Ruptured Cervical Radiculomedullary Artery Mycotic Aneurysm Presenting with Intracranial and Spinal Subarachnoid Haemorrhage: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Dec;26(4):266-70 | Epub 20 Nov 2023
Ruptured Cervical Radiculomedullary Artery Mycotic Aneurysm Presenting with Intracranial and Spinal Subarachnoid Haemorrhage: A Case Report
SH Liu1, NL Chan2, NR Mahboobani1, TL Poon2, WL Poon1
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Neurosurgery, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr SH Liu, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: lsh264@ha.org.hk
Submitted: 23 Feb 2022; Accepted: 11 Aug 2022.
Contributors: SHL, NRM and WLP designed the study. All authors acquired and analysed the data. SHL and NLC drafted the manuscript. NRM, TLP and WLP critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki and provided verbal consent for publication.
BACKGROUND
A 64-year-old Chinese man with no significant past
medical history presented to the Accident and Emergency
Department of our institution in December 2021 with a
1-day history of headache and vomiting. He reported no
head injury, seizure or loss of consciousness. Physical
examination revealed no focal neurological deficit.
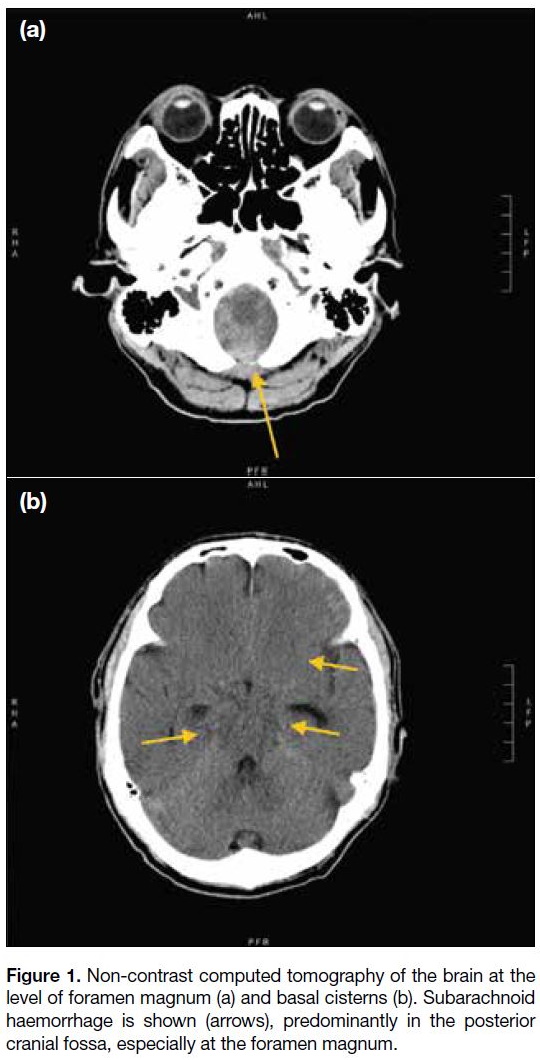
Non-contrast computed tomography (CT) of the brain
showed subarachnoid haemorrhage predominantly in
the posterior cranial fossa, extending to the spinal canal
at the C3 vertebral level, and to a lesser extent in the
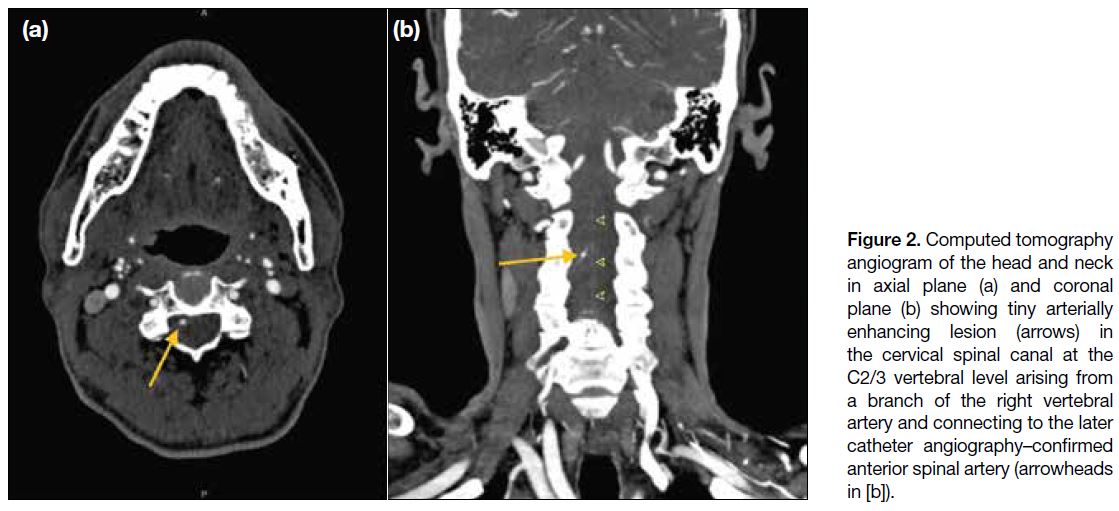
basal cisterns and at the inferior frontal regions (Figure 1). CT angiogram of the head and neck arteries revealed
a 2-mm arterial-enhancing lesion in the cervical spine
canal at the C2/3 vertebral level (Figure 2). It appeared
to arise from a branch of the right vertebral artery and
was connected to a prominent midline vasculature along
the anterior surface of the spinal cord. No intracranial
vascular lesion was identified.
Figure 1. Non-contrast computed tomography of the brain at the
level of foramen magnum (a) and basal cisterns (b). Subarachnoid
haemorrhage is shown (arrows), predominantly in the posterior
cranial fossa, especially at the foramen magnum.
Figure 2. Computed tomography
angiogram of the head and neck in axial plane (a) and coronal plane (b) showing tiny arterially enhancing lesion (arrows) in the cervical spinal canal at the C2/3 vertebral level arising from a branch of the right vertebral artery and connecting to the later catheter angiography–confirmed anterior spinal artery (arrowheads in [b]).
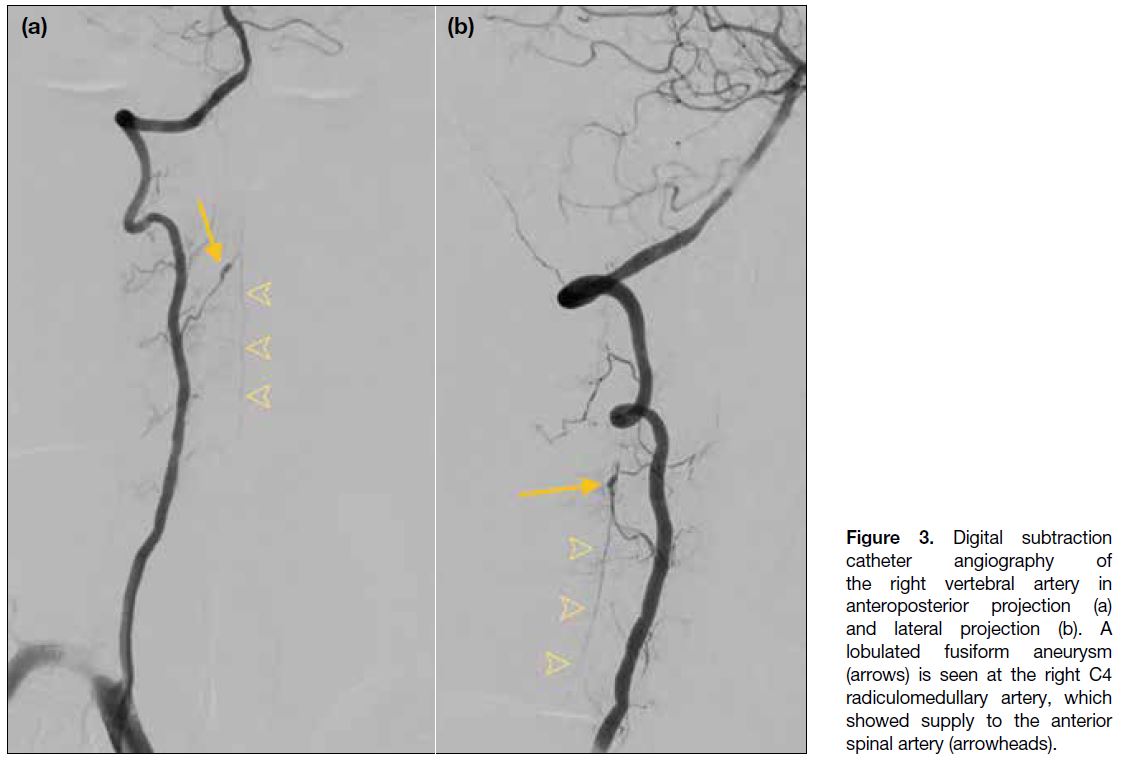
Diagnostic cerebral and cervical spine catheter
angiography was performed 3 days after admission. On right vertebral artery angiogram, there was a lobulated
fusiform aneurysm at the right C4 radiculomedullary
artery measuring 7.2 mm in length and 2.5 mm in
diameter (Figure 3). This radiculomedullary artery is
the dominant supply to the anterior spinal artery in the
cervical region and corresponds to the findings on CT
angiogram. There was no evidence of arteriovenous
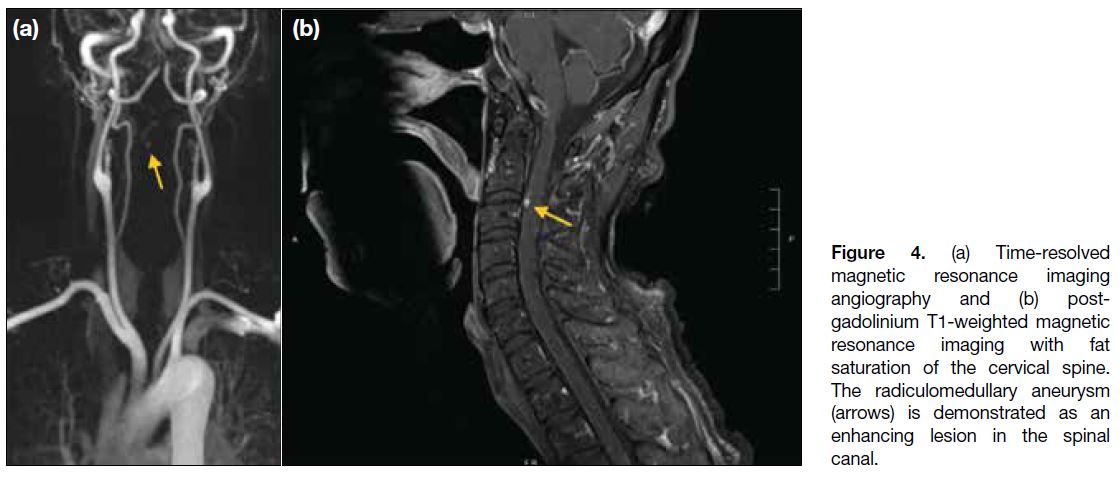
shunting. Subsequent magnetic resonance imaging of
the spine revealed a contrast-enhancing lesion within
the cervical spine canal (Figure 4), corresponding to the
CT- and angiography-detected aneurysm. There was
no evidence of spinal arteriovenous shunting, vascular
malformation or tumours.
Figure 3. Digital subtraction
catheter angiography of
the right vertebral artery in
anteroposterior projection (a)
and lateral projection (b). A
lobulated fusiform aneurysm
(arrows) is seen at the right C4
radiculomedullary artery, which
showed supply to the anterior
spinal artery (arrowheads).
Figure 4. (a) Time-resolved
magnetic resonance imaging
angiography and (b) post-gadolinium
T1-weighted magnetic
resonance imaging with fat
saturation of the cervical spine.
The radiculomedullary aneurysm
(arrows) is demonstrated as an
enhancing lesion in the spinal
canal.
The patient developed fever after admission. Initial
blood tests showed normal white blood cell count but
neutrophilia. Repeated blood tests revealed leucopenia
with persistent neutrophilia, thrombocytopenia and
elevated C-reactive protein level. Blood culture
yielded Klebsiella pneumoniae species. The patient
was prescribed antibiotics. Echocardiogram showed
no evidence of myocardial infarction, endocarditis or valvular vegetation. He remained stable with no
new neurological signs or symptoms. Laboratory tests
showed subsequent normalisation of white blood cell
counts, platelet count and C-reactive protein level.
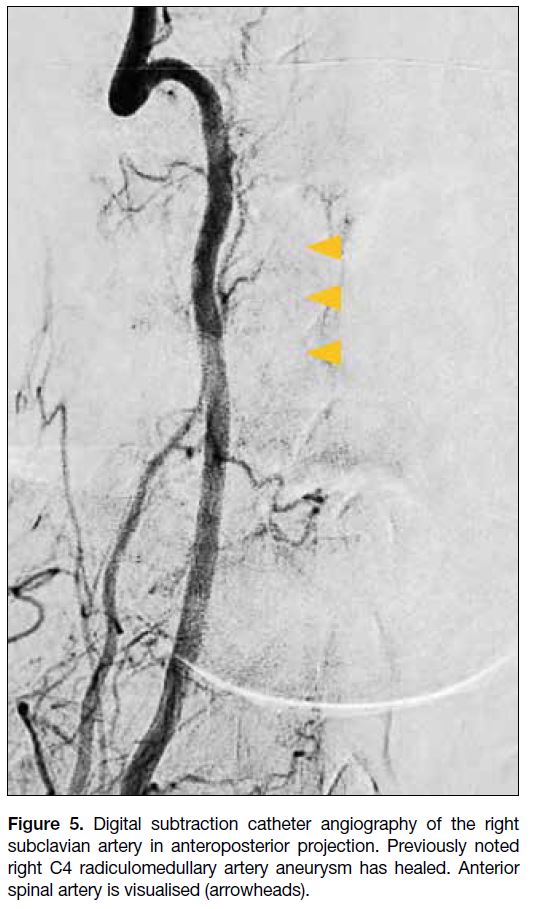
Follow-up diagnostic catheter angiography of the right
vertebral artery was performed 3 weeks after the first
catheter angiography. There was no opacification of
the aneurysm, suggesting that it is healed. The right C4
radiculomedullary artery remained patent, with supply to
the anterior spinal artery visualised (Figure 5).
Figure 5. Digital subtraction catheter angiography of the right
subclavian artery in anteroposterior projection. Previously noted
right C4 radiculomedullary artery aneurysm has healed. Anterior
spinal artery is visualised (arrowheads).
With the available clinical history, laboratory test results, and spontaneous occlusion of the aneurysm during antibiotic therapy, the most likely diagnosis for this
patient was ruptured mycotic aneurysm at the right C4
radiculomedullary artery. The patient remained stable
with neither altered consciousness nor focal neurological
deficit. He completed a 6-week course of antibiotics
as per the microbiologist’s recommendation and was
discharged uneventfully.
DISCUSSION
Spinal artery aneurysm is a rare cause of spinal or
intracranial subarachnoid haemorrhage. Diagnosis
of spinal artery aneurysms can be challenging and is
sometimes delayed due to the rarity of the condition.
These aneurysms have different morphological features
to those of intracranial origin. They are usually fusiform,
without a defined neck, and often not related to arterial
branching sites.[1] In addition, they are usually associated
with underlying vascular lesions such as arteriovenous
malformation[2] or fistula.[3] There are also reported cases
of association with tumours (e.g., haemangioblastoma),[4]
aortic coarctation2 and Moyamoya disease.[5] Other
causes include underlying vasculopathy such as
Behçet’s disease,[6] Sjögren’s syndrome,[7] systemic
lupus erythematosus,[8] and pseudoxanthoma elasticum.[9]
Mycotic aneurysms, as an alternative consideration, are
rarely reported.[1] [10]
There is no standardised treatment for ruptured
radiculomedullary artery aneurysm due to its rare
occurrence and varying aetiology.[11] [12] Management
should be tailored to each patient and take account of
the size of haematoma, size of aneurysm, neurological
symptoms, and feasibility and risk of intervention.
Vascular anatomy should be thoroughly evaluated prior
to endovascular intervention or surgery. Cross-sectional
imaging such as computed tomography or magnetic
resonance angiography and catheter angiography can
be adopted to delineate the morphology and size of the
aneurysm, size of the relevant arteries, and pattern of
vascular supply to the relevant segment of the spinal
cord.
Dabus et al[13] reported a case of spontaneous thrombosis of a posterior radiculomedullary artery dissecting aneurysm
with its parent artery in their case series, of which the
patient showed no neurological deterioration on follow-up.
This may have been due to the presence of good
anastomoses of the radiculopial and radiculomedullary
arteries. This emphasises the importance of angiographic
evaluation of the arterial supply to the spinal cord. If the culprit artery shows a dominant supply to the relevant
segment of cord, thrombosis or inadvertent injury
during intervention may jeopardise the spinal cord blood
supply. This has to be taken into account when deciding the suitable treatment strategy for each patient.
Endovascular intervention for small fusiform aneurysm
without surgical neck at a very small size vessel can be technically challenging and carries the potential risk of
arterial thrombosis causing spinal ischaemia. In the case
of large aneurysm or large haematoma with considerable
mass effect, surgery such as clipping or resection may
be a better option to reduce compression on the adjacent
spinal cord and/or nerve roots.
Cases of resolution or thrombosis of spinal artery
aneurysms have been reported,[1] [6] [7] [13] [14] [15] either with a
conservative approach or with medical treatment when
an underlying cause is determined. In cases with small
haematoma, minimal or mild neurological symptoms,
and technically challenging and high-risk intervention, a
conservative approach or medical treatment to target the
underlying cause are reasonable options.
In our patient, apart from headache and vomiting,
there was no focal neurological deficit. Aetiology
was presumed to be a mycotic aneurysm. There was
potentially high surgical risk including postoperative
neurological deterioration. Medical treatment with
antibiotic therapy was adopted.
CONCLUSION
Radiologists should remain alert for spinal artery mycotic
aneurysm as a rare cause of subarachnoid haemorrhage.
Treatment varies with the size, location and morphology
of the aneurysm, vascular anatomy of the spinal
arteries, presenting symptoms, and risk of intervention
including potential neurological deterioration. Decision
for intervention should be based on the balance of risks and benefits. In patients with mild symptoms and
high surgical risk, medical treatment with antibiotics
is a reasonable treatment choice. Follow-up imaging
including catheter angiography should be considered to
monitor progress and guide further management.
REFERENCES
1. Berlis A, Scheufler KM, Schmahl C, Rauer S, Götz F, Schumacher M. Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: potential etiology and treatment
strategy. ANJR Am J Neuroradiol. 2005;26:405-10.
2. Rengachary SS, Duke DA, Tsai FY, Kragel PJ. Spinal arterial aneurysm: case report. Neurosurgery. 1993;33:125-9; discussion 129-30. Crossref
3. Malek AM, Halbach VV, Phatouros CC, Higashida RT, Dowd CF, Wachhorst S, et al. Spinal dural arteriovenous fistula with an associated feeding artery aneurysm: case report. Neurosurgery. 1999;44:877-80. Crossref
4. Berlis A, Schumacher M, Spreer J, Neumann HP, van Velthoven V. Subarachnoid haemorrhage due to cervical spinal cord
haemangioblastomas in a patient with von Hippel-Lindau disease.
Acta Neurochir (Wien). 2003;145:1009-13. Crossref
5. Walz DM, Woldenberg RF, Setton A. Pseudoaneurysm of the anterior spinal artery in a patient with Moyamoya: an unusual cause of subarachnoid hemorrhage. AJNR Am J Neuroradiol.
2006;27:1576-8.
6. Bahar S, Coban O, Gürvit IH, Akman-Demir G, Gökyiğit A. Spontaneous dissection of the extracranial vertebral artery with spinal subarachnoid haemorrhage in a patient with Behçet’s disease.
Neuroradiology. 1993;35:352-4. Crossref
7. Klingler JH, Gläsker S, Shah MJ, Van Velthoven V. Rupture of a spinal artery aneurysm attributable to exacerbated Sjögren syndrome: case report. Neurosurgery. 2009;64:E1010-1; discussion E1011. Crossref
8. Fody EP, Netsky MG, Mrak RE. Subarachnoid spinal hemorrhage in a case of systemic lupus erythematosus. Arch Neurol.
1980;37:173-4. Crossref
9. Kito K, Kobayashi N, Mori N, Kohno H. Ruptured aneurysm of the anterior spinal artery associated with pseudoxanthoma elasticum. Case report. J Neurosurg. 1983;58:126-8. Crossref
10. Nakamura H, Kim P, Kanaya H, Kurokawa R, Murata H, Matsuda H. Spinal subarachnoid hemorrhage caused by a mycotic aneurysm of the radiculomedullary artery: a case report and review of literature. NMC Case Rep J. 2015;2:49-52. Crossref
11. Crobeddu E, Pilloni G, Zenga F, Cossandi C, Garbossa D, Lanotte M. Dissecting aneurysm of the L1 radiculomedullary artery associated with subarachnoid hemorrhage: a case report. J Neurol Surg A Cent Eur Neurosurg. 2022;83:99-103. Crossref
12. van Es AC, Brouwer PA, Willems PW. Management considerations
in ruptured isolated radiculopial artery aneurysms. A report of two
cases and literature review. Interv Neuroradiol. 2013;19:60-6. Crossref
13. Dabus G, Tosello RT, Pereira BJ, Linfante I, Piske RL. Dissecting
spinal aneurysms: conservative management as a therapeutic
option. J Neurointerv Surg. 2018;10:451-4. Crossref
14. Yang TK. A ruptured aneurysm in the branch of the anterior spinal
artery. J Cerebrovasc Endovasc Neurosurg. 2013;15:26-9. Crossref
15. Longatti P, Sgubin D, Di Paola F. Bleeding spinal artery aneurysms.
J Neurosurg Spine. 2008;8:574-8. Crossref