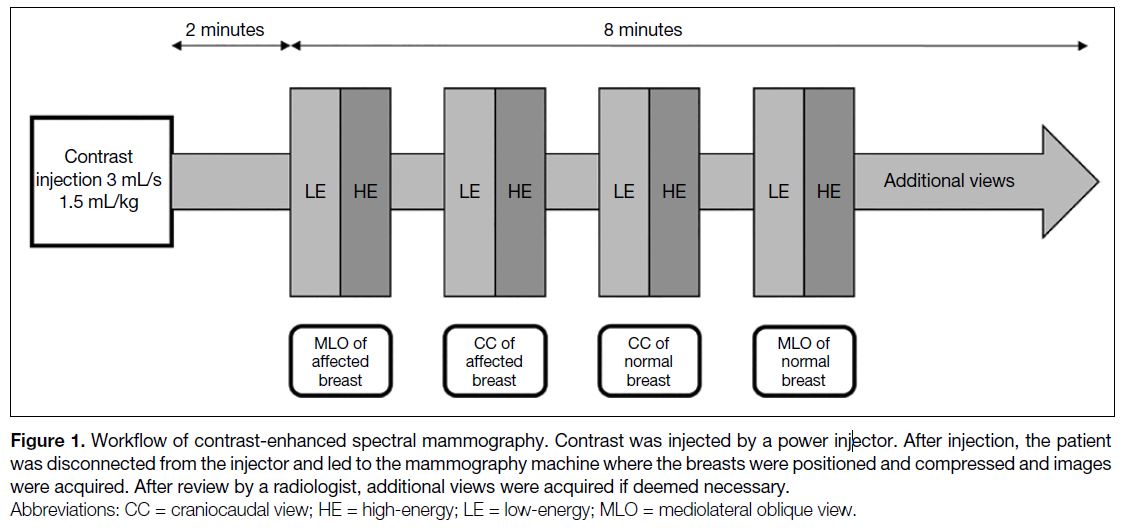
power injector. CESM images were then acquired 2
minutes after contrast injection and completed within
10 minutes, allowing an 8-minute window for image
acquisition (Figure 1).[11] CESM high-energy (45-49 kV)
and low-energy (28-33 kV) images were obtained in
the craniocaudal and mediolateral oblique projections
for each breast. CESM high-energy images were
used to produce subtracted images; the low-energy
and subtracted images were displayed for review by
radiologists. The CESM images were then immediately
reviewed by the session radiologist, and additional views,
e.g., magnified and compression views, were obtained if
deemed necessary (Figure 1). The time required for each
CESM study was recorded, starting at contrast injection
and concluding at the last image acquired.
Figure 1. Workflow of contrast-enhanced spectral mammography. Contrast was injected by a power injector. After injection, the patient was disconnected from the injector and led to the mammography machine where the breasts were positioned and compressed and images were acquired. After review by a radiologist, additional views were acquired if deemed necessary.
Magnetic Resonance Imaging
MRI was performed utilising the Siemens-Avanto 1.5T
MRI scanner (Erlangen, Germany), with patients in
the prone position. Gadoterate meglumine (Dotarem;
Guerbet, Villepinte, France) was used as contrast agent.
Sequences acquired included fat-suppressed axial T2-weighted sequence, coronal T2-weighted sequence,
axial T1-weighted sequence with dynamic contrast
(first sequence before contrast administration and seven
acquisitions after contrast agent administration each
spaced 1 minute apart), and axial diffusion-weighted
sequences. The time required for each MRI study was
recorded.
Imaging Interpretation
CESM and MRI images were interpreted by two
radiologists: a specialist radiologist with > 7 years’
experience in breast imaging, and a trainee radiologist
with 1 year of experience in breast imaging. During
image interpretation, the radiologists were blinded to the
measurements of the other imaging modality as well as
the measurements of the other radiologist. The images
from different patients were interpreted in a randomised
sequence and were interpreted twice by each radiologist
with a 2-month interval. In CESM, both low-energy
and subtracted images were reviewed (Figure 2). The
largest lesion in each breast was measured, disregarding
peritumoral calcifications. In MRI, the single largest
lesion was assessed in different sequences and planes,
where the largest dimension was recorded (Figures 3 and 4). The presence of any satellite lesions was also recorded on both CESM and MRI, and, if the entire
lesion was included, the largest dimension of the satellite
lesion was measured (Figure 5). Reading time for each
imaging study was also recorded, defined as the time
between opening and closing the images on the viewing
programme after recording the dimension of the largest lesion.
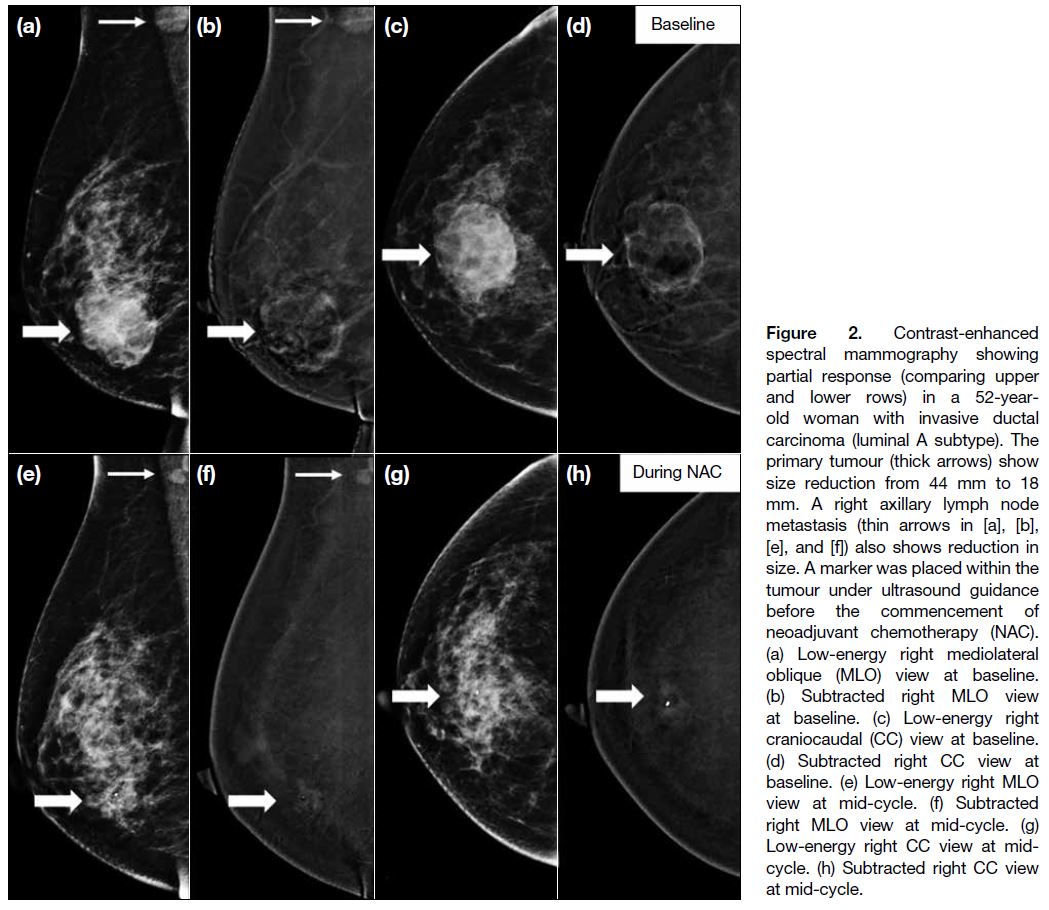
Figure 2. Contrast-enhanced
spectral mammography showing partial response (comparing upper and lower rows) in a 52-year-old woman with invasive ductal carcinoma (luminal A subtype). The primary tumour (thick arrows) show size reduction from 44 mm to 18 mm. A right axillary lymph node metastasis (thin arrows in [a], [b], [e], and [f]) also shows reduction in size. A marker was placed within the tumour under ultrasound guidance before the commencement of neoadjuvant chemotherapy (NAC). (a) Low-energy right mediolateral oblique (MLO) view at baseline. (b) Subtracted right MLO view at baseline. (c) Low-energy right craniocaudal (CC) view at baseline. (d) Subtracted right CC view at baseline. (e) Low-energy right MLO view at mid-cycle. (f) Subtracted right MLO view at mid-cycle. (g) Low-energy right CC view at mid-cycle. (h) Subtracted right CC view at mid-cycle.
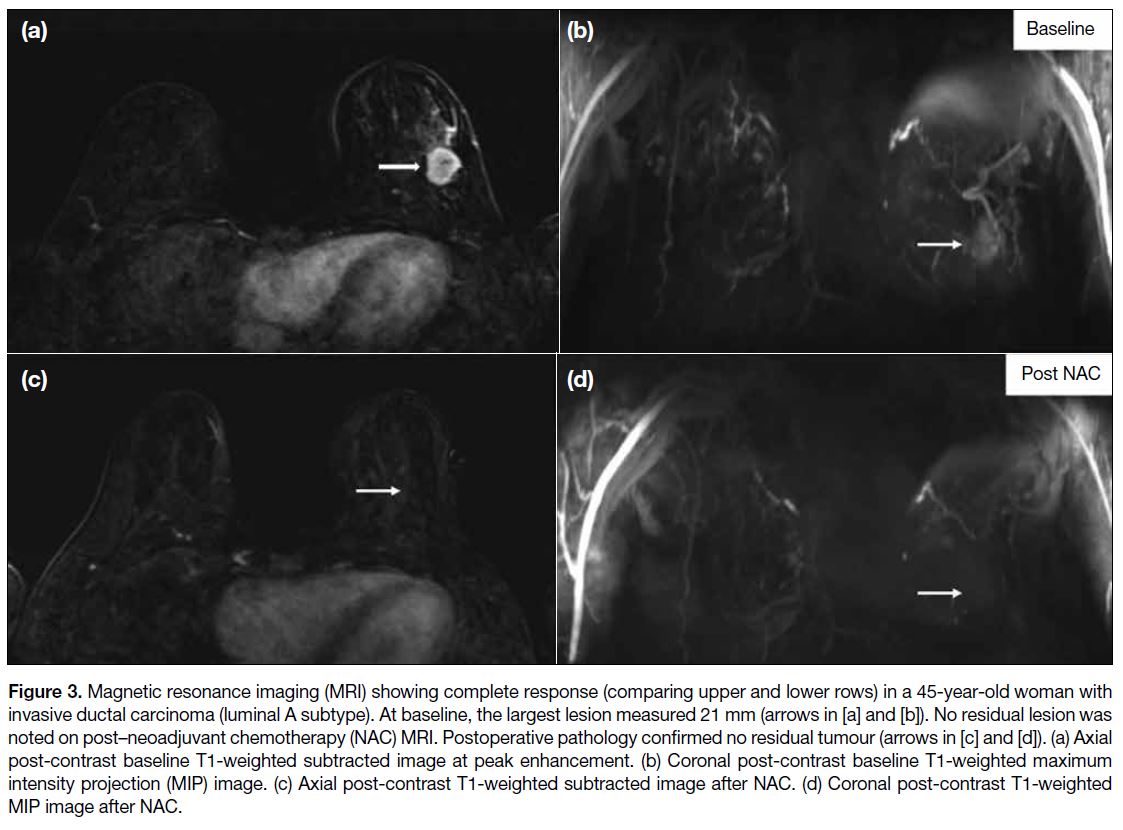
Figure 3. Magnetic resonance imaging (MRI) showing complete response (comparing upper and lower rows) in a 45-year-old woman with
invasive ductal carcinoma (luminal A subtype). At baseline, the largest lesion measured 21 mm (arrows in [a] and [b]). No residual lesion was
noted on post–neoadjuvant chemotherapy (NAC) MRI. Postoperative pathology confirmed no residual tumour (arrows in [c] and [d]). (a) Axial
post-contrast baseline T1-weighted subtracted image at peak enhancement. (b) Coronal post-contrast baseline T1-weighted maximum
intensity projection (MIP) image. (c) Axial post-contrast T1-weighted subtracted image after NAC. (d) Coronal post-contrast T1-weighted
MIP image after NAC.
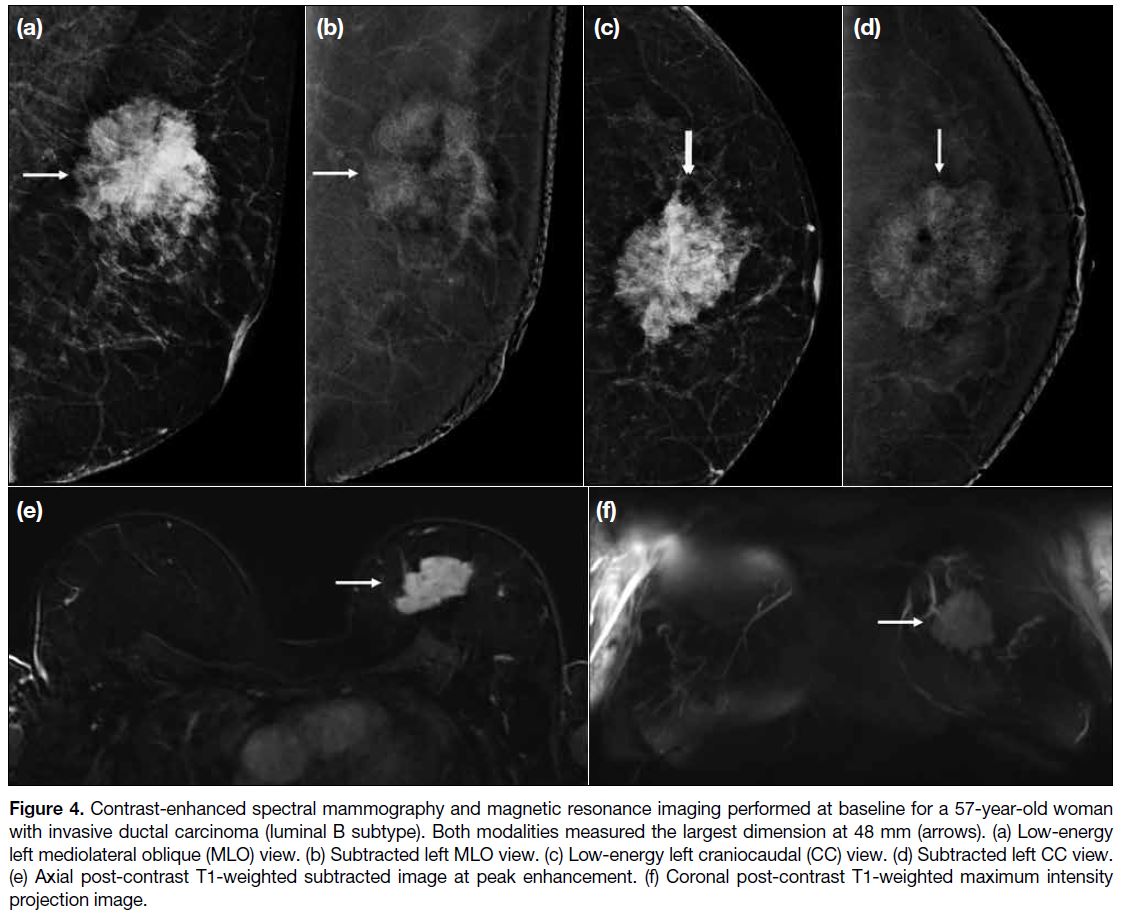
Figure 4. Contrast-enhanced spectral mammography and magnetic resonance imaging performed at baseline for a 57-year-old woman with invasive ductal carcinoma (luminal B subtype). Both modalities measured the largest dimension at 48 mm (arrows). (a) Low-energy left mediolateral oblique (MLO) view. (b) Subtracted left MLO view. (c) Low-energy left craniocaudal (CC) view. (d) Subtracted left CC view. (e) Axial post-contrast T1-weighted subtracted image at peak enhancement. (f) Coronal post-contrast T1-weighted maximum intensity projection image.
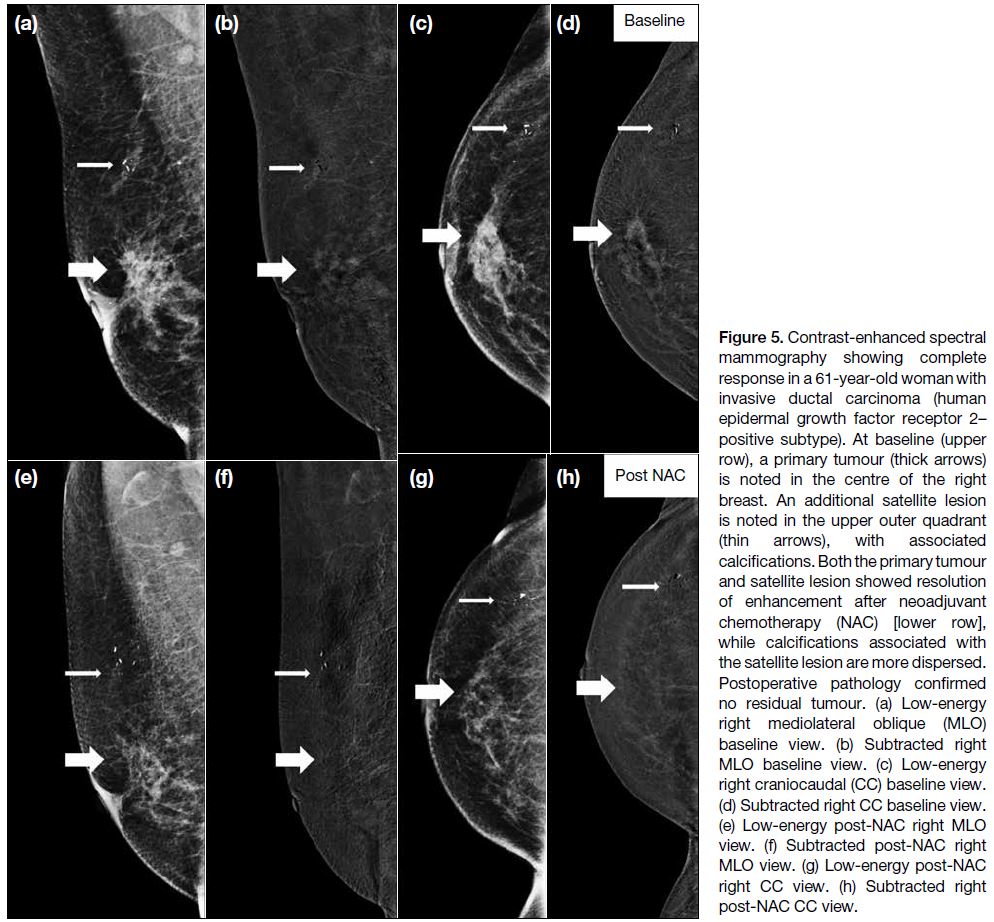
Figure 5. Contrast-enhanced spectral mammography showing complete response in a 61-year-old woman with invasive ductal carcinoma (human epidermal growth factor receptor 2–positive subtype). At baseline (upper
row), a primary tumour (thick arrows) is noted in the centre of the right breast. An additional satellite lesion is noted in the upper outer quadrant (thin arrows), with associated calcifications. Both the primary tumour and satellite lesion showed resolution of enhancement after neoadjuvant chemotherapy (NAC) [lower row], while calcifications associated with the satellite lesion are more dispersed. Postoperative pathology confirmed no residual tumour. (a) Low-energy right mediolateral oblique (MLO) baseline view. (b) Subtracted right MLO baseline view. (c) Low-energy right craniocaudal (CC) baseline view. (d) Subtracted right CC baseline view. (e) Low-energy post-NAC right MLO view. (f) Subtracted post-NAC right MLO view. (g) Low-energy post-NAC right CC view. (h) Subtracted right post-NAC CC view.
Statistical Analysis
The intraclass correlation coefficient (ICC) was used
to evaluate the intra- and inter-observer agreements of
both CESM and MRI results, using a two-way mixed
model testing for absolute agreement with a 95%
confidence interval (CI). Intra-observer agreement was
analysed for both radiologists, while inter-observer agreement was analysed using the first measurements
by both radiologists. Subgroup analysis was performed
by dividing the CESM studies into baseline studies and
non-baseline studies, i.e., mid-cycle and end-of-cycle
studies. Intra- and inter-observer agreements of these
subgroups was calculated by ICC. Lin’s concordance
correlation coefficient (CCC) and Bland–Altman
plots were used to evaluate for agreement between
the CESM and MRI studies in the cases of 10 patients
who had undergone both baseline imaging studies
within 3 days. The Mann–Whitney U test was used to
compare the scanning and reading times of CESM and
MRI. Statistical analysis was performed using SPSS
(Windows version 20.0; IBM Corp, Armonk [NY], US).
RESULTS
All 12 cases were female, with a mean age of 50.7
years (range, 32-66). Eleven cases were of invasive
ductal carcinoma and there was one case of invasive
lobular carcinoma. There were six cases of luminal A
subtype, three of luminal B subtype, and three of human
epidermal growth factor receptor 2–positive subtype.
There were no cases of TNBC. Out of the 12 cases, one
patient presented with urticaria after iodinated contrast
administration during CESM. None of the patients
presented with contrast reactions after gadolinium
contrast administration.
In total, 20 CESM studies and 18 MRI studies
were interpreted by the two radiologists. Individual
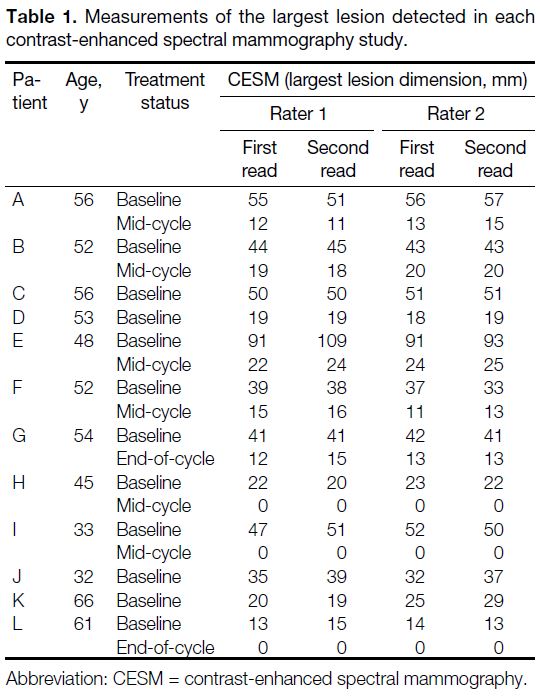
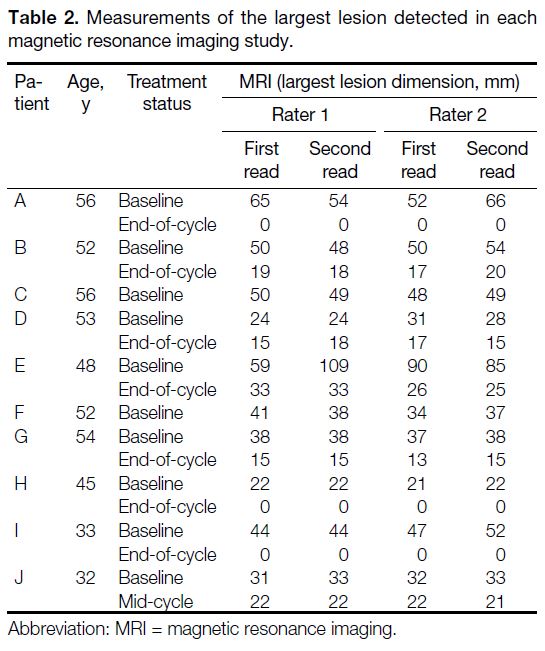
measurements for the largest lesion detected for each
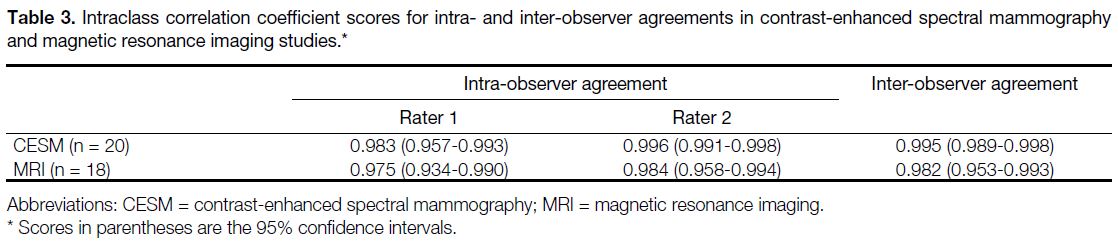
study are summarised in Tables 1 and 2. Overall, the ICCs
for both CESM and MRI were high, as summarised in
Table 3. The results for the subgroup analysis comparing
baseline and non-baseline CESM studies are summarised
in Table 4.
Table 1. Measurements of the largest lesion detected in each contrast-enhanced spectral mammography study.
Table 2. Measurements of the largest lesion detected in each magnetic resonance imaging study.
Table 3. Intraclass correlation coefficient scores for intra- and inter-observer agreements in contrast-enhanced spectral mammography and magnetic resonance imaging studies.
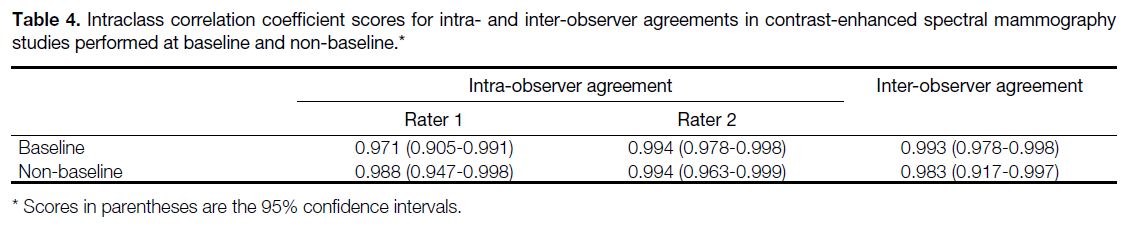
Table 4. Intraclass correlation coefficient scores for intra- and inter-observer agreements in contrast-enhanced spectral mammography studies performed at baseline and non-baseline.
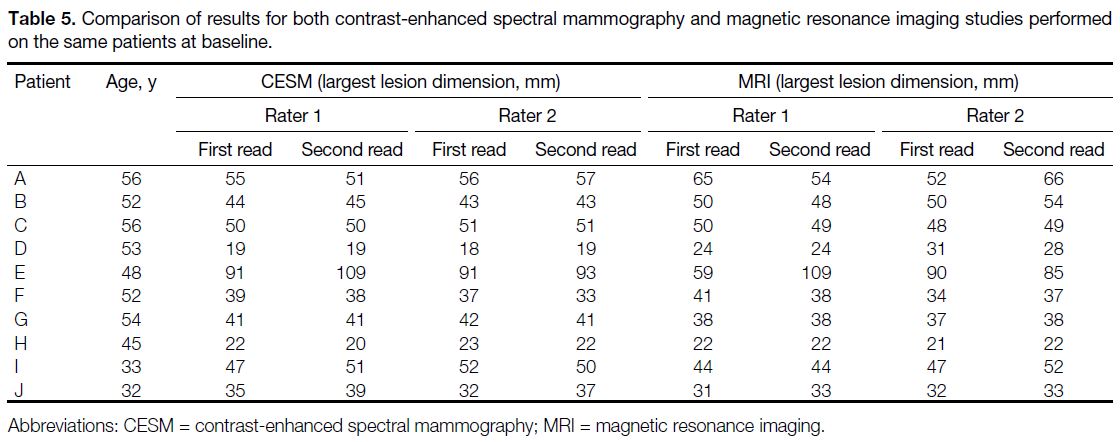
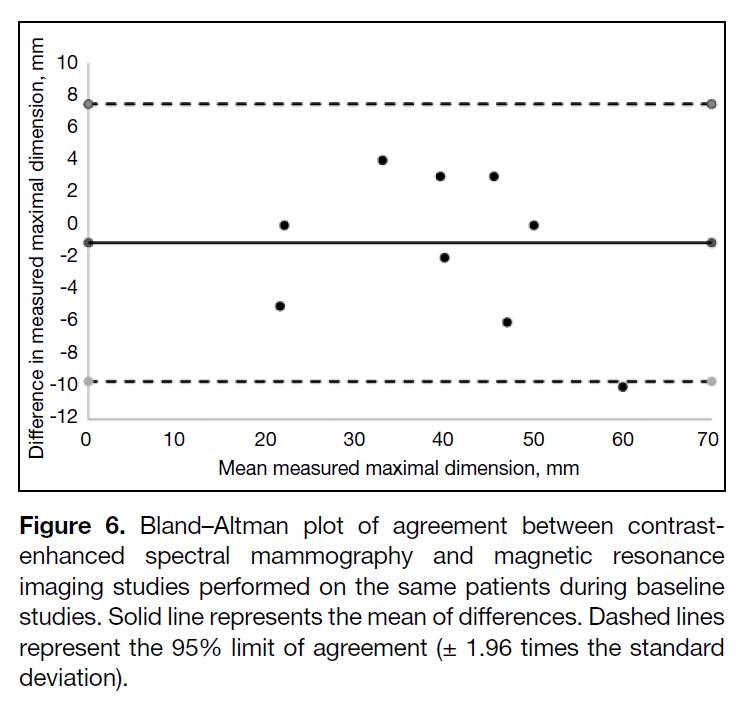
Table 5 summarises the results of the baseline CESM
and MRI studies. The CCC for comparing between the
two sets of baseline CESM and MRI studies was 0.972
(95% CI = 0.893-0.993; n = 10). The Bland–Altman plot
showed a bias of -1.1 mm and limits of agreement of -9.7
to 7.5 mm between modalities (Figure 6).
Table 5. Comparison of results for both contrast-enhanced
spectral mammography and magnetic resonance imaging studies
performed on the same patients at baseline.
Figure 6. Bland–Altman plot of agreement between contrast-enhanced spectral mammography and magnetic resonance imaging studies performed on the same patients during baseline studies. Solid line represents the mean of differences. Dashed lines represent the 95% limit of agreement (± 1.96 times the standard deviation).
A satellite lesion was only identified in one CESM
study. Both radiologists identified the lesion, with
measurements of 12 mm and 14 mm, respectively. MRI
was not performed on this patient; therefore, comparison
cannot be made. On the end-of-cycle CESM study
performed on this patient, both radiologists concurred
that the satellite lesion had resolved (Figure 5).
The median scanning time for CESM was 4.9 minutes
(interquartile range [IQR] = 1.3), while that for MRI
was 43.9 minutes (IQR = 10.8). The median reading
time for measuring the dimension of the largest lesion
on CESM was 54.5 seconds (IQR = 17.5), while that
for MRI was 96 seconds (IQR = 55). The results of the Mann–Whitney U test showed that both the scanning and reading times for CESM were significantly shorter (p < 0.001).
DISCUSSION
Contrast-enhanced imaging has substantial advantages
in assessing NAC response. Contrast enhancement is
based on abnormal angiogenesis in malignant tumours,
which results in the leakage of contrast medium from
the immature tumour vessels into the interstitial spaces.
MRI, which benefits from contrast-enhanced imaging,
has been proven to be a superior imaging modality to
traditional mammography and ultrasound.[5] [6] CESM is
another emerging modality which also benefits from
contrast-enhanced imaging. Intravenous iodinated
contrast is administered to the patient, and after 2
minutes contrast material reaches the breast tissues,[11]
allowing image acquisition to begin. It utilises a dual-energy technique, obtaining two spectral images using
different energy levels in quick succession while the
breast remains compressed. In the low-energy setting, the
energy is below the k-edge of iodine and contrast material
is not imaged, and the image can be interpreted as a
standard mammogram. In the high-energy setting, which
is above the k-edge of iodine, a non-interpretable image
is produced. Using subtraction, an image depicting only
areas of contrast enhancement results. The low-energy
image and the subtracted image are then interpreted
together by a radiologist. Owing to the subtraction
method and contrast-enhanced imaging, CESM has
been shown to be superior to standard mammography in
cancer diagnosis even in dense breasts.[12]
Along with the advancements in CESM, several
studies have compared the performance of CESM to
MRI in monitoring NAC response. One study showed that even though the use of CESM and MRI both led
to underestimation of the extent of residual tumour
compared to histology findings, CESM demonstrated
pathologic complete responses to NAC better than MRI.[8]
Other studies showed that CESM had good correlation
and agreement with histopathology comparable to MRI,
also showing high positive predictive values.[9] [10] Despite
the positive results, these studies did not thoroughly
compare intra- and inter-observer agreements in CESM
and MRI when assessing NAC. As shown by our results,
the intra- and inter-observer agreements for CESM were
excellent, all showing ICCs > 0.98. These results are
comparable with the intra- and inter-observer agreements in MRI, which showed ICCs > 0.97. Despite the fact that
one of the radiologists in the current study was a trainee
radiologist with only 1 year of experience in breast
imaging, the inter-observer agreement remained high.
This concurred with findings in previous studies,[12] [13]
suggesting that CESM techniques could be easily learned
by breast radiologists, which may be attributed to its
findings being akin to basic mammography observations.
Subgroup analysis was performed comparing the intra- and
inter-observer agreements in studies performed at
baseline prior to the commencement of NAC, and in
studies during or after NAC. It was thought that after
NAC, responding tumours may show shrinkage in both size and enhancement, possibly affecting the agreement
in size measurement as lesions may be less conspicuous.[8] Results showed that even though there was in fact a
slight drop in ICC, inter-observer agreement remained
excellent in the non-baseline group with an ICC of 0.983
(95% CI = 0.917-0.997), meaning that measurement
reproducibility remained high during or after NAC. Ten
of the patients in the current study underwent both CESM
and MRI prior to the commencement of NAC. Agreement
between the measurements of the two modalities was
high: CCC was 0.972 and mean difference was only
1.1 mm. This provides further support for CESM as a
viable alternative.
One of the advantages of CESM over MRI is the fact
that the scanning time is much shorter. This was proven
in the current study; scanning time in CESM was
significantly shorter than MRI (p < 0.001). In fact, when
considering the median scanning time required, more
than eight CESM studies can be performed during the
time required for one MRI study. Reading time was also
significantly shorter in CESM than MRI (p < 0.001).
This could be attributed to the fact that there are more
sequences and images in an MRI study compared with
CESM. However, it is important to keep in mind that
the reading time measured in the current study is only
regarding the measurement of the dimension of the
largest lesion, disregarding background and incidental
findings. Regardless, in the common scenario where
there are large numbers of patients, the potential time
saved through both scanning time and reading time is a
major advantage of CESM over MRI.
Despite the excellent results shown in the current study, there are limitations to CESM. One of the patients
presented with iodinated contrast allergy in the form of
urticaria. Additionally, the rate of adverse reactions in
iodinated contrast, such as nausea and headache, were
found to be significantly higher than those of gadolinium
contrast.[14] Thorough history taking and explanation
must be performed with patients before proceeding to
CESM. Radiation exposure is also an additional factor
to consider.
The ability to evaluate microcalcifications is an
advantage of CESM over MRI. Since this study only
measured the dimension of the largest lesion, the
effect of microcalcifications was not examined. Some
studies concluded that residual microcalcifications
after NAC correlated poorly with tumour size on
final pathology.[15] [16] Others showed that by including
both contrast enhancement and residual calcifications
in post-NAC CESM, sensitivity in residual disease detection increased but the false positive rate also
increased.[17] Therefore, the effect and reporting of
residual microcalcifications on post-NAC CESM
requires further research.
A satellite lesion was only detected in one case in the
current study. Correct identification of satellite lesions
is important for monitoring NAC response since it
may impact subsequent surgical planning. Multifocal
or multicentric disease are also known to be associated
with higher risk of locoregional recurrence after breast-conserving surgery.[18] The performance of CESM on detecting satellite lesions may require further research.
Limitations
There were several limitations of the current study.
It was a single-institution study with a small patient
population. The lack of TNBC within the molecular
subgroups may limit the applicability of our results to the
general population, given the fact that TNBC is one of
the indications for NAC. Due to the retrospective design
and constraint in resources (as shown in Tables 1 and 2), patients could not be arranged to undergo both CESM
and MRI at each point of the NAC cycle, therefore
agreement in results of the two modalities could not
always be compared to the non-baseline studies. Due
to the same reason, comparison with postoperative
histopathology could not be performed since most
patients did not undergo both CESM and MRI as end-of-cycle imaging evaluations. Further research with
histopathological correlation would be beneficial. Lastly,
when evaluating intra-observer agreement, despite a
2-month interval between image evaluation, recognition
of cases may produce measurement bias.
CONCLUSION
The current results showed that CESM had excellent
intra- and inter-observer agreements which were
comparable with MRI. Excellent agreement was found
when comparing baseline CESM and MRI studies.
Scanning and reading times were both significantly
shorter in CESM. These results provided further evidence
for CESM as a viable alternative to MRI for tumour size
monitoring in assessing NAC response.
REFERENCES
1. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin
Oncol. 2021;39:1485-505. Crossref
2. Ditsch N, Untch M, Thill M, Müller V, Janni W, Albert US, et al.
AGO recommendations for the diagnosis and treatment of patients
with early breast cancer: update 2019. Breast Care (Basel).
2019;14:224-45. Crossref
3. Morigi C. Highlights of the 16th St Gallen International Breast
Cancer Conference, Vienna, Austria, 20-23 March 2019:
personalised treatments for patients with early breast cancer.
Ecancermedicalscience. 2019;13:924. Crossref
4. Werutsky G, Untch M, Hanusch C, Fasching PA, Blohmer JU,
Seiler S, et al. Locoregional recurrence risk after neoadjuvant
chemotherapy: a pooled analysis of nine prospective neoadjuvant
breast cancer trials. Eur J Cancer. 2020;130:92-101. Crossref
5. Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R,
Cirillo S, et al. Monitoring response to primary chemotherapy in
breast cancer using dynamic contrast-enhanced magnetic resonance
imaging. Breast Cancer Res Treat. 2004;83:67-76. Crossref
6. Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M,
Schipper R, et al. The role of magnetic resonance imaging in
assessing residual disease and pathologic complete response in
breast cancer patients receiving neoadjuvant chemotherapy: a
systematic review. Insights Imaging. 2013;4:163-75. Crossref
7. Ali-Mucheru M, Pockaj B, Patel B, Pizzitola V, Wasif N, Stucky CC, et al. Contrast-enhanced digital mammography in the surgical management of breast cancer. Ann Surg Oncol.
2016;23(Suppl 5):649-55. Crossref
8. Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R,
et al. Contrast-enhanced spectral mammography in neoadjuvant
chemotherapy monitoring: a comparison with breast magnetic
resonance imaging. Breast Cancer Res. 2017;19:106. Crossref
9. Barra FR, Sobrinho AB, Barra RR, Magalhães MT, Aguiar LR,
de Albuquerque GF, et al. Contrast-enhanced mammography (CEM)
for detecting residual disease after neoadjuvant chemotherapy: a
comparison with breast magnetic resonance imaging (MRI).
Biomed Res Int. 2018;2018:8531916. Crossref
10. Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE, Lobbes M, et al. Contrast-enhanced spectral mammography is
comparable to MRI in the assessment of residual breast cancer
following neoadjuvant systemic therapy. Ann Surg Oncol.
2018;25:1350-6. Crossref
11. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C,
Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital
mammography: feasibility and comparison with conventional
digital mammography and MR imaging in women with known
breast carcinoma. Radiology. 2013;266:743-51. Crossref
12. Cheung YC, Lin YC, Wan YL, Yeow KM, Huang PC, Lo YF,
et al. Diagnostic performance of dual-energy contrast-enhanced
subtracted mammography in dense breasts compared to
mammography alone: interobserver blind-reading analysis. Eur
Radiol. 2014;24:2394-403. Crossref
13. Lalji UC, Houben IP, Prevos R, Gommers S, van Goethem M,
Vanwetswinkel S, et al. Contrast-enhanced spectral mammography
in recalls from the Dutch breast cancer screening program:
validation of results in a large multireader, multicase study. Eur
Radiol. 2016;26:4371-9. Crossref
14. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of
adverse effects of iodinated and gadolinium contrast materials:
retrospective review of 456,930 doses. AJR Am J Roentgenol.
2009;193:1124-7. Crossref
15. Weiss A, Lee KC, Romero Y, Ward E, Kim Y, Ojeda-Fournier H,
et al. Calcifications on mammogram do not correlate with tumor
size after neoadjuvant chemotherapy. Ann Surg Oncol.
2014;21:3310-6. Crossref
16. Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W.
Histopathologic correlation of residual mammographic
microcalcifications after neoadjuvant chemotherapy for locally
advanced breast cancer. Ann Surg Oncol. 2015;22:1111-7. Crossref
17. Iotti V, Ragazzi M, Besutti G, Marchesi V, Ravaioli S, Falco G, et al.
Accuracy and reproducibility of contrast-enhanced mammography
in the assessment of response to neoadjuvant chemotherapy
in breast cancer patients with calcifications in the tumor bed.
Diagnostics (Basel). 2021;11:435. Crossref
18. Vera-Badillo FE, Napoleone M, Ocana A, Templeton AJ, Seruga B,
Al-Mubarak M, et al. Effect of multifocality and multicentricity
on outcome in early-stage breast cancer: a systematic review and
meta-analysis. Breast Cancer Res Treat. 2014;146:235-44. Crossref