Treatment Outcomes of Stage II or III Gastric Cancer Treated with Adjuvant Chemotherapy with TS-1 or XELOX after Radical Surgery
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2023 Jun;26(2):91-9 | Epub 8 Jun 2023
Treatment Outcomes of Stage II or III Gastric Cancer Treated with Adjuvant Chemotherapy with TS-1 or XELOX after Radical Surgery
TCY So, KC Lee, ECY Wong
Department of Clinical Oncology, Pamela Youde Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr TCY So, Department of Clinical Oncology, Pamela Youde Eastern Hospital, Hong Kong SAR, China. Email: scy027@ha.org.hk
Submitted: 6 May 2022; Accepted: 2 Dec 2022.
Contributors: TCYS and ECYW designed the study. TCYS acquired, analysed the data and drafted the manuscript. KCL and ECYW critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present research are available from the corresponding author on reasonable request.
Ethics Approval: This research has been approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref. No.: HKECREC-2022-021) and was conducted in compliance with the Declaration of Helsinki. The requirement for patient consent
was waived by the Committee due to the retrospective nature of the study.
Abstract
Introduction
Capecitabine plus oxaliplatin (XELOX) and tegafur/gimeracil/oteracil (TS-1, also known as ‘S-1’) are two commonly used adjuvant chemotherapy regimens for gastric cancer in Hong Kong. This study aimed to review the outcomes of patients receiving these two regimens, to investigate important clinical factors that may impact on the risk of disease recurrence, and to explore the roles of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (PLR) in prognostication after radical surgery.
Methods
Patients who received adjuvant treatment (either XELOX or TS-1) for gastric cancer following radical surgical resection from January 2016 to December 2020 at our hospital were included. Patient demographics, overall survival (OS), and disease-free survival (DFS) were analysed.
Results
A total of 65 patients were included (XELOX: n = 40; TS-1: n = 25). XELOX appeared to have more favourable OS and DFS, although the result was confounded by older and frailer patients in the TS-1 group. An elevated PLR was associated with inferior OS after surgery (p = 0.036). Cox regression analysis showed that Eastern Cooperative Oncology Group (ECOG) performance status score of 2 and nodal stage of N2 to N3 were two independent factors associated with inferior OS. ECOG performance status score of 2, nodal stage of N2 to N3, and chemotherapy dose intensity <70% were significantly associated with a higher risk of relapse.
Conclusion
Poorer ECOG performance status and more advanced nodal stage are independent factors associated with inferior OS and DFS, and lower chemotherapy dose intensity (<70%) resulted in a higher risk of disease relapse. NLR and PLR is a simple clinical marker that may be further explored as a prognostic marker for gastric cancer after radical surgery.
Key Words: Blood platelets; Lymphocytes; Neutrophils; Prognosis; Stomach neoplasms
中文摘要
根治性手術後使用TS-1或XELOX輔助化療治療II期或III期胃癌的治療結果
蘇駿寅、李建忠、王晉彥
簡介
卡培他濱聯合奧沙利鉑(XELOX)及替加氟/吉美嘧啶/氧嗪酸(TS-1,又稱S-1)是香港兩種常用於胃癌的輔助化療方案。本研究旨在回顧接受這兩種方案的病人的結果,調查可能影響疾病復發風險的重要臨床因素,以及研究在根治性手術後嗜中性白血球與淋巴細胞比例(NLR)及血小板與淋巴細胞比例(PLR)在預測方面的角色。
方法
本研究包括於2016年1月至2020年12月期間在本院進行根治性手術切除後接受輔助治療(XELOX或TS-1)的胃癌病人,並分析了有關患者的人口特徵、整體存活及無疾病存活。
結果
本研究共包括65名患者(XELOX:n = 40;TS-1:n = 25)。雖然XELOX的整體存活及無疾病存活似乎較好,但這些結果受TS-1組別中年紀較大及較虛弱的患者影響。血小板與淋巴細胞比例上升與較差的術後整體存活相關(p = 0.036)。Cox迴歸分析顯示美國東岸癌症臨床研究合作組織(ECOG)身體功能狀態評分為2分及癌症分期為N2至N3,是與較差的整體存活相關的兩個獨立因素。ECOG身體功能狀態評分為2分、癌症分期為N2至N3及化療劑量強度<70%與較高復發風險顯著相關。
結論
較差的ECOG身體功能狀態及較晚期的癌症分期是與較差的整體存活及無疾病存活相關的獨立因素,而較低的化療劑量強度<70%造成較高的疾病復發風險。NLR和PLR是簡單的臨床標記,可成為日後的研究方向,以此比例作為根治性手術後胃癌的預後標記。
INTRODUCTION
Gastric cancer was the sixth commonest cancer in
Hong Kong, accounting for 3.7% of all new cancer
cases in 2019.[1] Although the incidence has been
gradually declining, compatible with global trends due
to efficacious Helicobacter pylori eradication therapy,[2]
gastric cancer remains more prevalent in Asian countries
than in the West.
Clear surgical resection with D2 lymphadenectomy
and chemotherapy is considered the standard of care
for resectable locoregionally advanced gastric cancer
nowadays,[3] and this has been advocated in several
international guidelines.[4] [5] Adjuvant chemoradiotherapy
(45 Gy over 25 fractions concurrent with 5-fluorouracil
and leucovorin) had once been widely adopted, but was
later criticised for the inclusion of a high proportion
of patients with D1 lymphadenectomy in the study
recommending it.[6]
The choice of chemotherapy regimen significantly
differs among different parts of the world. In European countries, perioperative chemotherapy, such as the
combination of epirubicin, cisplatin, and 5-fluorouracil[7]
or 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel,[8]
is frequently used, whereas in Hong Kong, clinicians tend
to use adjuvant chemotherapy as in most Asian countries.
The two most commonly used regimens of adjuvant
chemotherapy after radical surgery are capecitabine
plus oxaliplatin (XELOX) and tegafur/gimeracil/oteracil
(TS-1, also known as ‘S-1’). They both demonstrated
significant benefits when compared with surgery alone
in randomised clinical trials[9] [10] conducted in Asian
countries. Despite the two regimens having been widely
used, there are no prospective randomised clinical trials
directly comparing their efficacy.
Regarding the prognostic stratification of patients with
resected gastric cancer, several clinical and pathological
parameters have long been adopted to predict the
recurrence of gastric cancer including age, comorbidities,
tumour size, differentiation status, and presence of
lymphovascular or perineural invasion.[11] [12] [13] [14] In recent
years, the clinical utility of the peripheral neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte
ratio (PLR) as systemic inflammatory markers has been
addressed. In relation to cancer prognosis, several meta-analyses
showed that elevated NLR and PLR correlated
with tumour progression and poor survival in a number
of gastrointestinal cancers.[15] [16] However, what the same
observation connotes in the adjuvant setting remains
uncertain.
This retrospective study was conducted with three aims:
to compare the efficacy of adjuvant XELOX with TS-1
chemotherapy for patients with stage II or III gastric
cancer who received radical surgery in our locality; to
investigate important clinical factors that may impact
on the risk of disease recurrence; and to explore the
prognostic value of NLR and PLR as potentially useful
and easily available clinical parameters.
METHODS
Patients and Data Collection
Patients who received adjuvant treatment (XELOX: n =
40; TS-1: n = 25) for gastric cancer following radical
surgical resection from January 2016 to December 2020
at the Department of Clinical Oncology, Pamela Youde
Eastern Hospital, Hong Kong were included in the study.
Patients with metastatic disease at presentation (including
small-volume peritoneal metastasis) or double primary
cancers were excluded. Patients who received adjuvant
radiotherapy were also excluded. Relevant clinical and
pathological parameters were captured from clinical
notes and the Clinical Management System of Hospital
Authority.
Treatment
XELOX consists of oral capecitabine (1,000 mg/m2 twice daily on days 1-14 of each cycle) plus intravenous
oxaliplatin (130 mg/m2 on day 1 of each cycle) up to
8 cycles. TS-1 is oral chemotherapy (daily dose according
to body surface area [BSA]: patients with BSA <1.25 m2
received 80 mg daily, those BSA ranging from ≤1.25 m2
to 1.50 m2 received 100 mg daily, and those with BSA
≥1.50 m2 received 120 mg daily) given for 4 weeks
followed by 2 weeks of rest for a total of 9 cycles.
In practice, patients of an advanced age, borderline
Eastern Cooperative Oncology Group (ECOG)
performance status and pre-existing neuropathy would
be more likely to be given TS-1, as it is a non–self-financed
item under the institution.
Doses and schedule modifications were conducted based on patients’ ECOG performance status, organ functions,
and toxicities by clinicians’ decisions. Dose reduction
of chemotherapy was conducted in a stepwise manner
(75%-85% of the initial dose for 1st dose reduction, then
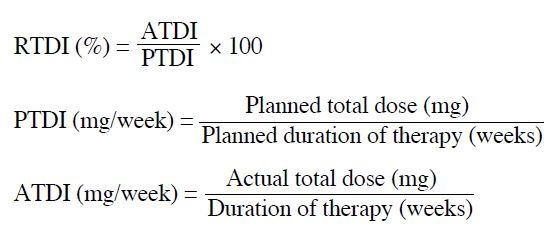
60%-70% for the 2nd dose reduction). The relative total
dose intensity (RTDI) is the ratio of the delivered actual
dose intensity (ATDI) to the standard planned dose
intensity (PTDI) for a chemotherapy regimen, which is
calculated as follows:
Follow-up and Assessment
Patients were seen by doctors prior to each cycle of
chemotherapy, when tolerance of chemotherapy and
results of blood tests would be recorded in the Clinical
Management System. Patients who had completed the
adjuvant chemotherapy would be followed up at an
interval of 3 to 6 months. Computed tomography was
performed if there was clinical suspicion of disease
relapse. Disease relapse was defined as any radiological
and/or histological confirmation of recurrence. Elevated
tumour markers alone were not considered as relapse
without proof of recurrent disease.
Statistical Analysis
Statistical analysis was performed using SPSS (Windows
version 22; IBM Corp, Armonk [NY], United States).
Clinical and pathological data were retrospectively
reviewed and analysed by descriptive statistics. Pearson’s
Chi squared test was used for testing any significant
correlations and differences between groups.
Treatment outcomes, including disease-free survival
(DFS, the time from surgery to disease relapse) and
overall survival (OS, the time from diagnosis of disease
to death from any cause) were analysed by the Kaplan-Meier method and the difference between groups
were tested with the log-rank test. Different clinical
parameters were tested for their impact on DFS and OS
by Cox regression analysis.
In order to have an accurate assessment of the baseline
NLR and PLR of our patients, the complete blood
counts right before the administration of first cycle of chemotherapy were recorded. This is to minimise
the effect due to postoperative inflammation and
chemotherapy on peripheral blood counts.
Using all-cause mortality as an endpoint for NLR and
PLR, the optimal cut-off values were determined by
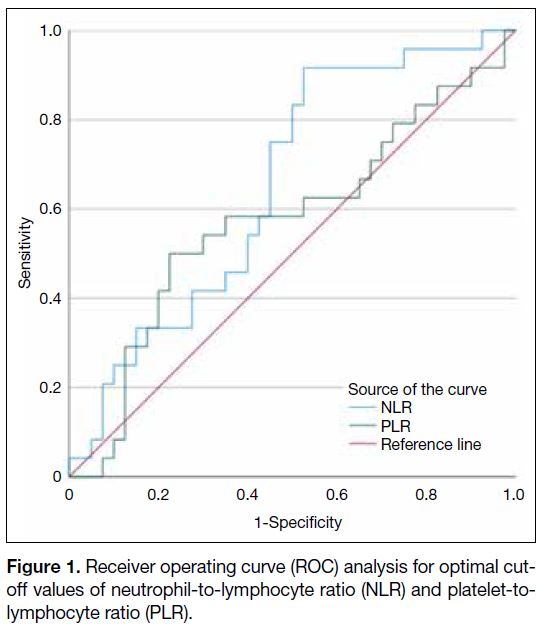
receiver operating curve analysis as shown in Figure 1.
The area under the curve of NLR and PLR was 0.653
and 0.575, respectively. The optimal cut-off values
determined by the Youden’s index for NLR and PLR
were 1.9 and 169, respectively.
Figure 1. Receiver operating curve (ROC) analysis for optimal cut-off values of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR).
RESULTS
Patient Characteristics
Sixty-five patients were identified and included in
the analysis. Forty patients received XELOX and 25
received TS-1. The median follow-up time for this study
was 33.7 months (range, 6.5-78.9).
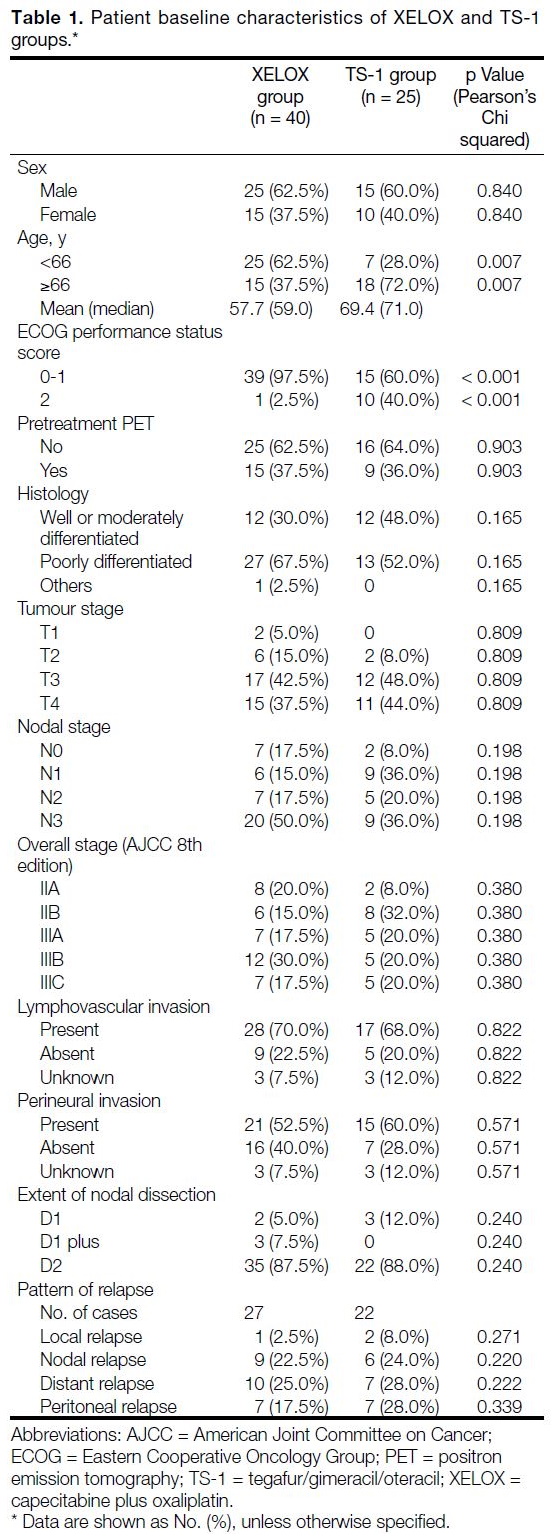
Patient baseline characteristics are summarised in Table 1. The median age of the entire cohort was 66.0 years.
The mean and median age in the XELOX group were
57.7 and 59.0 years respectively, compared to 69.4 and
71.0 years in the TS-1 group. Patients who received
TS-1 were significantly older, with 72.0% of them ≥66
years compared to 37.5% in XELOX group (p = 0.007). All included patients had an ECOG performance status
score of ≤2. There were significantly more patients with
ECOG performance status score ≤1 in the XELOX group
(97.5%) than in TS-1 group (60.0%) [p < 0.001].
Table 1. Patient baseline characteristics of XELOX and TS-1 groups.
Overall and Disease-Free Survival
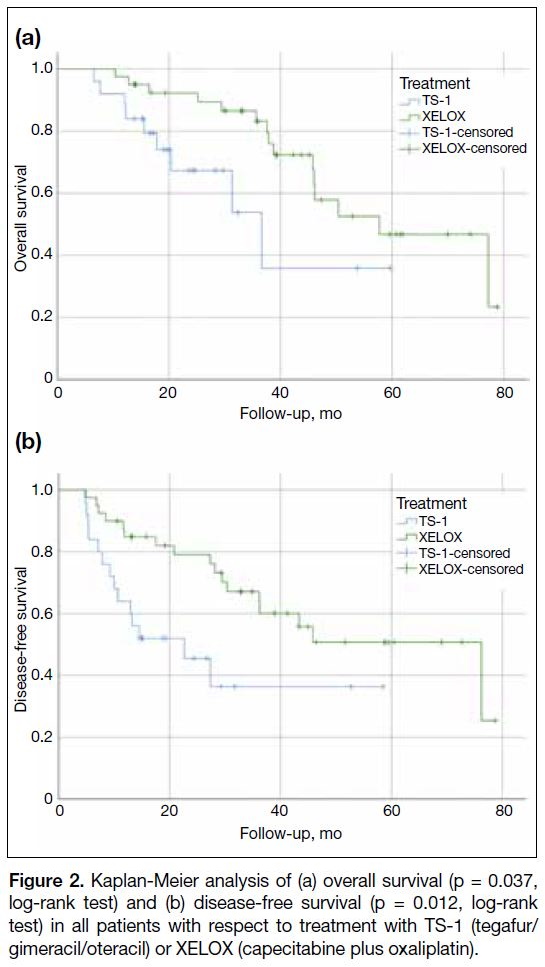
The median OS was 38.9 months for the XELOX group
and 22.9 months for TS-1 group. The observed OS and
DFS in the XELOX group were significantly longer than
those in the TS-1 group (Figure 2) [p = 0.037 and 0.012,
respectively]. However, it should be interpreted carefully
as the baseline patients’ characteristics suggested a bias
towards prescribing TS-1 in the older age-group and less
fit patients. These factors likely confound the survival
analysis.
Figure 2. Kaplan-Meier analysis of (a) overall survival (p = 0.037,
log-rank test) and (b) disease-free survival (p = 0.012, log-rank
test) in all patients with respect to treatment with TS-1 (tegafur/gimeracil/oteracil) or XELOX (capecitabine plus oxaliplatin).
Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio
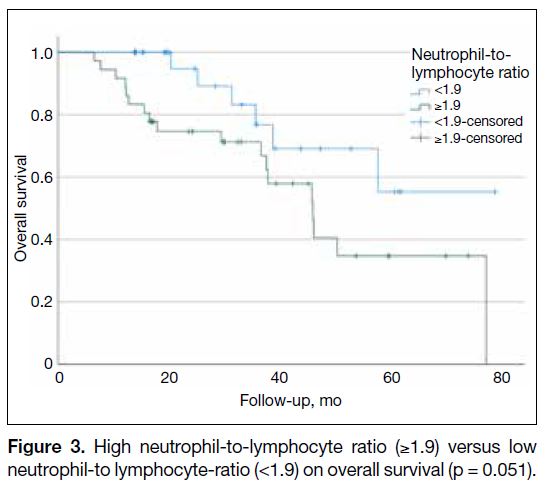
Overall survival analysis showed that patients with high
NLR (≥1.9) before adjuvant chemotherapy had shorter
OS than those with low NLR (<1.9), although the
difference was marginally significant (p = 0.051; Figure 3). The same analysis also demonstrated that patients
with high PLR (≥169) before adjuvant chemotherapy
had significantly shorter OS than those with low PLR
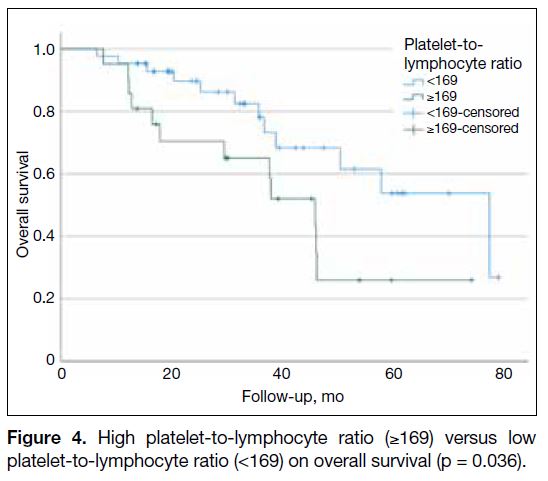
(<169) [p = 0.036; Figure 4].
Figure 3. High neutrophil-to-lymphocyte ratio (≥1.9) versus low neutrophil-to lymphocyte-ratio (<1.9) on overall survival (p = 0.051).
Figure 4. High platelet-to-lymphocyte ratio (≥169) versus low platelet-to-lymphocyte ratio (<169) on overall survival (p = 0.036).
In relation to clinical characteristics, patients with
elevated NLR correlated with female gender (borderline p value of 0.049) and elevated PLR was associated with more advanced disease (p = 0.012) [Tables 2 and 3].
Table 2. Clinical characteristics of patients with high and low
neutrophil-to-lymphocyte ratio.
Table 3. Clinical characteristics of patients with high and low platelet-to-lymphocyte ratio.
Clinical and Pathological Parameters on
Overall Survival and Disease-Free Survival
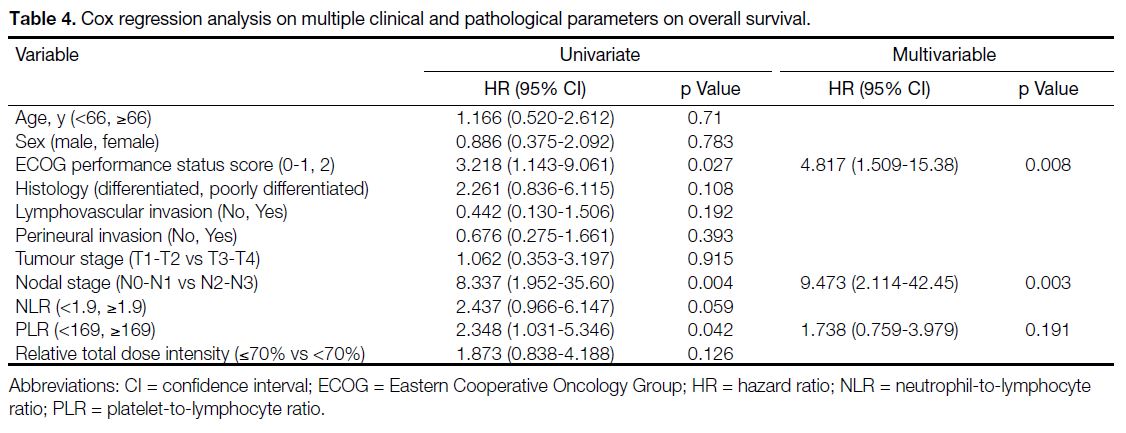
As shown in Table 4, univariate Cox regression analysis
showed that ECOG performance status score of 2, nodal
stage of N2 to N3, and elevated PLR (≥169) were adverse
prognostic factors for OS, while ECOG performance
status score of 2, nodal stage of N2 to N3, and RTDI
of chemotherapy <70% were adverse factors associated
with disease relapse.
Table 4. Cox regression analysis on multiple clinical and pathological parameters on overall survival.
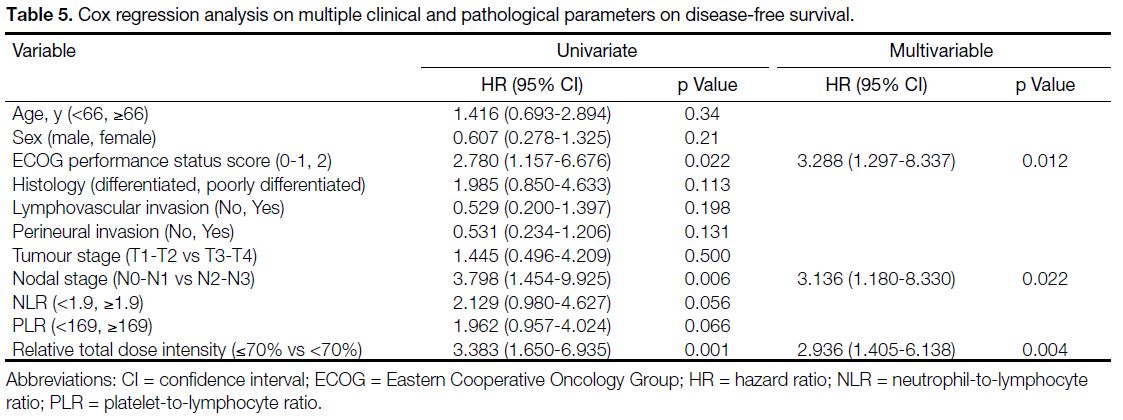
Multivariable Cox regression analysis demonstrated that
ECOG performance status score of 2 and nodal stage of N2 to N3 were the two independent adverse prognostic
factors for OS (Table 4). For DFS, ECOG performance
status score of 2, nodal stage of N2 to N3, and RTDI of
chemotherapy <70% were the three independent factors
associated with disease relapse (Table 5).
Table 5. Cox regression analysis on multiple clinical and pathological parameters on disease-free survival.
DISCUSSION
Our study revealed that the XELOX group had more
favourable oncological outcomes (both DFS and OS) than
the TS-1 group. However, it should be noted that patients
included in the TS-1 group in out centre were older
(p = 0.007) and of worse ECOG performance status
(p < 0.001). This is largely due to the fact that the
institutional guideline recommends TS-1 as the
treatment of choice for older patients with anticipated poor tolerance to XELOX and that under such
circumstances, only the drug costs of TS-1 would be
covered by the institution. There has not been any
randomised controlled trial comparing the efficacy of
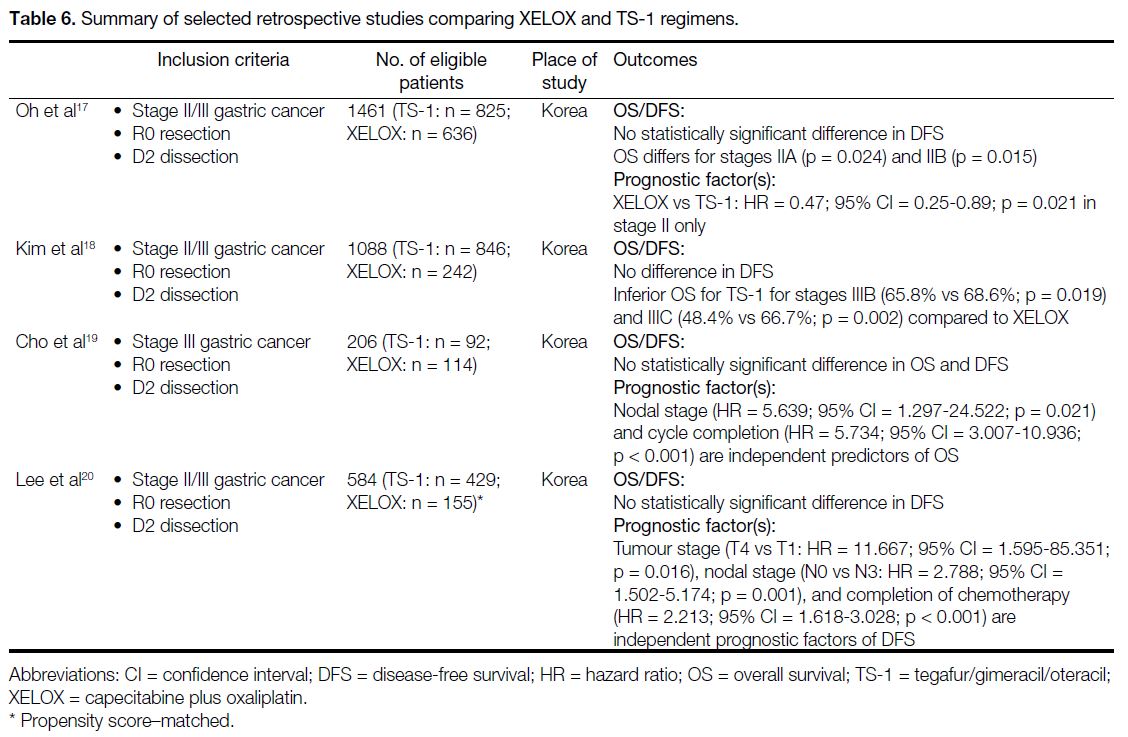
the two regimens. Retrospective studies[17] [18] [19] [20] did not
demonstrate statistically significant differences in DFS
between adjuvant TS-1 and XELOX (Table 6). In the
subgroup analysis, one study[17] demonstrated the use
of XELOX in stage II disease was associated with
better OS while another study[18] suggested the same
but in stage IIIB/C disease only. Apart from XELOX,
combination chemotherapy with more than three agents
has shown superior treatment outcomes in recent years.
Combination of TS-1 with oxaliplatin[21] or docetaxel[22] is
considered a preferred option for high-risk patients and
is increasingly recognised as a new standard of care.
Table 6. Summary of selected retrospective studies comparing XELOX and TS-1 regimens.
In our cohort, elevated PLR is associated with inferior
OS after curative surgery and there was a similar trend for NLR despite not reaching statistical significance (p = 0.051). NLR and PLR are important parameters indicating
systemic inflammation. It is observed that a chronic
inflammatory state confers unfavourable oncological
outcomes.[23] Several meta-analyses revealed that elevated
NLR and PLR were associated with tumour progression
and poor survival in gastrointestinal cancers.[15] [16]
Microscopically, various inflammatory cytokines and
growth factors in the tumour microenvironment are
known to dampen hosts’ anti-tumour immune response.
In tumour models, inflammatory cytokines such as
interleukin 6 (IL-6), IL-8 and IL-11 are associated
with chemotherapy resistance in gastric cancer through
mechanisms such as inhibition of apoptosis pathways,
increasing efflux of chemotherapeutic agents, and evasion
of DNA damage.[24] [25] [26] We therefore postulated that in an
adjuvant setting, the persistent inflammatory state after
curative surgery possibly led to tumour evasion from
immunosurveillance and enhanced chemoresistance of micrometastases.[27] NLR and PLR are two readily
accessible clinical parameters and may serve as simple
prognostic tools in addition to performance status, stage,
and age.
Our study revealed that the RTDI is an independent
prognostic factor for disease recurrence. Inadequate
chemotherapy dose intensity is either attributed to
excessive dose reduction or failure to complete scheduled
cycles within the planned time interval. It is noteworthy
that severe adverse events of chemotherapy (≥ Grade 3)
have been shown to be quite uncommon (≤6%) with TS-1
in a large-scale clinical trial,[28] although these patients
were generally frailer and older. For elderly patients
who may be more vulnerable to chemotherapy toxicity,
proper geriatric assessments (such as comorbidity and
frailty indices) are needed, as biological age is not a
reliable indicator for chemotherapy dose adjustment, and
an adaptive dose optimisation approach is recommended
based on patients’ tolerance of each cycle.
This study has several limitations. First, it is only a
single-centre retrospective study in which the small
sample size limits its statistical power. Second, there is imbalance between the baseline characteristics of the
two groups of patients. Similar to the Korean studies,[17] [18] [19] [20]
patients in the TS-1 group were generally older and had a
worse ECOG performance status. There is a tendency for
clinicians to prescribe a more conservative chemotherapy
dosage in this group of patients, which may explain the
lower dose intensity of TS-1 than XELOX. Propensity
score matching should be performed in a larger cohort
to reduce the bias due to these confounding variables.
Third, a much large sample size is needed to further
evaluate the prognostic power of NLR and PLR on OS
and DFS in the adjuvant setting. In our cohort, high PLR
appeared to correlate with patients with more advanced
disease (stage III), which is an important confounding
factor.
CONCLUSION
In conclusion, we compared the OS and DFS between
adjuvant XELOX and TS-1 in our local gastric cancer
patients. Clinical outcomes were statistically better with
XELOX- than TS-1–treated patients. However, the
results should be viewed with caution because of the
limited sample size and obvious imbalance in baseline
characteristics. ECOG performance status score of 2 and advanced nodal stage of N2 to N3 are independent
adverse prognostic factors associated with poor OS
and a higher rate of disease recurrence. NLR and
PLR are readily available markers that may be further
explored as prognostic markers for gastric cancer after
radical surgery. We also speculated that the RTDI of
chemotherapy of <70% might affect the risk of disease relapse.
REFERENCES
1. Centre for Health Protection, Department of Health, Hong Kong SAR Government. Stomach cancer. Available from: https://www.chp.gov.hk/en/healthtopics/content/25/55.html. Accessed 2 May
2022.
2. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. Crossref
3. Randle RW, Swords DS, Levine EA, Fino NF, Squires MH, Poultsides G, et al. Optimal extent of lymphadenectomy for gastric adenocarcinoma: a 7-institution study of the US gastric cancer
collaborative. J Surg Oncol. 2016;113:750-5. Crossref
4. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-49. Crossref
5. Hakkenbrak, NA, Jansma EP, van der Wielen N, van der Peet DL, Straatman J. Laparoscopic versus open distal gastrectomy for gastric cancer: a systematic review and meta-analysis. Surgery. 2022;171:1552-61. Crossref
6. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach
or gastroesophageal junction. N Engl J Med. 2001;345:725-30. Crossref
7. Cunningham D, Allum WH, Stenning SP, Weeden S. Perioperative
chemotherapy in operable gastric and lower oesophageal cancer:
final results of a randomised, controlled trial (the MAGIC trial,
ISRCTN 93793971). J Clin Oncol. 2005;23(16 suppl):4001. Crossref
8. Al-Batran SE, Homann N, Schmalenberg H, Kopp HG, Haag GM, Luley KB, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma
(FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin
Oncol. 2017;35(15 suppl):4004. Crossref
9. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised
controlled trial. Lancet. 2012;379:315-21. Crossref
10. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-93. Crossref
11. Liang YX, Deng JY, Guo HH, Ding XW, Wang XN, Wang BG, et al. Characteristics and prognosis of gastric cancer in patients aged ≥70 years. World J Gastroenterol. 2013;19:6568-78. Crossref
12. Stiekema J, Cats A, Kuijpers A, van Coevorden F, Boot H,
Jansen EP, et al. Surgical treatment results of intestinal and diffuse
type gastric cancer. Implications for a differentiated therapeutic
approach? Eur J Surg Oncol. 2013;39:686-93. Crossref
13. Feng F, Liu J, Wang F, Zheng G, Wang Q, Liu S, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018;18:865. Crossref
14. Asplund J, Gottlieb-Vedi E, Leijonmarck W, Mattsson F, Lagergren J. Prognosis after surgery for gastric adenocarcinoma in the Swedish Gastric Cancer Surgery Study (SWEGASS). Acta
Oncol. 2021;60:513-20. Crossref
15. Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N,
Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte
ratio and platelet-to-lymphocyte ratio in oncologic
outcomes of esophageal cancer: a systematic review and meta-analysis.
Ann Surg Oncol. 2016;23:646-54 Crossref
16. Zhang J, Zhang HY, Li J, Shao XY, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget.
2017;8:68837-46. Crossref
17. Oh SE, An JY, Choi MG, Lee JH, Sohn TS, Bae JM. Comparison
of long-term efficacy in S-1 and capecitabine with oxaliplatin
as adjuvant chemotherapy for patients with gastric cancer after
curative surgery: a retrospective, single-center observational study.
Technol Cancer Res Treat. 2021;20:15330338211039679. Crossref
18. Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, et al. Efficacy of adjuvant S-1 versus XELOX chemotherapy for patients with gastric cancer after D2 lymph node dissection: a retrospective, multi-center observational study. Ann Surg Oncol. 2018;25:1176-83. Crossref
19. Cho JH, Lim JY, Cho JY. Comparison of capecitabine and oxaliplatin with S-1 as adjuvant chemotherapy in stage III gastric cancer after D2 gastrectomy. PLoS One. 2017;12:e0186362. Crossref
20. Lee CM, Yoo MW, Son YG, Oh SJ, Kim JH, Kim HI, et al. Long-term efficacy of S-1 monotherapy or capecitabine plus oxaliplatin as adjuvant chemotherapy for patients with stage II or III gastric cancer after curative gastrectomy: a propensity score-matched
multicenter cohort study. J Gastric Cancer. 2020;20:152-64. Crossref
21. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A
randomized phase III trial comparing adjuvant single-agent S1, S-1
with oxaliplatin, and postoperative chemoradiation with S-1 and
oxaliplatin in patients with node-positive gastric cancer after D2
resection: the ARTIST 2 trial. Ann Oncol. 2021;32:368-74. Crossref
22. Kodera Y, Yoshida K, Kochi M, Ichikawa W, Kakeji Y, Sano T,
et al. A randomized phase III study comparing S-1 plus docetaxel
with S-1 alone as a postoperative adjuvant chemotherapy for
curatively resected stage III gastric cancer (JACCRO GC-07 trial).
J Clin Oncol. 2018;36(15 suppl):4007. Crossref
23. Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11-20. Crossref
24. Ham IH, Oh HJ, Jin H, Bae A, Jeon SM, Choi KS, et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019;18:68. Crossref
25. Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18:979-85. Crossref
26. Ma J, Song X, Xu X, Mou Y. Cancer-associated fibroblasts promote the chemo-resistance in gastric cancer through secreting IL-11 targeting JAK/STAT3/Bcl2 pathway. Cancer Res Treat. 2019;51:194-210. Crossref
27. Olive KP. Fanning the flames of cancer chemoresistance: inflammation and anticancer therapy. J Oncol Pract. 2017;13:181-3. Crossref
28. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-20. Crossref