Conversion Surgery in Advanced Unresectable Gastric Cancer After Induction Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 5 March 2025
Conversion Surgery in Advanced Unresectable Gastric Cancer After Induction Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel
R Liu1, HLY Hou1, RPY Tse1, KK Yuen1, DLW Kwong2, WL Chan2
1 Department of Clinical Oncology, Queen Mary Hospital, Hong Kong SAR, China
2 Department of Clinical Oncology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Correspondence: Dr R Liu, Department of Clinical Oncology, Queen Mary Hospital, Hong Kong SAR, China. Email: lr638@ha.org.hk
Submitted: 15 August 2024; Accepted: 27 November 2024. This version may differ from the final version when published in an issue.
Contributors: RL and WLC designed the study. RL acquired the data. RL and HLYH analysed the data. RL drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, DLWK was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: UW24-514). The requirement for patient consent was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
Palliative chemotherapy is the standard treatment for unresectable locally advanced gastric cancer (GC) with poor prognosis. We evaluated the safety and efficacy of a multimodality approach with induction fluorouracil plus leucovorin, oxaliplatin, and docetaxel (FLOT) followed by attempted conversion surgery with additional FLOT at a tertiary hospital in Hong Kong.
Methods
Medical records of advanced GC patients treated with induction FLOT and attempted conversion surgery between 2017 and 2023 were reviewed. Patients suitable for surgery after chemotherapy underwent resection, followed by adjuvant FLOT for another four cycles. Safety, treatment outcomes and predictive factors for survival were analysed.
Results
Thirty-one patients (25 males, median age = 63 years) were included. The median follow-up time was 22.0 months. Disease control rate after induction FLOT was 87.1% (n = 27). Conversion surgery was performed in 23 patients (74.2%), with 20 achieving R0 resection. Patients with conversion surgery had longer median overall survival (OS) and event-free survival than those who could not undergo surgery. Multivariable analysis identified no conversion surgery, higher neutrophil-to-lymphocyte ratio, serum albumin level <35 g/L, body mass index <23 kg/m2, and clinical nodal stage N3 disease with a worse OS. No treatment-related deaths occurred. The incidence of grade ≥3 toxicities was 51.6%, with neutropenia (29.0%) and febrile neutropenia (12.9%) being most common.
Conclusion
Induction FLOT achieved high conversion rates and R0 resections, offering a favourable survival benefit and acceptable safety in unresectable GC. Prospective trials incorporating biomarker-driven therapy may further improve pathological complete response rates and survival.
Key Words: Adenocarcinoma; Induction chemotherapy; Stomach neoplasms
中文摘要
晚期不可切除胃癌在接受誘導氟尿嘧啶、亞葉酸鈣、奧沙利鉑及多西他賽治療後的轉化手術
廖文傑、侯力予、謝佩楹、袁國強、鄺麗雲、陳穎樂
引言
緩和性化療是預後差的不可切除局部晚期胃癌的標準治療。患者在接受誘導氟尿嘧啶、亞葉酸鈣、奧沙利鉑及多西他賽(FLOT)治療後嘗試進行轉化手術再配合FLOT是一個多模態治療方式,我們評估這治療方式在香港某所三級醫院的安全性及效用。
方法
我們對在2017至2023年間接受誘導FLOT及嘗試轉化手術治療的晚期胃癌患者的醫療紀錄進行回顧性研究。適合在化療後進行手術的患者接受切除,然後進行另外四個療程的輔助FLOT治療。我們分析了安全性、治療結果及存活的預測因素。
結果
本研究包括31名患者(25名男性,年齡中位數 = 63歲)。隨訪時間中位數為22.0個月。在接受誘導FLOT治療後的疾病控制率為87.1%(n = 27)。共有23名患者(74.2%)接受了轉化手術,當中20名達至完全切除(R0)。與不能接受手術的患者相比,接受了轉化手術的患者的整體存活時間及無事件存活時間均較長。多變量分析識別出沒有接受轉化手術、嗜中性白血球與淋巴球比例(NLR)較高、血清白蛋白水平<35 g/L、體重指標(BMI)<23 kg/m2及臨床第N3期胃癌的整體存活較差。本研究沒有發生與治療相關的死亡事件。毒性≥3的發生率為51.6%,當中以嗜中性白血球減少症(29.0%)及嗜中性白血球減少症合併發熱(12.9%)最為常見。
結論
誘導FLOT治療達至高轉化率及完全切除,為不可切除胃癌患者提供了有利的存活獲益及可接受的安全性。結合生物標記驅動治療的前瞻性試驗可進一步改善病理完全緩解率及存活。
INTRODUCTION
Gastric cancer (GC) is the sixth most common cancer
and the sixth leading cause of cancer deaths in Hong
Kong.[1] Worldwide, it is the fourth most common leading
cause of cancer deaths (7.7%).[2] While surgical resection
is the treatment of choice for operable GC, many patients
eventually relapse. Thus, combined modality treatment is
recommended for resectable GC classified as stage ≥IB
disease under the TNM (Tumour, Node and Metastasis)
system.[3] [4] Unresectable locally advanced/metastatic GC
has a poor prognosis with a median overall survival
(OS) of about 4 to 6 months with supportive care alone.[5]
With the use of various combination chemotherapeutic
regimens containing platinum, fluoropyrimidine, taxane,
irinotecan, and/or trastuzumab, the median survival time
improves to approximately 11 to 15 months.[5] [6] [7] [8] [9] [10] Recently,
the addition of immunotherapy to chemotherapy has
also been shown to improve survival in the first-line
advanced/metastatic setting, especially for those with
high expression of programmed death ligand 1.[11] [12]
Currently, the standard of care is palliative in nature, and newer multidisciplinary therapeutic approaches to
improve survival and offer a chance of cure for advanced
GC are being evaluated.
The role of surgery in advanced GC is a controversial
topic. Advanced GC is a heterogeneous disease with
varying extents of local invasion, varying locations and
extent of lymph node metastases, and diverse metastatic
patterns. The REGATTA randomised phase three trial
(Reductive Gastrectomy for Advanced Tumour in Three
Asian Countries)[13] failed to show a survival advantage
with gastrectomy followed by chemotherapy versus
chemotherapy alone in advanced GC. This could partly
be explained by the decreased tolerance to chemotherapy
after gastrectomy, which was similarly shown in the
perioperative setting in the MAGIC trial.[14] Conversion
surgery, in which patients with unresectable tumours
are given neoadjuvant chemotherapy in an attempt to
downstage the disease and achieve an R0 resection,
is an appealing approach in advanced GC.[15] Besides
downstaging the tumour itself, the rationale behind giving neoadjuvant chemotherapy is to eradicate occult
metastatic disease and to take advantage of the improved
tolerance of chemotherapy compared to the postoperative
setting. Most data on conversion therapy are from
single-arm phase two studies and retrospective cohort
studies.[16] [17] [18] [19] [20] [21] [22] [23] [24] Various neoadjuvant chemotherapy regimens
including docetaxel, capecitabine/S-1, and cisplatin/oxaliplatin, have been used as conversion therapy in
treating advanced GCs.[16] [17] [18] [19] [20] [21] [22] [23] [24] The median survival time
of patients following R0 resection is up to 41 to 57
months,[16] [18] [19] [23] dramatically better than that achieved
by palliative systemic treatment alone. Perioperative
chemotherapy regimens such as S-1 plus cisplatin, and
fluorouracil plus leucovorin, oxaliplatin, and docetaxel
(FLOT) have also been used in conversion therapy and
demonstrated favourable outcomes, especially in those
with limited metastatic advanced GC.[25] [26]
The multidisciplinary approach of using induction FLOT
for four cycles followed by attempted conversion surgery
for advanced GC has been used in our centre since 2017.
Our centre prefers to use FLOT due to our experience
with it in the perioperative setting for resectable GC.
Perioperative FLOT in resectable GC has been shown to
have a high pathological response rate with a reasonable
toxicity profile[27] [28] and is the preferred regimen in this
setting as recommended by the National Comprehensive
Cancer Network[4] and the European Society for Medical
Oncology.[3] This retrospective study evaluated the safety
and efficacy of incorporating FLOT and conversion
surgery in advanced GC.
METHODS
Patients
The cases of 31 consecutive patients with clinically
unresectable, locoregionally advanced GC (cT2-4bN0-3M0) treated with induction FLOT with the goal of
conversion surgery at Queen Mary Hospital, Hong
Kong between January 2017 and December 2023
were reviewed. All patients had undergone baseline
upper gastrointestinal endoscopy, were diagnosed with
histologically confirmed gastric or gastroesophageal
junction adenocarcinoma, and had received staging
18F-fluorodeoxyglucose positron emission tomography–computed tomography (18F-FDG PET/CT) whole-body
scans. Disease was staged according to the 7th and 8th
TNM staging system developed by the American Joint
Committee on Cancer.[29] [30] [31] Preoperative exploratory
laparoscopy to rule out peritoneal metastases was
not mandatory. All cases were deemed clinically
unresectable with locoregionally advanced disease after discussion in multidisciplinary meetings comprising
of surgeons, oncologists, and radiologists. Hitherto
‘unresectable’ features included invasion into adjacent
organs (clinical tumour stage T4b [stage cT4b] disease),
locoregionally advanced/bulky non–stage cT4b disease,
or extensive involvement of regional lymph nodes.
These groups of patients were targeted as they would
otherwise receive palliative systemic treatment. Patients
with grossly definite metastases (i.e., visible on 18F-FDG
PET/CT or histologically confirmed at surgery) or
distant metastases to visceral organs other than lymph
nodes were not included, as our centre would treat these
patients with multimodality treatment consisting of
intraperitoneal chemotherapy or systemic therapy with
palliative intent.
Treatment Overview
All patients received induction chemotherapy using
FLOT according to the protocol used in the FLOT4 trial.[28]
Each cycle of FLOT consisted of intravenous docetaxel
50 mg/m2, oxaliplatin 85 mg/m2, and leucovorin
200 mg/m2, followed by a 24-hour continuous infusion
of fluorouracil 2600 mg/m2 on day 1. This regimen
was given every 2 weeks for four cycles. The dose
was adjusted if patient had intolerable side-effects.
Prophylactic use of granulocyte colony-stimulating
factor (G-CSF) was used as needed.
Evaluation of Response
Clinical response was assessed after four cycles of
FLOT with upper gastrointestinal endoscopy and
18F-FDG PET/CT scan. Response measurement was
classified according to RECIST (Response Evaluation
Criteria in Solid Tumors) version 1.1.[32] All cases were
re-evaluated in multidisciplinary meetings. If the tumour
responded well and curative resection was judged
to be possible, conversion surgery was scheduled.
Patients with tumours that responded unsatisfactorily
and were unlikely amenable to curative resection were
administered palliative second-line treatments.
Conversion Surgery and Follow-up
All patients included were alive within 10 weeks from the
day of last administration of FLOT. Conversion surgery
was performed within 10 weeks from the day of last
FLOT. If surgical exploration did not reveal unresectable
features, R0 resection was attempted. Depending on
the location and size of the gastric or gastroesophageal
junction tumour, curative resection involved distal or
total gastrectomy plus or minus oesophagectomy, along
with D2 lymph node dissection. After surgery, another four cycles of adjuvant FLOT were administered to those
with R0 resection. For patients with R1 or R2 resections,
subsequent treatments were chosen at the discretion of
oncologists.
For patients who underwent conversion surgery and
completed adjuvant FLOT, the follow-up schedule
consisted of clinical visits at least once every 3 months
during the first 2 years, followed by at least once every 6
months. CT or 18F-FDG PET/CT was performed at least
once every 6 months for the first 3 years.
Outcome Measures and Statistical Analyses
Relevant clinical and pathological parameters were
recorded from clinical notes and the Clinical Management
System of the Hospital Authority. These data included
age, sex, body mass index (BMI), co-morbidities,
baseline blood tests, tumour characteristics, clinical
response, pathological response using the Modified Ryan
score,[33] and any adverse events from chemotherapy.
All statistical analyses were conducted using R version
4.3.1 (R Foundation for Statistical Computing).
Treatment outcomes included OS, defined as the period
from the time of starting induction FLOT to the date of
death or the last follow-up time; and event-free survival
(EFS), defined as the period from the time of starting
induction FLOT to the date of disease progression,
relapse, death or the last follow-up time. Safety was
evaluated according to the National Cancer Institute’s
CTCAE (Common Terminology Criteria for Adverse
Events) version 5.0.[34] OS and EFS of the groups
with and without conversion surgery were analysed
using the Kaplan-Meier method. The differences in
survival time were investigated by the log-rank test.
Simple and multivariable Cox proportional hazards
regression analyses were performed to identify clinical
and pathological variables in relation to survival. Each
clinical and pathological variable was assessed
individually using simple Cox regression and Kaplan-Meier curves with log-rank tests. Significant clinical
and pathological variables with p value ≤ 0.1 in
simple analysis were incorporated into a multivariable
regression model. Multivariable Cox regression model
was used to evaluate the independent effect of each factor
while adjusting for other variables. Hazard ratios were
presented with 95% confidence intervals (95% CIs). A
two-sided p value < 0.05 was considered as statistically
significant.
RESULTS
Patient Characteristics
During the study period, 31 patients (25 males and
6 females) with recently diagnosed locoregionally
advanced and clinically unresectable GC received
FLOT. The median age was 63 years (range, 34-80).
All patients had an Eastern Cooperative Oncology
Group performance status score of ≤2, with majority
(93.5%) scoring 1. Fourteen patients (45.2%) had
baseline anaemia requiring blood transfusion and seven
patients (22.6%) required either feeding tube insertion or
gastrojejunostomy for dysphagia. In total, two patients
(6.5%) had stage cT3 disease, 16 (51.6%) had stage
cT4a disease and 12 (38.7%) had stage cT4b disease.
Eight patients (25.8%) had clinical nodal stage N1 (stage
cN1) disease, 14 (45.2%) had stage cN2 disease and six
(19.4%) had stage cN3 disease. As stated above, reasons
for unresectability included invasion into adjacent organs
(stage T4b disease) in 12 patients (38.7%) [pancreas,
n = 6; liver, n = 3; colon, n = 3; heart, n = 1; spleen, n = 1],
locoregionally advanced/bulky non–stage cT4b disease
in 15 patients (48.4%), and extensive regional lymph
node involvement in 13 patients (41.9%). Seven patients
(22.6%) had two unresectable features and one (3.2%)
had three unresectable features. Table 1 summarises
the baseline characteristics of this study cohort. The
median follow-up time was 22.0 months (range, 5.8-
80.7). During the follow-up period, 19 (61.3%) of the 31
patients died.
Table 1. Baseline characteristics of patients who had induction fluorouracil plus leucovorin, oxaliplatin, and docetaxel (n = 31).
Clinical and Pathological Response and
Adverse Events
All patients except one (96.8%) completed the intended
four cycles of induction FLOT, with one patient
developing grade 3 encephalopathy and only received
three cycles. The disease control rate after induction
FLOT was 87.1% (n = 27), of which 23 patients (85.2%)
had a complete response (CR) or partial response (PR)
and four patients (14.8%) had stable disease (SD) on
imaging. Conversion surgery was performed in 23
patients (74.2%), with 20 underwent R0 resections, two
underwent R1 resections (microscopic distal duodenal
margin), and one underwent R2 resection (macroscopic
lymph nodes encasing major arteries). Total gastrectomy
was performed in seven patients (22.6%), distal
gastrectomy in nine (29.0%), and oesophagogastrectomy
in seven patients (22.6%). The median time between
end of chemotherapy and surgery was 5.4 weeks
(interquartile range, 3.8-6.8). Patients not undergoing conversion surgery included eight (25.8%) with stable
disease with unresolved unresectable features (n = 5)
and progressive disease (n = 3). Of these eight patients,
one underwent a palliative gastrojejunostomy and one a
palliative oesophagogastrectomy.
The pathological response rate was 60.9%, of which
two patients (8.7%) had pathological complete response
(pCR) or near complete response. All patients with
pathological response had radiological PR. Amongst
those with poor pathological response, seven had
radiological PR, one had radiological SD, and one had
radiological progressive disease.
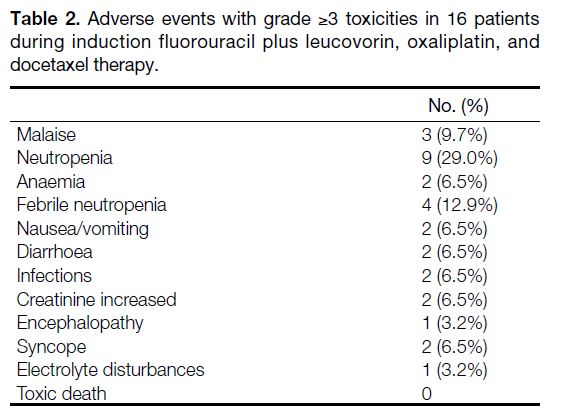
During preoperative FLOT, 20 patients (64.5%) received
prophylactic G-CSF support. The incidence of grade 3 or
4 toxicities during FLOT was 51.6%. Neutropenia (n = 9,
29.0%) and febrile neutropenia (n = 4, 12.9%) were the
most common; nine of these occurred in those without
prophylactic G-CSF. Non-haematologic adverse events
of grade ≥3 toxicities were not common in this cohort,
with malaise being the most common (9.7%). Treatment
was generally well tolerated, and no treatment-related deaths occurred. Table 2 shows adverse events with
grade ≥3 toxicities on FLOT.
Table 2. Adverse events with grade ≥3 toxicities in 16 patients during induction fluorouracil plus leucovorin, oxaliplatin, and docetaxel therapy.
Post Surgery
Postoperative complications were infrequent and
occurred in four patients (17.4%). They included
pneumonia, arrythmia, surgical emphysema, and
surgical site infections. No patients died within 30 days
after surgery.
Among 20 patients who underwent successful R0
conversion surgery, 19 (95.0%) started postoperative FLOT, with only one (5.3%) not completing the
intended four cycles due to malaise. One patient (5.0%)
in the R0 resection group did not start postoperative
FLOT and received TS-1 and cisplatin instead because
of the appearance of new perigastric lymph nodes while
on preoperative FLOT, though conversion surgery
was still deemed feasible. One of two patients with
R1 resection had a postoperative course of TS-1 and
chemoradiotherapy with TS-1 to the operative bed, while
the other one continued on FLOT. The patient with R2
resection continued on TS-1 alone.
Of the eight patients who did not undergo conversion
surgery, seven continued on chemotherapy (two receiving
FLOT, and one each receiving irinotecan/ramucirumab,
paclitaxel/ramucirumab, cisplatin/capecitabine/trastuzumab, TS-1/cisplatin, and capecitabine/oxaliplatin/pembrolizumab). The other patient received
palliative radiation therapy and best supportive care.
Survival
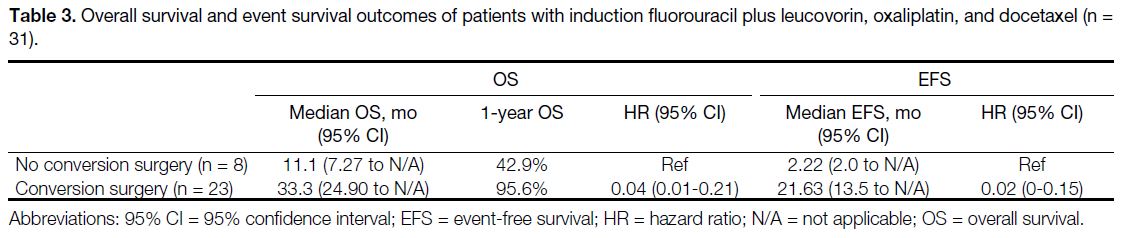
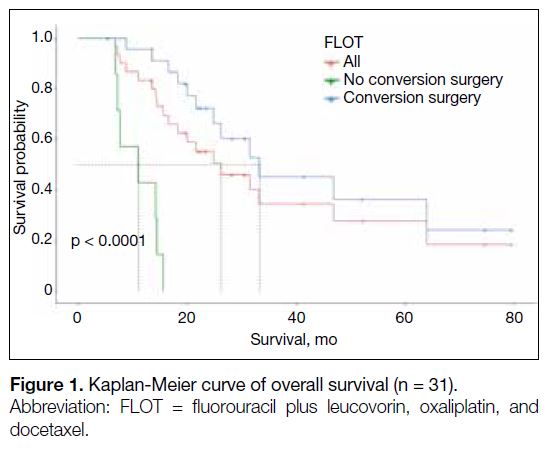
For the entire cohort of 31 patients, the median OS was
26.3 months (95% CI = 18.4 to not applicable) and median
EFS was 13.5 months (95% CI = 7.3-26.7). Patients
undergoing conversion surgery had a longer median OS
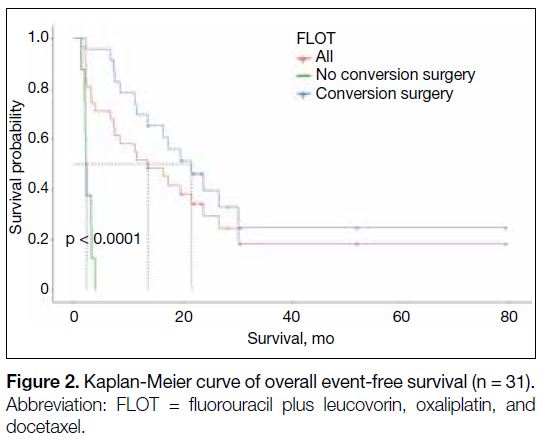
(median OS = 33.3 months vs. 11.1 months) [Table 3 and Figure 1] and EFS (median EFS = 21.63 months vs.
2.22 months) [Table 3 and Figure 2] than those who did not. The 1-year survival proportion was also higher in
patients with conversion surgery versus those without
(95.6% vs. 42.9%) [Table 3 and Figure 1]. Multivariable
analysis showed that low BMI, higher neutrophil-to-lymphocyte
ratio (NLR), low serum albumin level, stage
cN3 disease, and those without conversion surgery were
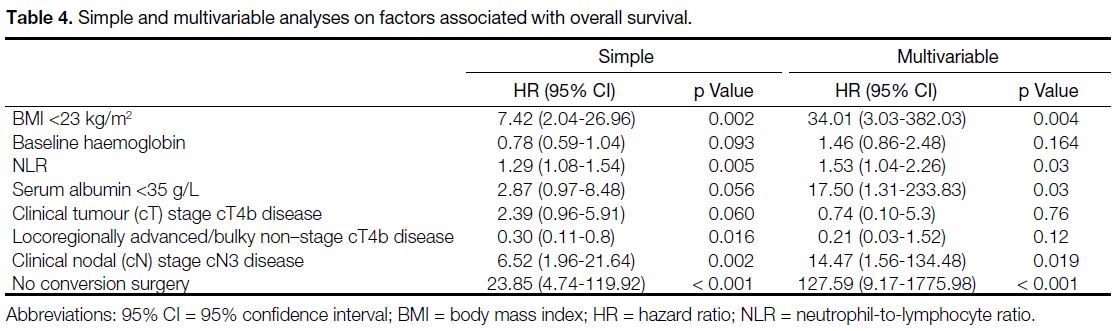
associated with worse OS (Table 4).
Table 3. Overall survival and event survival outcomes of patients with induction fluorouracil plus leucovorin, oxaliplatin, and docetaxel (n = 31).
Figure 1. Kaplan-Meier curve of overall survival (n = 31).
Figure 2. Kaplan-Meier curve of overall event-free survival (n = 31).
Table 4. Simple and multivariable analyses on factors associated with overall survival.
Pattern of Recurrence
Among 20 patients who underwent R0 conversion
surgery, 12 developed recurrences. Peritoneum was
the most common site of first relapse (n = 7), followed
by distant lymph nodes (n = 6) and liver (n = 3). Eight
patients went on to have active palliative systemic
treatment while four had palliative radiotherapy and best
supportive care.
DISCUSSION
This retrospective study demonstrated the high efficacy
of induction FLOT and subsequent conversion surgery
in unresectable advanced GC, offering a chance of cure
with long-term survival benefit with acceptable safety in
a hitherto palliative scenario.
Induction FLOT led to a high response rate with disease
control rate of 87.1% (85.2% CR/PR and 14.8% SD).
No patient died within 10 weeks of last induction
FLOT, and it achieved a high conversion rate of 74.2%.
Among the 23 patients who underwent conversion
surgery, 87.0% (n = 20) achieved R0 resection. These
appeared to be slightly better figures compared with the
AIO-FLOT3 trial (Arbeitsgemeinschaft Internistische
Onkologie-fluorouracil, leucovorin, oxaliplatin, and
docetaxel) done in Germany,[26] which reported a response
rate of 43% to 60% in patients not upfront resectable, and
a 60% conversion rate and 80.6% R0 resection in arm B
(patients with limited metastases). It is understandable
because our study focused on patients with relatively
less advanced stages compared to the AIO-FLOT3 trial
while the AIO-FLOT3 trial recruited stage IV patients
with more distant metastases.[26] Nevertheless, these are
encouraging results as it confirms that a majority of
patients may be able to have conversion surgery done
with upfront unresectable advanced GC, especially for
those with less bulky disease and metastatic burden. The
high R0 resection rate in our study is important because
R0 resection offers the best chance of cure in GC and
is a prognostic factor for survival for patients after
preoperative chemotherapy.[25] [35] [36] Patients with non-R0
tumour resection have a poor prognosis and non-R0
resection should be avoided in advanced GC.[37] [38]
Our study showed that induction FLOT was well
tolerated. All patients except one completed four cycles
of induction FLOT. For those with R0 conversion surgery
and planned postoperative FLOT, 95.0% completed
four cycles. The high postoperative chemotherapy
compliance rate is an important finding in this study as
previous trials have reported low compliance with other
postoperative chemotherapy regimens due to toxicity
after gastrectomy.[14] [39] [40] The REGATTA trial[13] did not
show a survival advantage with gastrectomy followed by
chemotherapy versus chemotherapy alone in advanced
GC, underscoring the importance and better tolerability
of induction chemotherapy. The incidence of grade 3 or
4 toxicities during FLOT was comparable to those in the
perioperative setting in resectable GCs.[28]
The FLOT4 trial[28] established perioperative FLOT as
standard of care in locally advanced resectable GC.
However, the role of perioperative chemotherapy in
unresectable advanced GC such as the ones reviewed
in this study is uncertain. Conversion therapy for GC is
a multimodality strategy garnering attention in recent
years.[15] However, definition of conversion therapy varies
widely, and studies have included different induction
chemotherapy regimens, surgical resection types, and
local and metastatic status. A systemic review and meta-analysis[41]
found that induction chemotherapy followed
by conversion surgery led to survival advantage when
compared with chemotherapy alone for advanced
GC. However, it also concluded that most patients in
the surgery group had only one non-curative clinical
factors versus more in the non-surgery group.[41] Those
in the surgery group were more often chemotherapy
responders and most patients underwent R0 resection.[41]
The CONVO-GC-1 study (International Retrospective
Cohort Study of Conversion Therapy for Stage IV
Gastric Cancer 1)[16] suggested that conversion therapy
is a promising approach even for those with stage IV
disease involving multiple sites and organs, given that
they have a response to chemotherapy and R0 resection
can be achieved. Likewise, the AIO-FLOT3 trial[26]
demonstrated improved survival in patients with limited
metastatic disease.
This study achieved quite a remarkable OS for those
patients undergoing conversion surgery after induction
FLOT. With this multimodality approach, patients
with conversion surgery had tripled the median OS of
33.3 months compared to 11.1 months in those without
conversion surgery. The hazard ratios of 0.04 in OS
and 0.02 in EFS are remarkable. A total of 95.6% of
patients undergoing conversion surgery survived the
1-year mark, more than double that of those who did
not undergo conversion surgery (42.9%). Patients who
did not undergo conversion surgery essentially had
survival time similar to that quoted in the literature for
advanced GC (11-15 months).[5] [6] [7] [8] [9] [10] The longer survival
for those who underwent conversion surgery may not
be fully attributable to the surgery itself. It can also
be a reflection of biological behaviour of the tumour:
patients with chemotherapy-sensitive tumours have
better survival than those with relatively chemotherapyresistant
tumours.
Several studies, mainly conducted in Japan, had reported
a wide range of median OS from 13 to 48 months with
induction chemotherapy followed by conversion surgery approach.[16] [19] [20] [24] [25] [26] Most of these studies were done on
stage IV advanced GC.[16] [20] [24] [25] [26] Factors associated with
longer survival times included negative para-aortic
lymph nodes,[20] R0 resection,[20] [24] [25] positive peritoneal cytology as the only non-curative factor,[25] downstaging,
pathological response,[24] and those with only
retroperitoneal lymph node metastases.[26] Our study
found that low BMI (<23 kg/m2), higher NLR, low serum
albumin level (<35 g/L), stage cN3 disease, and those
without conversion surgery were associated with worse
OS (Table 4). Most of these factors can be identified
at baseline and help with clinical decision making.
Hypoalbuminaemia was similarly found to be predictive
of worse outcomes in a retrospective study looking at
patients receiving CROSS (neoadjuvant carboplatin and
paclitaxel with radiotherapy) or FLOT in advanced GC.[42]
In the same study, low BMI was not associated with
survival.[42] Another study showed that BMI is predictive
of survival outcomes in gastroesophageal cancers.[43] This
inconsistent finding of BMI as a prognostic factor is
likely due to the fact that it may not be a perfect marker
for nutrition or that nutrition plays only a small role
in affecting survival. NLR is a known blood marker
representative of systemic inflammation response, which
influences tumour progression.[44] It has been shown to be
a prognostic marker in predicting tumour progression
for resectable GC.[45] Similarly, our study confirms higher
NLR was associated with worse OS.
One unique aspect of our study is that it focused
mostly on unresectable advanced GC with advanced
local tumour and nodal staging, with almost 40% stage
cT4b disease, and almost half with locoregionally
bulky non–stage cT4b or extensive regional lymph
node metastases. On the other hand, a lot of the studies
quoted previously in conversion therapy investigated
stage IV advanced GC as a whole, consisting of a lot
more patients with distant metastases.[1] [16] [17] [20] [21] [23] [24] [25] [26] The
absence of laparoscopic staging might have resulted
in inclusion of more upstaged patients with potentially
positive peritoneal cytology or small peritoneal implants,
which confer a worse prognosis. Given this limitation,
the median OS achieved in our study was still admirable.
In recent years, efforts to improve outcomes of resectable
GCs investigated the addition of immune checkpoint
inhibitor to perioperative FLOT. Data from both the
KEYNOTE-585[46] and MATTHERHORN trials[47]
demonstrated an increase in pCRs of about 10% with the
addition of pembrolizumab and durvalumab, respectively.
Our pCR rate of 8.7% is consistent with the FLOT-only arm of these two studies.[46] [47] Our study showed that poor
pathological response had a trend of worse survival.
Whether pCR translates to longer survival in advanced
GC remains controversial as compared to other tumour
types (e.g., triple-negative breast cancer or lung cancer)
where pCR is a surrogate for survival. The addition of
pembrolizumab showed favourable outcomes in EFS but
no difference in OS so far.[48] The MATTERHORN trial[47]
is still awaiting long-term results.
Limitations
This study has several limitations. First, it is a single-centre retrospective analysis and there was no control
group for comparison. Second, the sample size is small.
Third, quality-of-life of the patients and cost-effectiveness
were not measured. Taken all together, induction FLOT
followed by conversion surgery should be strongly
considered if patient has limited burden of baseline
incurable factors which responded to chemotherapy and
R0 resection is anticipated, as this offers a favourable
survival benefit for patients with advanced GC.
CONCLUSION
Our study showed that induction FLOT achieved
high conversion rates and R0 resections, providing
a favourable survival benefit with acceptable safety
in unresectable GC. Future research is warranted to
explore whether adding immune checkpoint inhibitors,
especially for patients with high programmed death
ligand 1 expression, or other more effective biomarkerdriven
therapy can further improve the conversion rate,
pCR rate, and survival.
REFERENCES
1. Centre for Health Protection, Department of Health, Hong Kong
SAR Government. Stomach cancer. 2024 Jan 12. Available from:
https://www.chp.gov.hk/en/healthtopics/content/25/55.html. Accessed 22 May 2024.
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I,
Jemal A, et al. Global cancer statistics 2020: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 2021;71:209-49. Crossref
3. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-20. Crossref
4. National Comprehensive Cancer Network. NCCN Guidelines for
Gastric Cancer Version 2.2024. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434. Accessed 29 Jul 2024.
5. Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane
Database Syst Rev. 2017;8:CD004064. Crossref
6. Enzinger PC, Burtness BA, Niedzwiecki D, Ye X, Douglas K, Ilson DH, et al. CALGB 80403 (Alliance)/E1206: a randomized phase II study of three chemotherapy regimens plus cetuximab in
metastatic esophageal and gastroesophageal junction cancers. J
Clin Oncol. 2016;34:2736-42. Crossref
7. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-21. Crossref
8. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L,
Sawaki A, et al. Trastuzumab in combination with chemotherapy
versus chemotherapy alone for treatment of HER2-positive
advanced gastric or gastro-oesophageal junction cancer (ToGA):
a phase 3, open-label, randomised controlled trial. Lancet.
2010;376:687-97. Crossref
9. Park H, Jin RU, Wang-Gillam A, Suresh R, Rigden C, Amin M, et al. FOLFIRINOX for the treatment of advanced gastroesophageal cancers: a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6:1231-40. Crossref
10. Van Cutsem E, Boni C, Tabernero J, Massuti B, Middleton G,
Dane F, et al. Docetaxel plus oxaliplatin with or without fluorouracil
or capecitabine in metastatic or locally recurrent gastric cancer: a
randomized phase II study. Ann Oncol. 2015;26:149-56. Crossref
11. Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C,
et al. First-line nivolumab plus chemotherapy for advanced gastric,
gastroesophageal junction, and esophageal adenocarcinoma: 3-year
follow-up of the phase III CheckMate 649 trial. J Clin Oncol.
2024;42:2012-20. Crossref
12. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al.
Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for HER2-negative advanced gastric cancer
(KEYNOTE-859): a multicentre, randomised, double-blind, phase
3 trial. Lancet Oncol. 2023;24:1181-95. Crossref
13. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M,
Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy
alone for advanced gastric cancer with a single non-curable factor
(REGATTA): a phase 3, randomised controlled trial. Lancet Oncol.
2016;17:309-18. Crossref
14. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de
Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J
Med. 2006;355:11-20. Crossref
15. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is
conversion therapy possible in stage IV gastric cancer: the proposal
of new biological categories of classification. Gastric Cancer.
2016;19:329-38. Crossref
16. Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J,
Li G, et al. International retrospective cohort study of conversion
therapy for stage IV gastric cancer 1 (CONVO-GC-1). Ann
Gastroenterol Surg. 2021;6:227-40. Crossref
17. Kano Y, Ichikawa H, Hanyu T, Muneoka Y, Ishikawa T,
Ishikawa T, et al. Conversion surgery for stage IV gastric cancer:
a multicenter retrospective study. BMC Surg. 2022;22:428. Crossref
18. Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, et al.
Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients
with unresectable locally advanced or metastatic gastric cancer.
Ann Surg Oncol. 2010;17:1024-32. Crossref
19. Inoue K, Nakane Y, Kogire M, Fujitani K, Kimura Y, Imamura H,
et al. Phase II trial of preoperative S-1 plus cisplatin followed by
surgery for initially unresectable locally advanced gastric cancer.
Eur J Surg Oncol. 2012;38:143-9. Crossref
20. Saito M, Kiyozaki H, Takata O, Suzuki K, Rikiyama T. Treatment
of stage IV gastric cancer with induction chemotherapy using S-1
and cisplatin followed by curative resection in selected patients.
World J Surg Oncol. 2014;12:406. Crossref
21. Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K,
Makino I, et al. Efficacy of conversion gastrectomy following
docetaxel, cisplatin, and S-1 therapy in potentially resectable stage
IV gastric cancer. Eur J Surg Oncol. 2015;41:1354-60. Crossref
22. Mitsui Y, Sato Y, Miyamoto H, Fujino Y, Takaoka T, Miyoshi J,
et al. Trastuzumab in combination with docetaxel/cisplatin/S-1
(DCS) for patients with HER2-positive metastatic gastric cancer:
feasibility and preliminary efficacy. Cancer Chemother Pharmacol.
2015;76:375-82. Crossref
23. Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N,
Tanaka Y, et al. The long-term survival of stage IV gastric cancer
patients with conversion therapy. Gastric Cancer. 2018;21:315-23. Crossref
24. Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T,
Fujikawa K, et al. Conversion therapy for inoperable advanced
gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS)
chemotherapy: a multi-institutional retrospective study. Gastric
Cancer. 2017;20:517-26. Crossref
25. Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, et al.
Phase II trial of combined treatment consisting of preoperative S-1
plus cisplatin followed by gastrectomy and postoperative S-1 for
stage IV gastric cancer. Gastric Cancer. 2012;15:61-9. Crossref
26. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM,
Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed
by surgical resection on survival in patients with limited metastatic
gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial.
JAMA Oncol. 2017;3:1237-44. Crossref
27. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM,
Luley KB, et al. Histopathological regression after neoadjuvant
docetaxel, oxaliplatin, fluorouracil, and leucovorin versus
epirubicin, cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): results from the phase 2 part of a multicentre, open-label,
randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-708. Crossref
28. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J,
Kasper S, et al. Perioperative chemotherapy with fluorouracil
plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or
capecitabine plus cisplatin and epirubicin for locally advanced,
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-57. Crossref
29. Washington K. 7th edition of the AJCC cancer staging manual:
stomach. Ann Surg Oncol. 2010;17:3077-9. Crossref
30. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC
Cancer Staging Manual: esophagus and esophagogastric junction.
Ann Surg Oncol. 2010;17:1721-4. Crossref
31. Edge SB, Byrd DR, Compton CC, editors. AJCC Cancer Staging
Manual (7th edition). Available from: https://www.facs.org/media/j30havyf/ajcc_7thed_cancer_staging_manual.pdf. Accessed 19 Feb 2025.
32. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D,
Ford R, et al. New response evaluation criteria in solid
tumours: revised RECIST guideline (version 1.1). Eur J Cancer.
2009;45:228-47. Crossref
33. Ryan R, Gibbons D, Hyland JM, Treanor D, White A,
Mulcahy HE, et al. Pathological response following long-course
neoadjuvant chemoradiotherapy for locally advanced rectal cancer.
Histopathology. 2005;47:141-6. Crossref
34. Department of Health and Human Services, United States
Government. Common Terminology Criteria for Adverse Events
(CTCAE) Version 5.0. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 14 Feb 2025.
35. Biondi A, Persiani R, Cananzi F, Zoccali M, Vigorita V, Tufo A,
et al. R0 resection in the treatment of gastric cancer: room for
improvement. World J Gastroenterol. 2010;16:3358-70. Crossref
36. Brenner B, Shah MA, Karpeh MS, Gonen M, Brennan MF,
Coit DG, et al. A phase II trial of neoadjuvant cisplatin-fluorouracil
followed by postoperative intraperitoneal floxuridine-leucovorin
in patients with locally advanced gastric cancer. Ann Oncol.
2006;17:1404-11. Crossref
37. Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA,
et al. Surgical outcomes in patients with T4 gastric carcinoma. J
Am Coll Surg. 2006;202:223-30. Crossref
38. Schmidt B, Look-Hong N, Maduekwe UN, Chang K, Hong TS,
Kwak EL, et al. Noncurative gastrectomy for gastric adenocarcinoma
should only be performed in highly selected patients. Ann Surg
Oncol. 2013;20:3512-8. Crossref
39. Macdonald JS, Bendetti J, Smalley S, Haller DG, Hundahl S,
Jessup J, et al. Chemoradiation of resected gastric cancer: a 10-year
follow-up of the phase III trial INT0116 (SWOG 9008). J Clin
Oncol. 2009;27(15_supp):4515. Crossref
40. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G,
et al. Perioperative chemotherapy compared with surgery alone
for resectable gastroesophageal adenocarcinoma: an FNCLCC and
FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-21. Crossref
41. Du R, Hu P, Liu Q, Zhang J. Conversion surgery for unresectable
advanced gastric cancer: a systematic review and meta-analysis.
Cancer Invest. 2019;37:16-28. Crossref
42. McNamee N, Nindra U, Shahnam A, Yoon R, Asghari R, Ng W,
et al. Haematological and nutritional prognostic biomarkers
for patients receiving CROSS or FLOT. J Gastrointest Oncol.
2023;14:494-503. Crossref
43. Deans DA, Wigmore SJ, de Beaux AC, Paterson-Brown S,
Garden OJ, Fearon KC. Clinical prognostic scoring system to
aid decision-making in gastro-oesophageal cancer. Br J Surg.
2007;94:1501-8. Crossref
44. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-503. Crossref
45. Arigami T, Uenosono Y, Matsushita D, Yanagita S, Uchikado Y,
Kita Y, et al. Combined fibrinogen concentration and neutrophil-lymphocyte
ratio as a prognostic marker of gastric cancer. Oncol
Lett. 2016;11:1537-44. Crossref
46. Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M,
et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy
in locally advanced gastric or gastro-oesophageal cancer
(KEYNOTE-585): an interim analysis of the multicentre, double-blind,
randomised phase 3 study. Lancet Oncol. 2024;25:212-24. Crossref
47. Janjigian YY, Al-Batran SE, Wainberg ZA, Van Cutsem E,
Molena D, Muro K, et al. LBA73 Pathological complete
response (pCR) to durvalumab plus 5-fluorouracil, leucovorin,
oxaliplatin and docetaxel (FLOT) in resectable gastric and
gastroesophageal junction cancer (GC/GEJC): interim results of
the global, phase III MATTERHORN study [abstract]. Ann Oncol.
2023;34(suppl_2):S1315-6. Crossref
48. Al-Batran SE, Shitara K, Folprecht G, Moehler MH, Goekkurt E,
Ben-Aharon I, et al. Pembrolizumab plus FLOT vs FLOT as
neoadjuvant and adjuvant therapy in locally advanced gastric and
gastroesophageal junction cancer: interim analysis of the phase
3 KEYNOTE-585 study [abstract]. J Clin Oncol. 2024;42(3_suppl):247. Crossref