Breast Ductography: A Hidden Diagnostic Gem for Patients with Abnormal Nipple Discharge
PICTORIAL ESSAY
Hong Kong J Radiol 2023 Dec;26(4):271-82 | Epub 7 Dec 2023
Breast Ductography: A Hidden Diagnostic Gem for Patients with Abnormal Nipple Discharge
FFY Wan1, EPY Fung2, KM Kwok2, KM Wong2, LW Lo2, WS Mak2 WP Cheung2
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
Correspondence: Dr FFY Wan, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: wfy471@ha.org.hk
Submitted: 1 Oct 2022; Accepted: 28 Nov 2022.
Contributors: All authors designed the study, acquired and analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: Ethics approval has been obtained from the Kowloon Central Cluster/Kowloon East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: IRB-2022-176). The requirement for informed consent from patients was waived by the Committee due to retrospective nature of the study.
INTRODUCTION
Nipple discharge is one of the commonest breast disease
symptoms[1] and it can be physiological or pathological.
Physiological nipple discharge typically involves
multiple ducts in both breasts. Its common causes
include pregnancy, lactation, endocrine disorders, and
side-effects from medications.[2] Nipple discharge is
considered pathological when it is unilateral single-duct
discharge that is spontaneous, bloody, serous, or
clear, with or without an associated palpable mass. The
commonest aetiology is intraductal papilloma, which is
seen in approximately 35% to 48% of patients, followed
by ductal ectasia, which is the cause in 17% to 36% of
patients.[1] Malignancy, most commonly ductal carcinoma
in situ (DCIS), is found in 5% to 15% of patients.[3]
Breast ductography is a valuable investigation for
assessment of single-duct nipple discharge. It involves
administration of iodinated contrast into the duct,
followed by mammographic examination. Ductography
can unveil the cause of nipple discharge, including duct ectasia and fibrocystic changes. In the presence
of filling defects suggestive of tumour, ductography
assists in subsequent surgical planning by localising
the abnormalities. The aim of this pictorial review is to
illustrate the techniques for a successful examination and
classical radiological findings of pathologies detected by
ductography.
TECHIQUES FOR PERFORMING
DUCTOGRAPHY
Indications and Contraindications
Pathological nipple discharge (PND) from single duct is
the indication for ductography. The discharge must be
observed during the examination so that the discharging
duct can be appropriately identified and cannulated.
Ductography is not recommended in lactating women
or patients with active mastitis. Allergic reactions to
the contrast injected into the ductal system are rarely
reported. Nonetheless, patients with a history of mild or
moderate allergic reactions to iodinated contrast should
still be premedicated with steroids. Patients with a history of severe allergic reactions (e.g., anaphylaxis) should
not undergo ductography and alternative investigation
such as magnetic resonance imaging (MRI) should be
considered.[3]
Patient Preparation Prior to the Examination
Patient preparation is crucial for a successful examination.
Patients should be reminded not to squeeze the nipple 1
day prior to the procedure. This ensures that adequate
discharge is available on the day of examination for
localisation and cannulation of the discharging duct
orifice. Similar to mammography, patients should avoid
applying deodorant, talcum powder, or lotion in their
axillae or on their breasts, since these substances may
masquerade as microcalcifications on mammography.
Review of Relevant Imaging
Before the examination, any recent breast imaging,
including mammography and ultrasound, should be
reviewed for any suspicious findings. If not recently
performed, mammography with craniocaudal (CC)
and mediolateral (ML) views should be performed for
reference prior to duct cannulation.
Discharging Duct Cannulation and Contrast
Injection
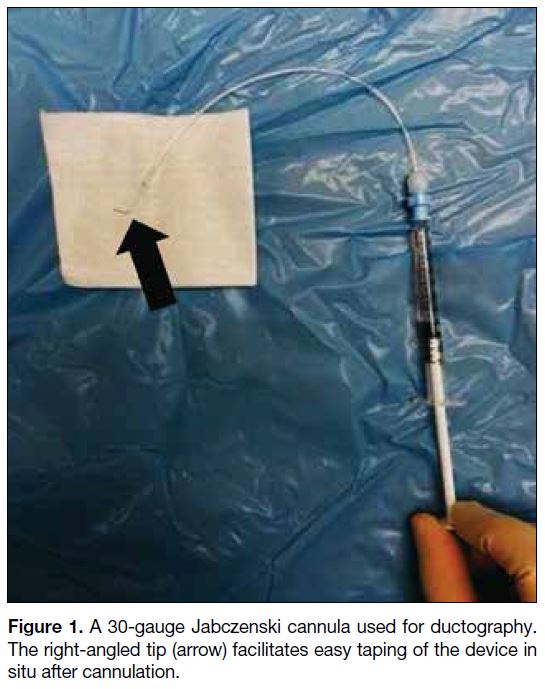
In our centre, the contrast injection system consists
of a 30-gauge Jabczenski cannula (Cook Medical,
Bloomington [IN], United States) with right-angled tip
connected via small-volume extension tubing to a 1-mL
syringe filled with 350 mg/mL non-ionic iodinated
contrast material (Figure 1). The use of non-diluted
contrast is advised for optimal ductal opacification. The
extension tubing and cannula should be properly primed
with contrast, and any air bubbles should be expelled
from the system to avoid artefacts.
Figure 1. A 30-gauge Jabczenski cannula used for ductography. The right-angled tip (arrow) facilitates easy taping of the device in situ after cannulation.
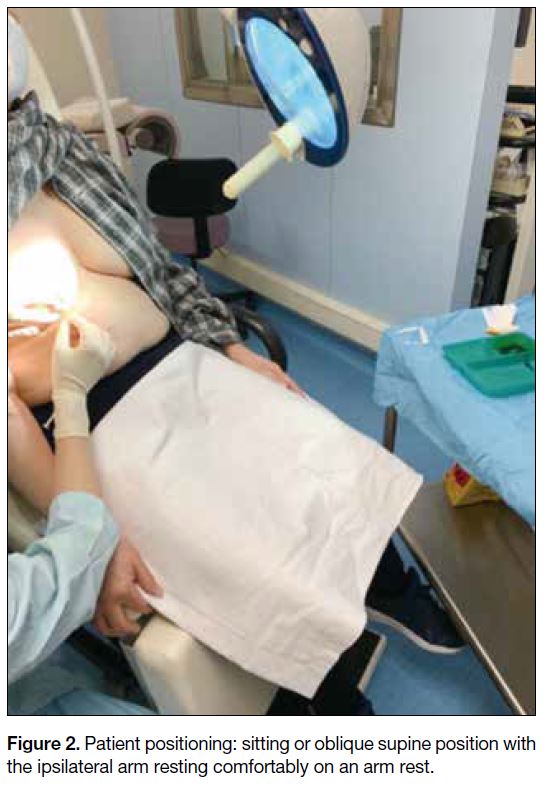
Depending on the location of the duct opening, the patient
is placed in the sitting or oblique supine position with
the ipsilateral arm resting comfortably on an arm rest
(Figure 2). The nipple is cleansed to remove any dried
secretions and given a sterile prep. Gentle pressure is
applied in the periareolar region to elicit nipple discharge.
Identification of the ‘trigger point’, i.e., the area which
repeatedly produces nipple discharge when compressed,
is helpful. Once nipple discharge is elicited, it is prudent
to confirm that the discharge comes from a single pore
since ductography is not the appropriate investigation for
multi-pore discharge. In case of difficulty in localising
the discharging pore, ‘spreading’ the nipple with
the fingers on the adjacent skin can help visualise the discharging orifice. With careful inspection, the orifice
of the discharging duct may appear relatively patulous
and slightly erythematous. Once the location of the
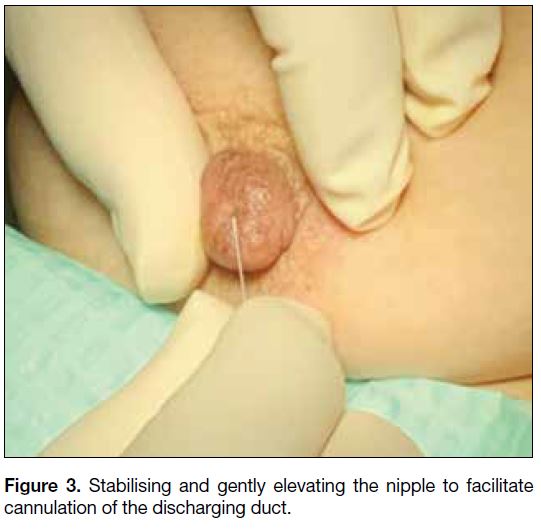
discharging orifice is confirmed, the nipple is stabilised
between the thumb and the index finger with gentle
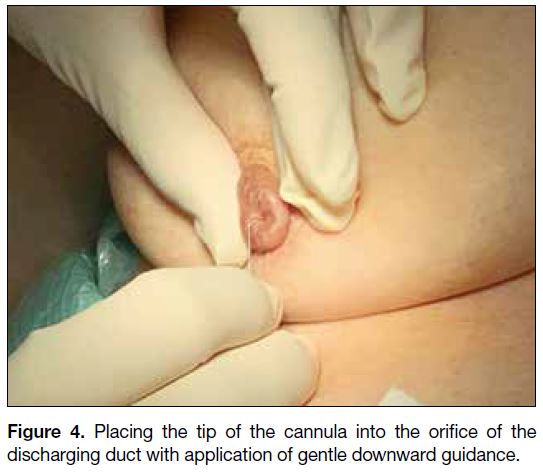
elevation (Figure 3). The tip of the cannula is placed
with application of gentle downward guidance (Figure 4). In case of difficulty in cannulating the discharging
duct, gentle probing with careful rotation or angulation
along the pore may result in successful cannulation. If
the most superficial part of the orifice is cannulated but
resistance is encountered during further insertion, it is
advised to maintain gentle pressure with careful rotation or angulation; forceful cannulation should always be
avoided due to risk of ductal perforation.
Figure 2. Patient positioning: sitting or oblique supine position with
the ipsilateral arm resting comfortably on an arm rest.
Figure 3. Stabilising and gently elevating the nipple to facilitate cannulation of the discharging duct.
Figure 4. Placing the tip of the cannula into the orifice of the discharging duct with application of gentle downward guidance.
After successful cannulation, the cannula should be held in position against the nipple. Approximately 0.2 to
0.4 mL of non-ionic contrast is introduced by slow and
gentle injection until contrast reflux, high resistance, or
pain occurs. Small lesions may be obscured if too much
contrast is injected; it is therefore recommended to begin
with small amounts. Because the ducts are fragile, pain
or a burning sensation may indicate duct perforation or
contrast extravasation. Either symptom is an indication
to stop further contrast injection. The cannula position
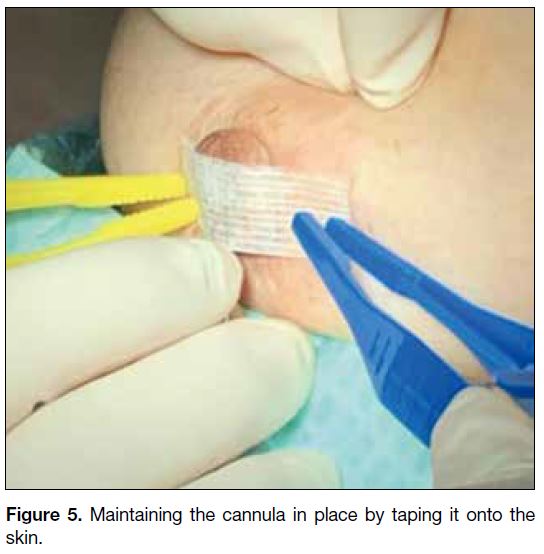
is maintained in place by taping it onto the skin (Figure 5), which is facilitated by the right-angled tip of the
cannula. This renders further contrast injection feasible
and reduces contrast leakage upon subsequent breast
compression for mammographic acquisition.
Figure 5. Maintaining the cannula in place by taping it onto the skin.
Mammographic Acquisition
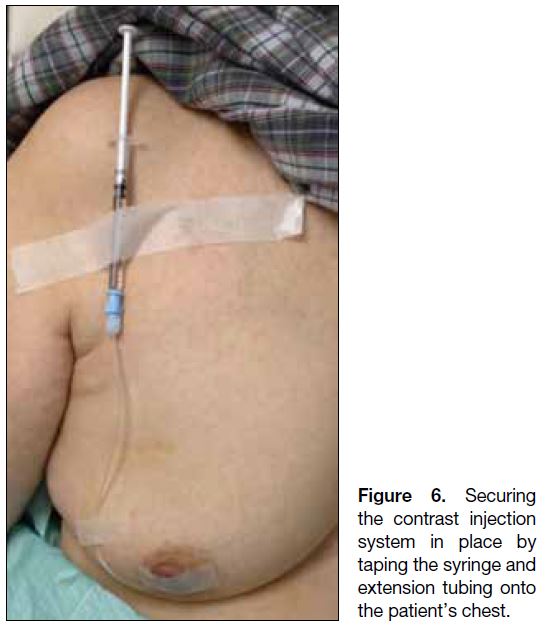
The contrast injection system should be held in place and can be secured by taping the syringe and extension tubing
onto the patient’s chest (Figure 6). Attention should be
paid when transferring the patient to the mammography
department to prevent the cannula from slipping out.
Figure 6. Securing the contrast injection system in place by taping the syringe andextension tubing onto the patient’s chest.
Mammograms with CC and ML views are subsequently
performed. An additional magnification view is
useful for detecting faint or subtle microcalcifications
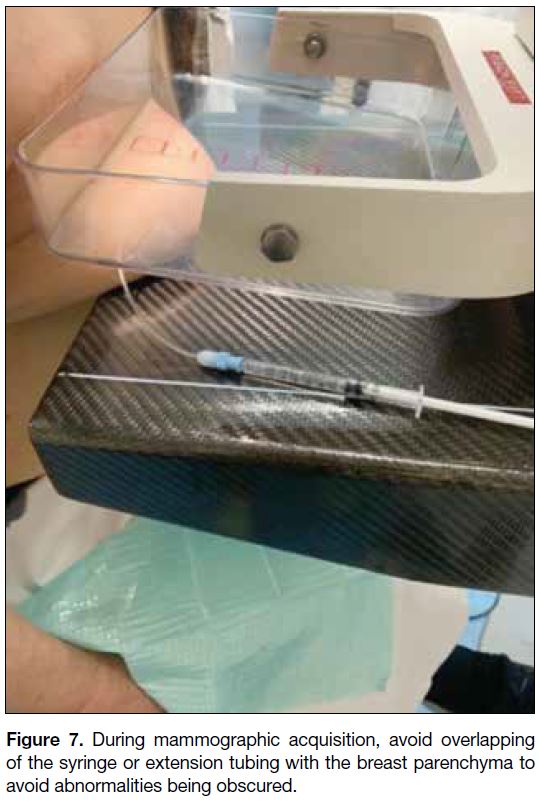
associated with the abnormal ductal system. One suggestion when performing mammography is to avoid
overlapping of the syringe or extension tubing with
breast parenchyma during mammographic acquisition to
avoid abnormalities being obscured (Figure 7). If there
is significant superimposition of the opacified ducts, a
standard ML oblique view or a rolled CC view can be
considered. Spot compression views can also be acquired
if needed.
Figure 7. During mammographic acquisition, avoid overlapping of the syringe or extension tubing with the breast parenchyma to avoid abnormalities being obscured.
Supplementary Ultrasound
After the mammographic examination, supplementary
ultrasound is performed with particular attention to
any retroareolar or ductal abnormalities and areas with
corresponding mammographic abnormalities. It is
helpful to perform ultrasound first with the cannula still
in situ. The advantage of doing so is that the discharging
ductal system may still be distended by the contrast,
making any intraductal lesions more conspicuous, and the
relationship of the distended ducts and adjacent lesions
may be better delineated. Afterwards, the cannula can be
removed and the retroareolar region can be scrutinised
again.
Because the orifices on the nipple can be closely related and there may be communication between different ducts, this renders the possibility of cannulating the
wrong duct, resulting in suboptimal assessment of the
ductal system harbouring the pathology. This highlights
the importance of careful identification and precise
cannulation of the discharging orifice.
Patient Selection
A total of 125 consecutive patients referred to our
institution for ductography from January 2016 to July
2022 were reviewed. The procedure was not performed
in 20 patients with no nipple discharge during the
examination (16.0%) and in five patients with discharge
from multiple ducts (4.0%). The examination could not
be completed in five patients with failed cannulation
(4.0%), two patients with resistance on contrast injection
(1.6%), and six patients with contrast extravasation
(4.8%). Among patients who completed the procedure,
intraductal papilloma (24.1%) was the commonest
pathology, followed by duct ectasia (21.8%), DCIS
(10.3%), fibrocystic change (4.6%), duct adenoma
(1.1%), and invasive carcinoma (1.1%). The rest (36.8%) had no abnormal findings. Cases with variable
normal and pathological ductographic appearances were
selected for demonstration.
Imaging Findings
Normal Ductographic Appearances
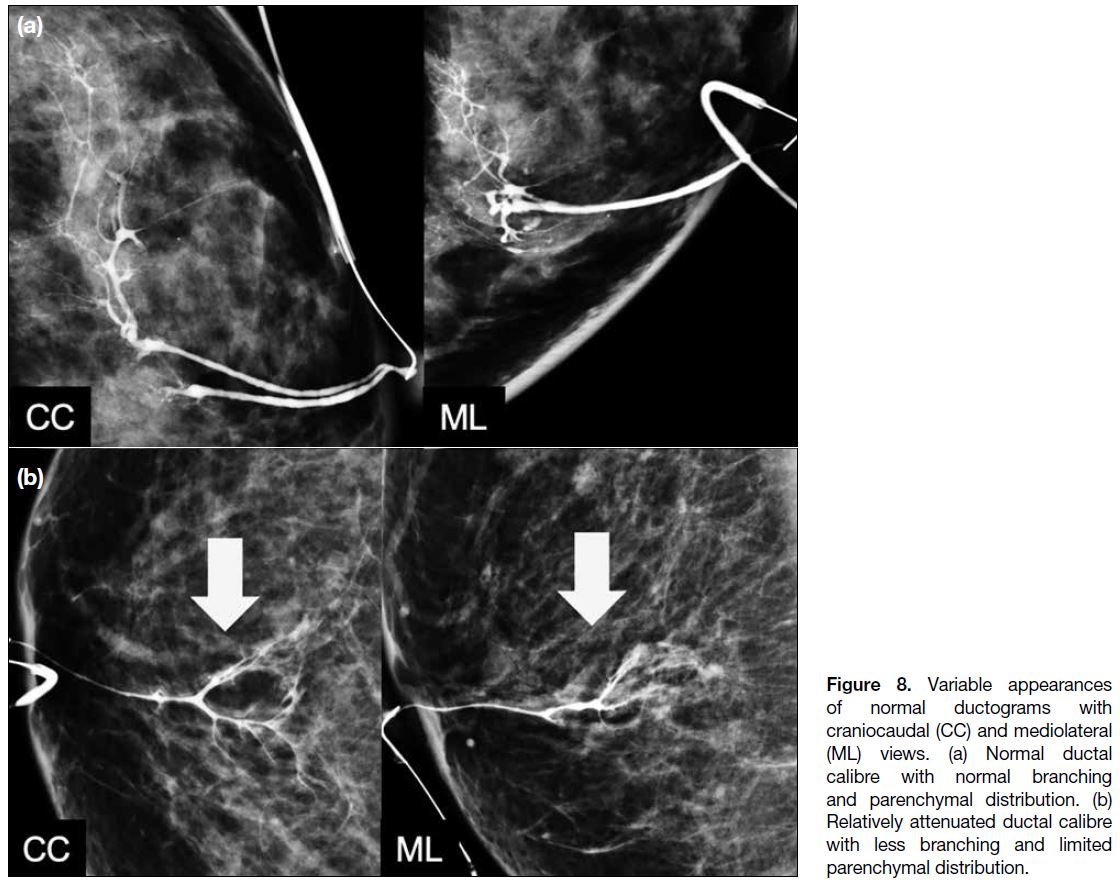
A normal duct arborises from a single-entry point on the nipple into smaller ducts extending peripherally. Normal
ducts are thin and smooth-walled with no filling defects
or wall irregularities. Normal ductograms may show
variability in ductal calibre, branching patterns, and
parenchymal distribution as shown in Figure 8. However,
the significance of different branching patterns, extent of
ductal distribution, and ductal calibres is unknown.
Figure 8. Variable appearances of normal ductograms with craniocaudal (CC) and mediolateral (ML) views. (a) Normal ductal calibre with normal branching and parenchymal distribution. (b) Relatively attenuated ductal calibre with less branching and limited parenchymal distribution.
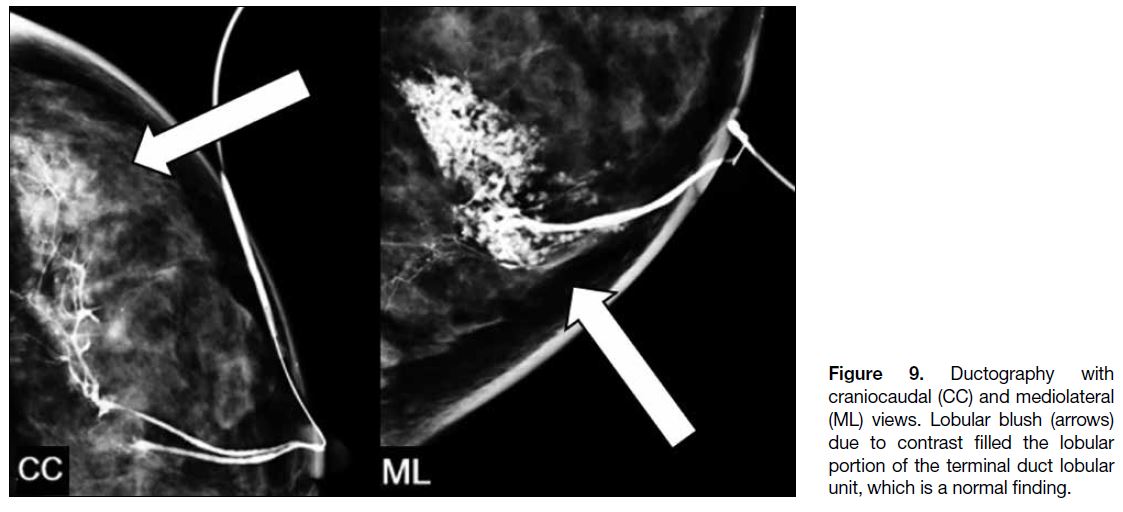
Lobular blush is caused by contrast filling the lobular
portion of the terminal duct lobular unit and is a finding
of no clinical significance (Figure 9). It occurs when the
ductal system has reached its maximum pressure and there is risk of extravasation with additional contrast
administration.
Figure 9. Ductography with
craniocaudal (CC) and mediolateral (ML) views. Lobular blush (arrows) due to contrast filled the lobular portion of the terminal duct lobular unit, which is a normal finding.
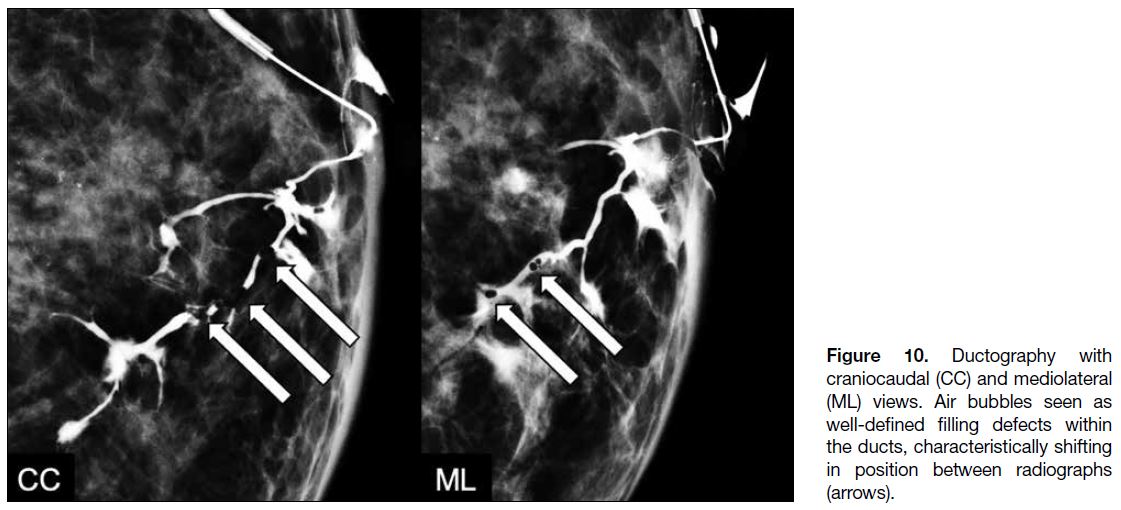
Air bubbles can occasionally be seen within the ducts.
Their round morphology and change in position between
radiographs are usually sufficient for differentiating
them from genuine lesions (Figure 10).
Figure 10. Ductography with
craniocaudal (CC) and mediolateral (ML) views. Air bubbles seen as well-defined filling defects within the ducts, characteristically shifting in position between radiographs (arrows).
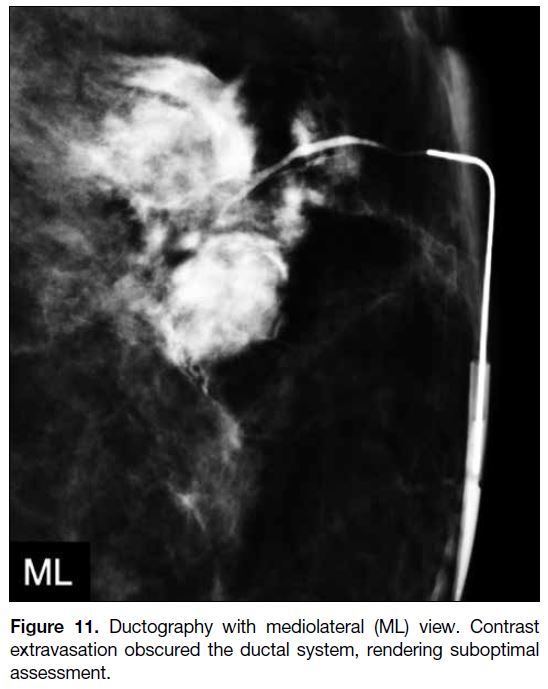
Extravasation
In the event of contrast extravasation (Figure 11), patients
usually complain of pain or a burning sensation, but
some may be asymptomatic. Common causes include
administration of too much contrast material, forceful
contrast administration, or too-vigorous manipulation
of the cannula causing wall perforation. Infrequently,
malignancy causing destruction of ductal wall integrity
may lead to extravasation. Since the presence of
extravasation may obscure the underlying pathology, the
procedure should be rescheduled 7 to 14 days later.
Figure 11. Ductography with mediolateral (ML) view. Contrast extravasation obscured the ductal system, rendering suboptimal assessment.
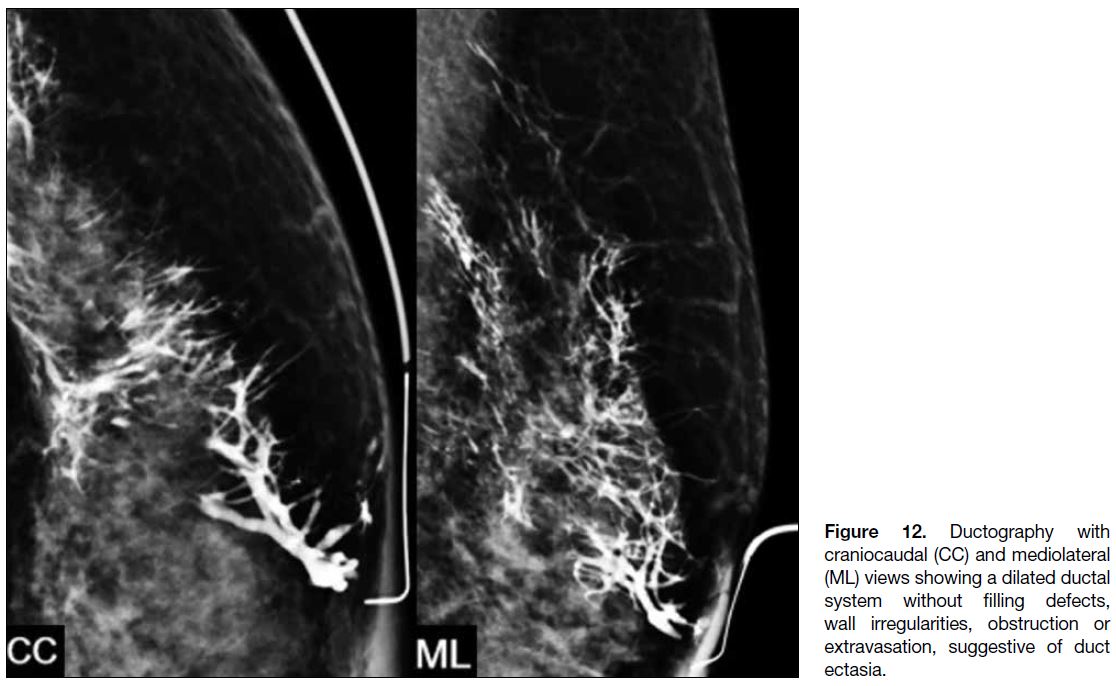
Duct Ectasia
Duct ectasia refers to non-specific dilatation of mammary ducts and is defined as ductal calibre more than 3 times
the width of the cannula.[4] It can cause both physiological
and PND. Ductography typically demonstrates a dilated
ductal system without intraductal filling defects, ductal
wall irregularities, ductal obstruction, or periductal
contrast extravasation (Figure 12).
Figure 12. Ductography with
craniocaudal (CC) and mediolateral (ML) views showing a dilated ductal system without filling defects, wall irregularities, obstruction or extravasation, suggestive of duct ectasia.
Fibrocystic Change
Fibrocystic change is benign alteration in the terminal
ductal lobular unit with or without associated fibrosis. As one of the primary components of fibrocystic change,
cysts develop from progressive lobular distension. Cysts
communicating with ducts could lead to nipple discharge
by decompression of cyst fluid into ducts. Ductography
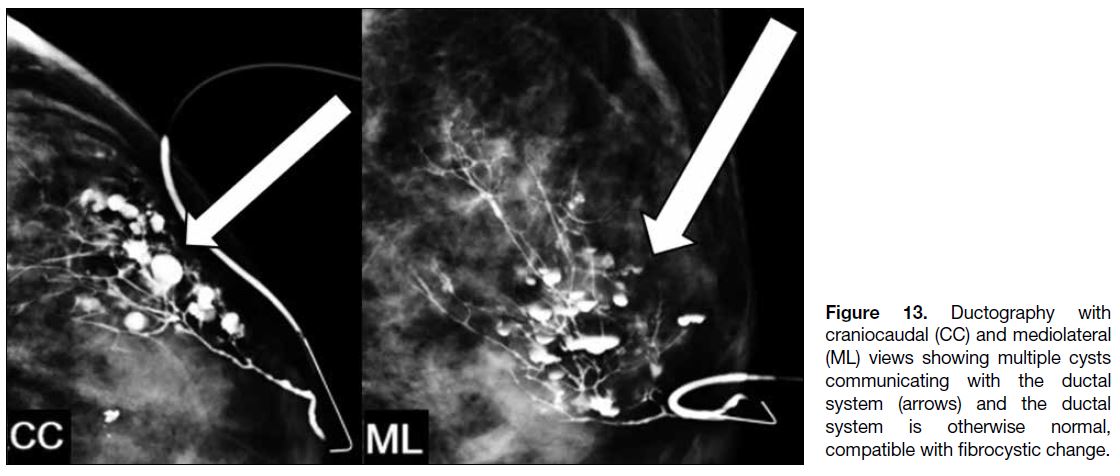
shows normal ducts communicating with cysts (Figure 13).
Figure 13. Ductography with
craniocaudal (CC) and mediolateral (ML) views showing multiple cysts communicating with the ductal system (arrows) and the ductal system is otherwise normal, compatible with fibrocystic change.
Intraductal Papilloma
Papillomas are benign masses of breast duct epithelium
with a fibrovascular stalk attached to the duct wall.
They may be single or multiple and may extend along
the ducts. When large, they can appear to be encysted and multilobulated. This is the commonest cause of
spontaneous unilateral single-orifice nipple discharge,
accounting for 35% to 48% of cases.[1] The mammogram
is frequently negative and ductography could be useful
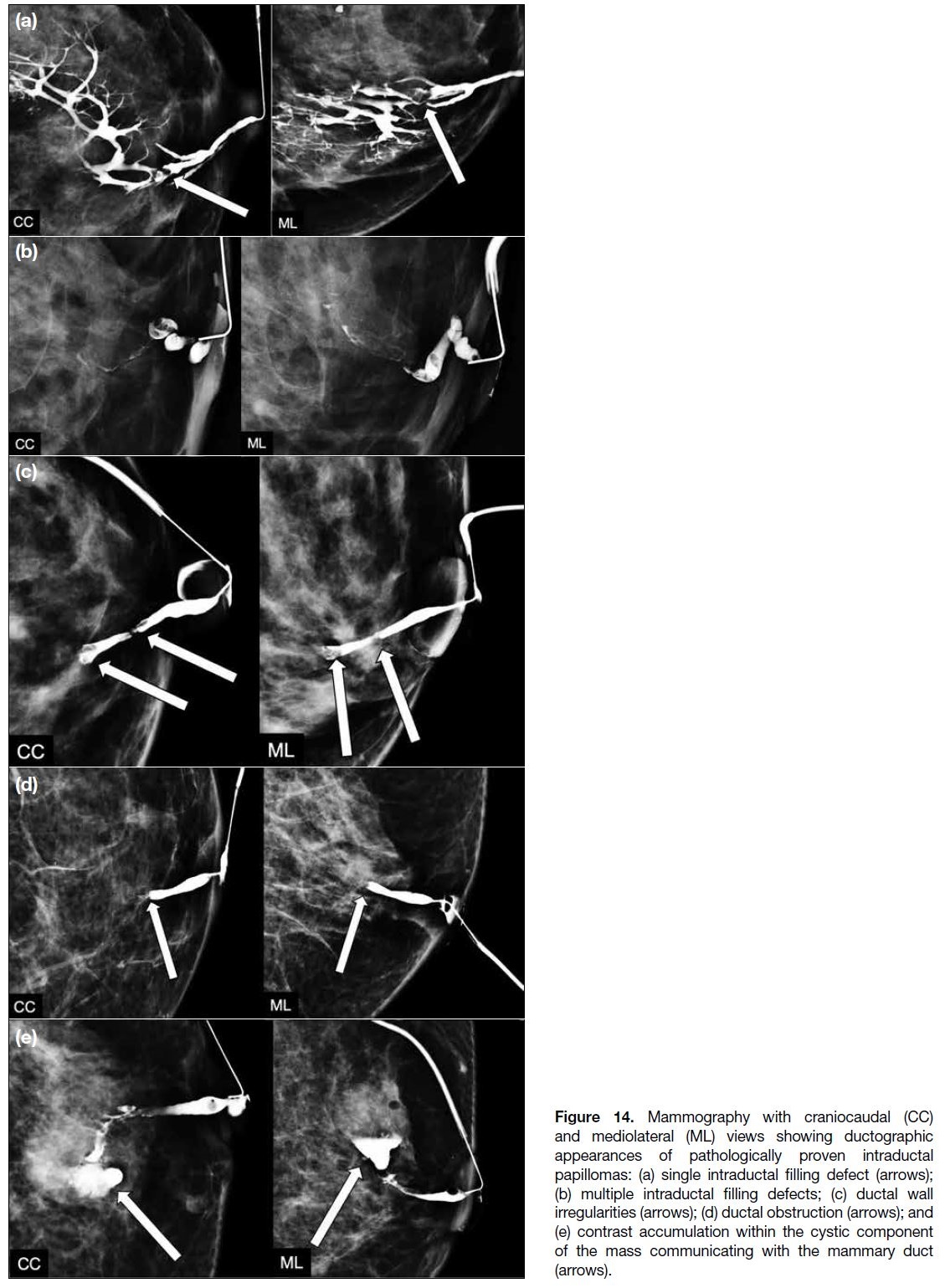
for its detection. Ductographic findings include single
intraductal filling defects, multiple intraductal filling
defects, ductal wall irregularities, and ductal obstruction
(Figure 14a, 14b, 14c, and 14d, respectively). Rarely,
contrast may be seen to accumulate within the cystic
component of the mass which communicates with
the duct (Figure 14e). Although these findings can be
non-specific and seen in other entities, malignancy in
particular, ductography is still useful in assessing the
number, extent and location of the abnormalities. Surgical
excision of papillomas with atypia is widely accepted
with an upgrade rate to malignancy ranging from 21%
to 38%.[2] However, the management of asymptomatic
papillomas without atypia is more controversial, with
an upgrade rate to malignancy of 2% to 12%.[2] Although
some clinicians still recommend surgical excision of all
papillary lesions, ultrasound-guided vacuum-assisted
excision has been proven to be a safe and effective
alternative with high rate of successful lesion removal.[5]
Figure 14. Mammography with craniocaudal (CC)
and mediolateral (ML) views showing ductographic
appearances of pathologically proven intraductal
papillomas: (a) single intraductal filling defect (arrows);
(b) multiple intraductal filling defects; (c) ductal wall
irregularities (arrows); (d) ductal obstruction (arrows); and
(e) contrast accumulation within the cystic component of the mass communicating with the mammary duct (arrows).
Ductal Carcinoma In Situ
Cancer is found in 5% to 15% of patients with PND,
the commonest type being DCIS. Up to 12% of patients
with DCIS present with nipple discharge.[6] Ductographic
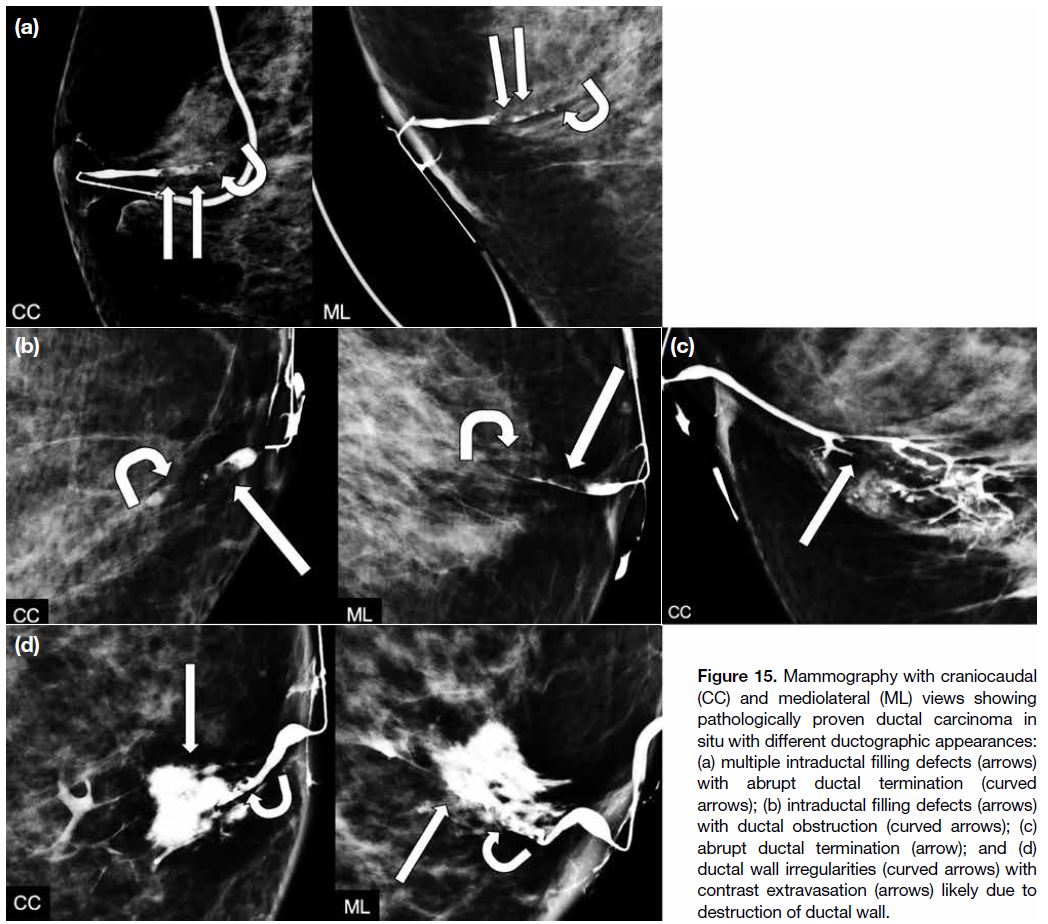
findings of malignancy, most commonly DCIS, may
mimic those of intraductal papillomas, including
filling defects, abrupt ductal termination, ductal wall
irregularities, and periductal contrast extravasation
(Figure 15). Histological assessment would be helpful in
differentiation of malignancy from other benign entities
including papillomas.
Figure 15. Mammography with craniocaudal
(CC) and mediolateral (ML) views showing
pathologically proven ductal carcinoma in
situ with different ductographic appearances:
(a) multiple intraductal filling defects (arrows)
with abrupt ductal termination (curved
arrows); (b) intraductal filling defects (arrows)
with ductal obstruction (curved arrows); (c)
abrupt ductal termination (arrow); and (d)
ductal wall irregularities (curved arrows) with
contrast extravasation (arrows) likely due to
destruction of ductal wall.
Duct Adenoma
Duct adenomas are uncommon benign glandular
tumours which usually fill and distend the ductal lumen.
They are usually single, occasionally multiple, nodular
lesions occupying medium- and large-sized breast ducts
but not major subareolar ducts. Because of their location,
they more commonly present as palpable lumps, unlike
intraductal papillomas which are more likely associated
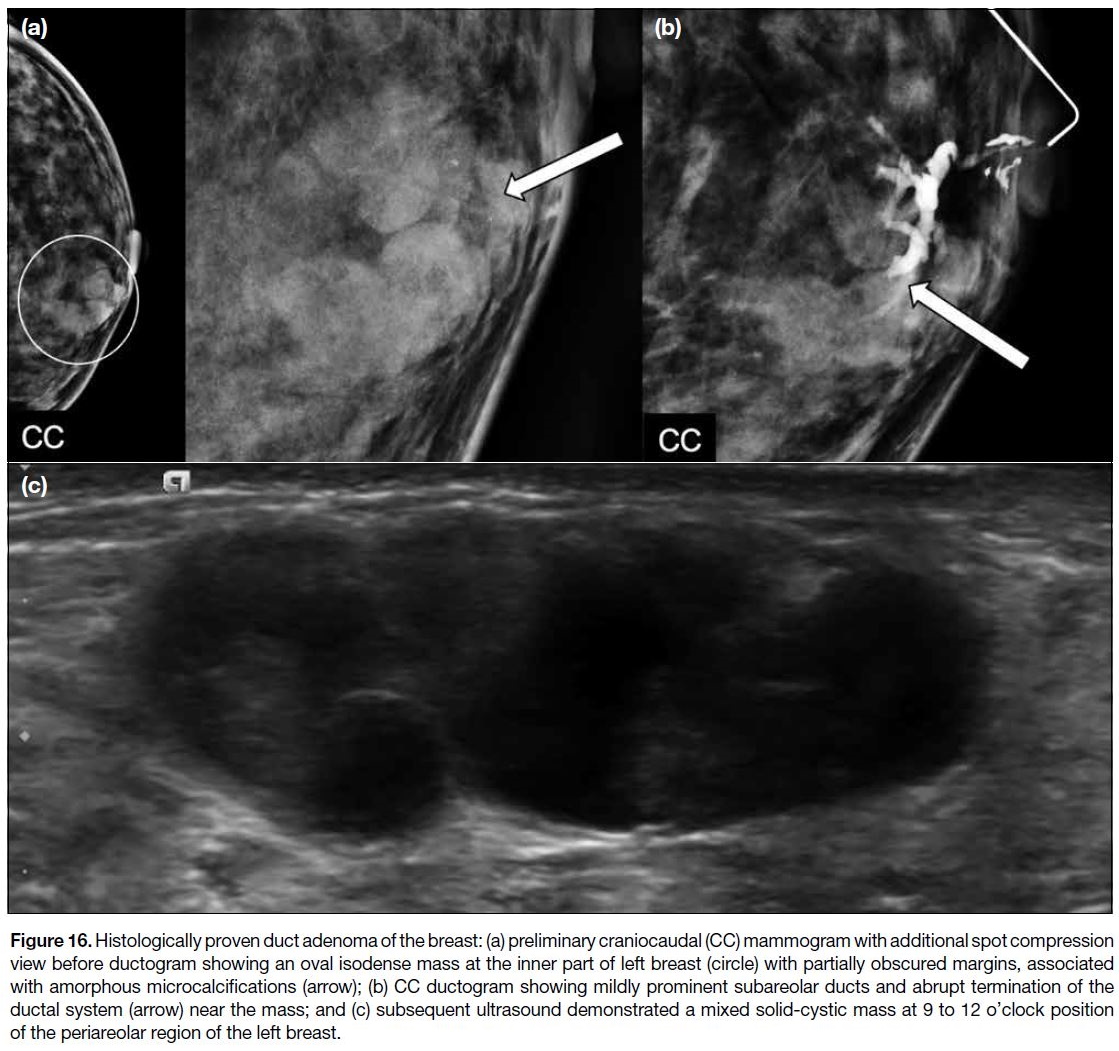
with nipple discharge. Figure 16 illustrates a rare case of
duct adenoma presenting with nipple discharge.
Figure 16. Histologically proven duct adenoma of the breast: (a) preliminary craniocaudal (CC) mammogram with additional spot compression
view before ductogram showing an oval isodense mass at the inner part of left breast (circle) with partially obscured margins, associated
with amorphous microcalcifications (arrow); (b) CC ductogram showing mildly prominent subareolar ducts and abrupt termination of the
ductal system (arrow) near the mass; and (c) subsequent ultrasound demonstrated a mixed solid-cystic mass at 9 to 12 o’clock position
of the periareolar region of the left breast.
DISCUSSION
In patients with PND, mammography and ultrasound are
first-line investigations for women who are ≥ 30 years
of age.[1] Mammographic abnormalities were found to be
positive in 50% to 90% of patients with breast cancer
and in < 50% of patients with intraductal papilloma.[7]
Mammography often fails to demonstrate lesions that
are small, lack calcifications, or are located entirely
within the duct.[8] Nevertheless, it is still a crucial initial imaging modality. DCIS is the commonest malignancy
associated with PND and it may present as suspicious
microcalcifications on mammography. Underlying
invasive cancer may also present as mass or architectural
distortion. Mammography may be complementary to
ultrasound in women < 30 years of age if they are BRCA-positive
or have other gene mutation predisposing to
breast cancer. In particular, it should be considered in
women < 30 years of age who present with suspicious
masses on ultrasound. This is because mammography
can detect any calcifications associated with the mass or
the ducts. If present, the extent, pattern, and morphology
of the calcifications are best assessed on mammography.[2]
Apart from mammography, ultrasound also plays
an important role in the initial evaluation of PND.
Ultrasound can identify sub-centimetre ductal
abnormalities and associated ductal changes which are
occult on mammography, especially in dense breasts. In
a study, ultrasound examination in patients with PND
but negative mammographic findings led to detection of
malignancy in 15% of cases.[9]
If no abnormality explaining the nipple discharge can
be detected on both mammography and ultrasound,
ductography is usually the next step in imaging
examinations in our centre. The value of ductography
as a second-line investigation is controversial. It is an
invasive examination, although it is actually rather
minimally invasive. Despite the possible events of
contrast extravasation and ductal perforation, these
are minor complications with no reported long-term consequences. The primary goal of ductography is to
localise intraductal lesions and assist in surgical planning.
Since there is considerable overlap in ductographic
findings of papillary lesions and malignancy, histological
correlation is usually required to ascertain the benign or
malignant nature of an intraductal abnormality.[1]
Ductography is more sensitive than mammography
and ultrasound but has lower specificity than those
two modalities.[1] In cases of negative findings with
conventional imaging, ductography has been shown to
localise 76% of otherwise occult high-risk and malignant
lesions.[9] However, a negative ductographic examination cannot be used to exclude the possibility of underlying malignancy, with a false negative rate reported to be 20% to 30%.[9]
The management approach for evaluating PND is
evolving. Because of its high sensitivity in detecting
breast malignancy and its capability for biopsy, breast
MRI has emerged as the most sensitive modality in
detecting malignancy. Contrast-enhanced breast MRI
has been proposed for investigation when conventional
imaging modalities have failed to identify the underlying
cause of PND. It offers an alternative means when
ductography is not performed due to risk of iodinated
contrast reaction, failure to cannulate the duct, or
patients’ preference. MRI can enable detection of lesions in peripheral ducts that are beyond the area
normally encompassed by ductography or targeted
ultrasound. In contrast to ductography, which only
detects abnormalities in discharging ducts, MRI allows
evaluation of the entire ductal system at the same time
and enables identification of additional cancers in both
the ipsilateral and contralateral breast. With increasing
availability of MRI scanners and growing experience
in MRI interpretation, there have been more reports
showing the high sensitivity and negative predictive
value of MRI for breast cancer. The European Society of
Mastology guidelines evaluated 10 papers on the use of MRI in PND and concluded that there is still insufficient
evidence to support routine use of MRI for these
patients.[10] Its relatively high cost and poor accessibility
in less developed countries, as well as patient factors
(e.g., claustrophobia, severe obesity, and implantable
devices not compatible with MRI examination), could be
possible causes of its limited use in many departments.
Nonetheless, patients with persistent symptoms after
unremarkable or failed ductographic examination may
benefit from MRI. Furthermore, it is recommended to
perform MRI in BRCA mutation carriers and other high-risk
patients to minimise radiation exposure.
A new area of research involves MR ductography with
use of a three-dimensional heavily T2-weighted fat-suppressed
sequence. It is non-invasive and does not
require use of contrast. The discharging duct is often
dilated with fluid and can be seen on heavily T2-weighted
images. The presence of intraluminal filling defects,
ductal wall irregularities, or ductal obstruction can be
assessed. Compared with conventional ductography, MR
ductography can show the distal part of a duct obstructed
by an intraductal mass. On the other hand, it cannot
reveal a non-dilated duct. According to a feasibility study
involving 21 patients,[11] the indirect MR ductography
sequence did not show significantly better performance
when compared with conventional ductography. More
large-scale studies with refinement of the sequence
or fusion imaging with contrast-enhanced MRI could
potentially be a fruitful area of research.
Apart from MRI, the addition of digital breast
tomosynthesis (DBT) to conventional ductography has
been investigated as a technique in the evaluation of
PND. This three-dimensional reconstruction can provide
sectional images from different projection angles,
thus reducing overlap of the ductal system and tissue
superimposition. Retrospective studies have compared
the diagnostic performance of DBT-ductography and
digital ductography, revealing higher sensitivity for
DBT-ductography without compromise in specificity.[12] [13]
A recent prospective study by Tao et al[14] evaluated
128 patients with PND and concluded that DBT-ductography
increases the sensitivity and specificity of
lesion detection by enhancing the image quality without
significant increase in the radiation dose. Further studies
may be helpful in validating and generalising the findings
of DBT-ductography in patients with PND.
Surgical duct excision has been the standard of care
to exclude underlying malignancy. There has been
increasing trend in adopting surveillance for patients
with unremarkable findings on combined assessment
using mammogram, ultrasound and ductograpohy.[2]
Despite being the reference standard, microdochectomy
cannot detect all malignancies, especially those located
far from the nipple. Sanders and Daigle[15] examined
the role of MRI as an alternative to microdochectomy.
Among the 85 patients who underwent MRI prior to duct
excision, eight malignant lesions (9.4%) were detected
and seven out of these eight malignancies (87.5%) were
identified on MRI. The authors proposed that a negative
MRI study may obviate the need for microdochectomy
in most patients.
CONCLUSION
Ductography is a practical, valuable, and cost-effective
procedure in the diagnosis of intraductal lesions.
Gaining familiarity with the procedure and including
it in the evaluation of patients with PND may facilitate
management for these patients. If ductography is
technically unfeasible, MRI should be considered as an
ancillary tool to investigate for the possible causes.
REFERENCES
1. Lee SJ, Trikha S, Moy L, Baron P,
diFlorio RM, Green ED, et al. ACR Appropriateness Criteria® evaluation of
nipple discharge. J Am Coll Radiol. 2017;14(5S):S138-53. Crossref
2. Gupta D, Mendelson EB, Karst I. Nipple discharge: current clinical and imaging evaluation. AJR Am J Roentgenol. 2021;216:330-9. Crossref
3. Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-60. Crossref
4. Cardenosa G. Clinical breast imaging: the essentials. Pennsylvania
(PA): Wolters Kluwer; 2014: 381.
5. Chau CM, Fung EP, Wong CW, Kwok KM, Leung AY,
Wong LK, et al. Ultrasound-guided vacuum-assisted excision of
papillary breast lesions as an alternative to surgical excision: 7-year
experience. Hong Kong J Radiol. 2020;23:253-60. Crossref
6. Barreau B, de Mascarel I, Feuga C, MacGrogan G, Dilhuydy MH,
Picot V, et al. Mammography of ductal carcinoma in situ of
the breast: review of 909 cases with radiographic-pathologic
correlations. Eur J Radiol. 2005;54:55-61. Crossref
7. Orel SG, Dougherty CS, Reynolds C, Czerniecki BJ, Siegelman ES,
Schnall MD. MR imaging in patients with nipple discharge: initial
experience. Radiology. 2000;216:248-54. Crossref
8. Yoon JH, Yoon H, Kim EK, Moon HJ, Park YV, Kim MJ.
Ultrasonographic evaluation of women with pathologic nipple
discharge. Ultrasonography. 2017;36:310-20. Crossref
9. Morrogh M, Morris EA, Liberman L, Borgen PI, King TA.
The predictive value of ductography and magnetic resonance
imaging in the management of nipple discharge. Ann Surg Oncol.
2007;14:3369-77. Crossref
10. Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ,
et al. Magnetic resonance imaging of the breast: recommendations
from the EUSOMA working group. Eur J Cancer. 2010;46:1296-316. Crossref
11. Nicholson BT, Harvey JA, Patrie JT, Mugler JP 3rd. 3D-MR
ductography and contrast-enhanced MR mammography in patients
with suspicious nipple discharge: a feasibility study. Breast J.
2015;21:352-62. Crossref
12. Moschetta M, De Ruvo V, Drago A, Troiano N, Paolicelli S,
Rubini G, et al. DBT-galactography: a promising tool for improving
the diagnostic workup of nipple discharge. Eur Radiol Exp.
2020;4:40. Crossref
13. Lee JY, Jang M, Kim SM, Yun BL. Galactography using digital
breast tomosynthesis for the evaluation of pathologic nipple
discharge: a comparison with 2D synthetic digital mammography.
J Korean Soc Breast Screen. 2019;16:60-9.
14. Tao J, Liao H, Liu Y, Peng Q, Zhu W, Peng S, et al. Evaluation of
breast galactography using digital breast tomosynthesis: a clinical
exploratory study. Diagnostics (Basel). 2021;11:2060. Crossref
15. Sanders LM, Daigle M. The rightful role of MRI after negative
conventional imaging in the management of bloody nipple
discharge. Breast J. 2016;22:209-12. Crossref