Pembrolizumab with or without Concurrent Chemotherapy in Metastatic Non–Small-Cell Lung Cancer with High Programmed Death Ligand 1 Expression
ORIGINAL ARTICLE
Hong Kong J Radiol 2024;27:Epub 19 November 2024
Pembrolizumab with or without Concurrent Chemotherapy in Metastatic Non–Small-Cell Lung Cancer with High Programmed Death Ligand 1 Expression
DHZ Lui1, ELM Yu2, MY Lim1
1 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
2 Clinical Research Centre, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr DHZ Lui, Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China. Email: lhz067@ha.org.hk
Submitted: 27 January 2024; Accepted: 26 April 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. DHZL acquired the data. DHZL and ELMY analysed the data. All authors drafted the manuscript
and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong
[Ref No.: KW/EX-23-056 (187-05)]. The requirement for patient consent was waived by the Committee due to the retrospective nature of the
research.
Supplementary Material: The supplementary material was provided by the authors and some information may not have been peer reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by the Hong Kong College of Radiologists. The
Hong Kong College of Radiologists disclaims all liability and responsibility arising from any reliance placed on the content.
Abstract
Introduction
We evaluated overall survival (OS), time on treatment (ToT), and prognostic factors in patients with
metastatic non–small-cell lung cancer (NSCLC) with programmed death ligand 1 (PD-L1) expression of ≥50%
receiving first-line pembrolizumab with or without chemotherapy.
Methods
Patients receiving at least one cycle of pembrolizumab in a single tertiary oncology centre in Hong Kong
from January 2018 to December 2022 were included. OS and ToT were assessed by Kaplan-Meier curves. Prognostic
factors, including clinical-biochemical prognostic indices (PIs) at selected cut-offs, were assessed. The sensitivities
and specificities of neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and advanced lung cancer inflammation index (ALI) were evaluated.
Results
A total of 133 consecutive cases were included, with 112 receiving pembrolizumab alone and 21 receiving
pembrolizumab in combination with chemotherapy. The median OS and ToT were 17.8 months (95% confidence
interval [CI] = 13.4-23.5) and 8.0 months (95% CI = 5.5-12.0), respectively, with no significant difference between
the two groups. ALI outperformed other PIs in 6-month, 1-year, and 2-year OS predictions. For 1-year OS prediction,
ALI had an area under the curve of 0.813 (95% CI = 0.731-0.895), and 85.7% sensitivity and 71.9% specificity for
ALI values ≤17.4. All PIs, low body weight, Eastern Cooperative Oncology Group performance status score of 2, and
presence of liver metastasis were significant independent poor prognostic factors in multivariable regression analyses.
Conclusion
In patients with metastatic NSCLC with high PD-L1 expression receiving first-line pembrolizumab,
OS and ToT were similar independent of chemotherapy use. ALI served as a simple effective index with the highest
hazard ratio in stratifying prognosis.
Key Words: Carcinoma, non–small-cell lung; Lung neoplasms; Prognosis; Survival
中文摘要
使用帕博利珠單抗或帕博利珠單抗聯合化療治療高表達程序性死亡配體1轉移性非小細胞肺癌
呂活證、余洛汶、林美瑩
引言
我們的研究對象是接受第一線帕博利珠單抗或帕博利珠單抗聯合化療的程序性死亡配體1(PD-L1)水平≥50%的轉移性非小細胞肺癌患者,找出他們的整體存活期、治療時間及預後因素。
方法
本研究納入於2018年1月至2022年12月期間曾在香港一所三級腫瘤科中心接受最少一個帕博利珠單抗療程的患者。我們使用Kaplan-Meier曲線評估整體存活期及治療時間,並分析了預後因素(包括於不同選定截斷點的臨床生化學預後指數)。我們找出以下四個指數的敏感度和特異度:嗜中性白血球與淋巴性白血球比例(NLR)、衍生的嗜中性白血球與淋巴性白血球比例(dNLR)、血小板與淋巴性白血球比例(PLR)及晚期肺癌炎症指數(ALI)。
結果
本研究共包括133個連續個案,當中112名患者只接受帕博利珠單抗治療,21名患者則同時接受化療。整體存活期及治療時間中位數分別為17.8個月(95%置信區間 = 13.4-23.5)及8.0個月(95%置信區間 = 5.5-12.0);兩組患者在統計學上沒有顯著差別。ALI在6個月、1年及兩年整體存活期預測的表現較其他三個指數佳。在1年整體存活期預測方面,ALI的曲線下面積為0.813(95%置信區間 = 0.731-0.895),而ALI數值≤17.4的敏感度和特異度則分別為85.7%及71.9%。所有預後指數、體重輕、美國東岸癌症臨床研究合作組織體能狀態為2分及有肝轉移在多變項迴歸分析中是重要的獨立不佳預後因素。
結論
在接受第一線帕博利珠單抗治療的高表達PD-L1轉移性非小細胞肺癌患者中,不論是否有同時接受化療,他們的整體存活期及治療時間均相若。ALI是簡單而有效的指數,在為預後進行分層方面的風險比最高。
INTRODUCTION
Lung cancer is the leading cancer in Hong Kong.[1]
Although epidermal growth factor receptor (EGFR)
mutation and anaplastic lymphoma kinase (ALK)
translocation are especially common in the Asian
population, approximately half of lung cancer patients
suffer from disease without an actionable driver
mutation.[2] [3]
Since the release of the KEYNOTE-024 (KN-024)
study, first-line pembrolizumab monotherapy has
become one of the standards of care for metastatic non–small-cell lung cancer (NSCLC) with high expression of
programmed death ligand 1 (PD-L1), defined as having
a tumour proportion score ≥50%.[4] [5] Subsequently, the
KEYNOTE-189 (KN-189) and KEYNOTE-407 (KN-407) trials found that a pembrolizumab-chemotherapy
combination was effective regardless of the level of
PD-L1 expression.[6] [7] [8] [9] [10]
For those with PD-L1 expression of ≥50%, pembrolizumab alone has a response rate of
approximately 45% (44.8% in the KN-024 trial).[4]
The KN-189 and KN-407 trials found that adding
chemotherapy to pembrolizumab increased the response
rates to >60% in both non-squamous (62.1% in the KN-189 trial)[7] and squamous cell carcinoma (64.4% in the KN-407 trial),[10] although the survival outcomes were
comparable. From the latest 5-year update report,[8] the
median overall survival (OS) and 5-year OS rates were
similar with pembrolizumab monotherapy (26.3 months
and 31.9%),[5] combined with pemetrexed and carboplatin
in non-squamous carcinoma (27.7 months and 29.6%),[8]
and combined with paclitaxel/nab-paclitaxel and
carboplatin (19.9 months and 23.3%).[10] Therefore, the
optimal choice of first-line treatment remains unsettled.
There is an unmet need to identify those who will
be durable responders, as well as those who are
expected to have futile and non-sustained responses
to pembrolizumab treatment. Early combination with
chemotherapy as an intensified treatment may benefit some patients, whereas early symptomatic care may
be more appropriate. Careful selection and prudent
decisions should be made together with patients after
considering all available factors.
Clinical, biochemical, pathological, and radiological
parameters are assessed for their predictive value as
well as their adverse effects. Consensus on the preferred
markers is difficult to arrive at in many tumours due to the
complex interplay between tumour microenvironment
and host immune system.[11] [12] The proinflammatory status
of the patient may promote cancer cell progression and
immune resistance.[13] Clinical-biochemical parameters
have been proposed and some are commonly used to
reflect a dysfunctional host immune state, such as a
neutrophil-to-lymphocyte ratio (NLR) ≥5, a derived
neutrophil-to-lymphocyte ratio (dNLR) ≥3, a platelet-to-lymphocyte ratio (PLR) ≥200, and an advanced lung
cancer inflammation index (ALI) ≤18. Utilisation of
such indices can serve as a simple but effective tool to
assist clinical decision.[14] [15] [16] [17] [18] [19] [20]
We aimed to review the clinical outcomes of patients
with metastatic NSCLC with high PD-L1 expression
receiving first-line pembrolizumab with or without
concurrent chemotherapy and to evaluate the utility of
several commonly used clinical-biochemical prognostic
indices.
METHODS
Study Population
This was a retrospective cohort study conducted in a
single tertiary oncology centre in Hong Kong, which
provides cluster-based oncology services to the most
densely populated districts in the city. The cases of
patients aged ≥18 years with pathologically confirmed
metastatic NSCLC, without sensitising EGFR mutation
or ALK translocation, and with ≥50% PD-L1 expression,
who had received at least one cycle of pembrolizumab
with or without concurrent chemotherapy in a first-line
setting between January 2018 and December
2022 were included. Cases with Eastern Cooperative
Oncology Group (ECOG) performance status score
of ≥3, and baseline blood results, body weight, or
height not recorded within 28 days from the first cycle
of pembrolizumab were excluded. The case list was
generated from the Clinical Data Analysis and Reporting
System of the Hospital Authority.
Assessment
Both electronic and physical records were reviewed. Relevant demographic, clinical, laboratory, treatment,
and outcome data were extracted. The tumour proportion
scores of PD-L1 were analysed by immunohistochemistry
using 22C3 or SP263 antibodies. Response evaluation
was performed as per the physician’s assessment and
investigator’s review based on clinical symptoms,
physical examination, chest radiography, computed
tomography, and carcinoembryonic antigen levels.
The data cut-off date was 30 April 2023. The duration
of follow-up was calculated from the date of initiating
the first cycle of pembrolizumab to the date of death or
the date of the last follow-up if the date of death was
unavailable.
Study Endpoints
The primary endpoint was OS, which was defined
as the time from the beginning of the first cycle of
pembrolizumab to the date of death from any cause
or the last date of follow-up if the date of death was
unavailable. Only one patient was lost to follow-up.
The key secondary endpoints included the following: (1)
time on treatment (ToT), which was defined as the time
of the initiation of the first cycle of pembrolizumab to
the last efficacious date after discontinuation (date of last
pembrolizumab injection plus 21 or 42 days for every
3- or 6-week regimen, respectively) for any reason or
death, whichever was earlier; (2) reasons for treatment
discontinuation; (3) calculation of the four prognostic
indices, namely NLR, dNLR, PLR and ALI, where
baseline blood results within 28 days of pembrolizumab
were used for analysis (online supplementary Table);
and (4) negative prognostic factors for OS, including
concurrent chemotherapy, male sex, age ≥70 years, ever-smoker,
ECOG performance status score of 2, histology
of squamous cell carcinoma, baseline brain metastasis,
baseline pleural metastasis or pleural effusion, baseline
liver metastasis, previous radiotherapy treatment, and
NLR, dNLR, PLR and ALI at their respective cut-offs.
Statistical Analysis
Differences in baseline characteristics between the
pembrolizumab alone (immunotherapy [IO]-alone)
group and concurrent platinum-based chemotherapy
(IO-combination) group were compared with the
Pearson’s Chi squared test or Fisher’s exact test for
categorical variables and an independent t test or the
Mann-Whitney U test for continuous variables. OS and
ToT were calculated using the Kaplan-Meier method
and compared with the log-rank test.
Time-dependent receiver operating characteristic (ROC)
curves were used to evaluate the discriminative ability of
the four prognostic indices on OS. The time-dependent
area under the curve (AUC), sensitivity, and specificity
with 95% confidence interval (95% CI) were calculated
at 6 months, 1 year, and 2 years after therapy initiation.
The cut-off of the prognostic indices was determined
by the highest achievable sensitivity and specificity
based on stepwise testing of incremental improvement.
Sensitivity ≥70% for 6-month and 1-year OS prediction
was required, and specificity ≥60% for 6-month and
1-year OS prediction was attempted. Escalation of
sensitivity and specificity testing at 5% increments were
attempted alternately to achieve the best cut-off. After
achieving a minimum sensitivity of ≥75% and specificity
of ≥65% for 6-month and 1-year OS prediction, further
increments focused on 1-year OS prediction to attain
the highest achievable combination of sensitivity and
specificity when selecting the representative cut-off.
Univariate Cox proportional hazards regression analyses
were performed to identify potential prognostic factors
associated with OS. The association of OS with each
prognostic index was examined using a multivariable
Cox regression model adjusting for chemotherapy
and other factors with p value < 0.1 in the univariate
analyses. The variance inflation factor was used to check
multicollinearity among independent variables in the
regression model, and the Akaike information criterion
was used to compare the performance of different
regression models.
All statistical analyses were carried out using RStudio
version 4.3.1 with packages ‘survival’, ‘timeROC’,
and ‘performance’. A p value < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
After excluding 9 cases with ECOG performance status
score ≥3, and 31 cases without baseline blood results,
body weight, or height recorded within 28 days from
the first cycle of treatment, a total of 133 cases of
pathologically confirmed metastatic NSCLC with ≥50%
PD-L1 expression, and no sensitising EGFR mutation
or ALK translocation, who had received at least one
cycle of pembrolizumab with or without concurrent
chemotherapy in a first-line setting were included.
Molecular tests on EGFR and ALK were routinely
performed for all adenocarcinoma and NSCLC but not squamous cell carcinoma as per institutional practice.
Additional molecular tests were arranged based on the
individual physician’s decisions. All tested patients
were negative for EGFR exon 19 deletion, EGFR exon
21 L858R mutation, and ALK translocation. Fifteen
patients had rare mutations detected, including ROS1
translocation (n = 1), EGFR exon 20 insertion (n = 3), human epidermal growth factor receptor 2 exon
20 insertion (n = 1), rearranged during transfection
rearrangement (n = 1), KRAS (Kirsten rat sarcoma
virus) mutation (G12C, G12D/S, and G12X; n = 3),
MET (mesenchymal epithelial transition receptor) exon
14 skipping (n = 5), and co-mutation of KRAS G12C
mutation and MET exon 14 skipping (n = 1).
Among the 133 consecutive cases, 112 received
pembrolizumab alone and 21 received pembrolizumab
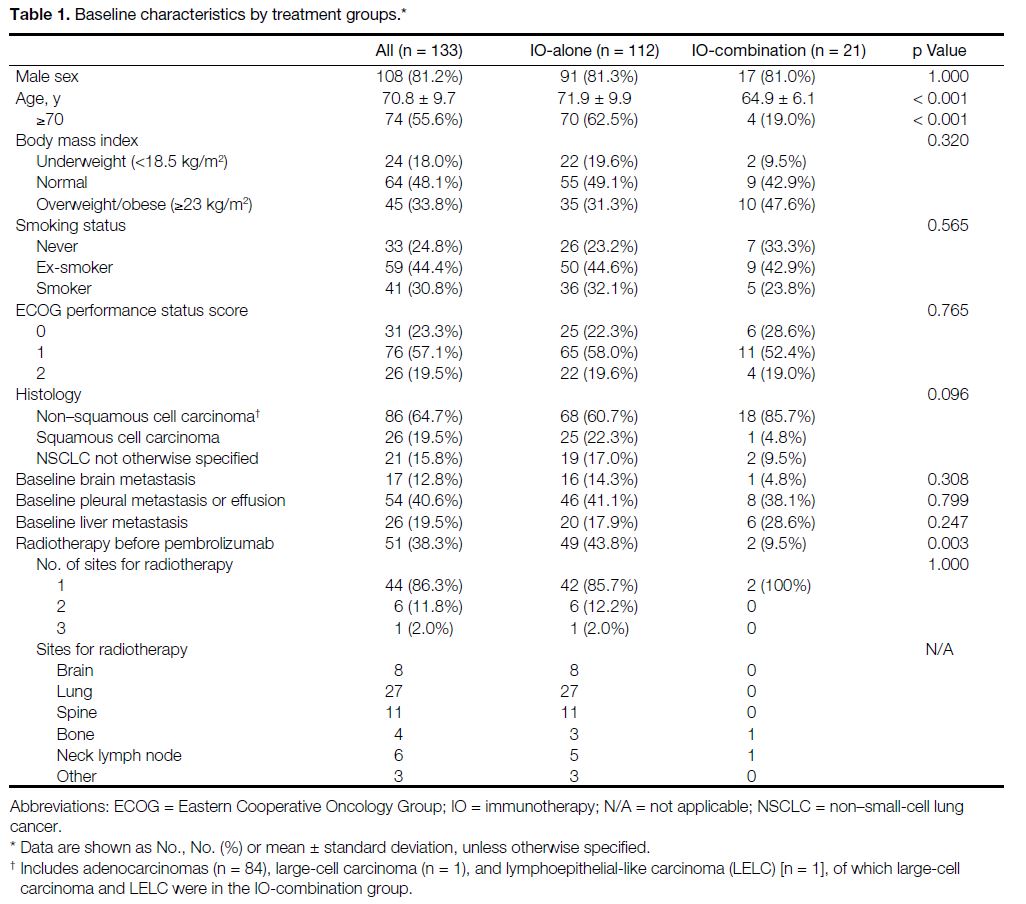
with chemotherapy. Baseline characteristics are
summarised in Table 1. Both groups were balanced
except for age and radiotherapy treatment prior to
pembrolizumab. The combination group was younger
(mean age, 64.9 ± 6.1 years; p < 0.001) and had less
radiotherapy before pembrolizumab (n = 2, 9.5%; p = 0.003).
Table 1. Baseline characteristics by treatment groups
Treatment
Pembrolizumab with or without Chemotherapy
The dosing and frequency of pembrolizumab varied and
depended on financial and funding issues. A total of
78.9% (n = 105) of patients started one of two standard
fixed-dose regimens (94 at 200 mg every 3 weeks and 11
at 400 mg every 6 weeks), while the remaining 21.1% (n
= 28) received a weight-based regimen (three at 80 mg,
24 at 100 mg, and one at 120 mg every 3 weeks). Twenty-eight
patients had a change of dose and frequency during
the treatment course. Most patients changed from every
3 weeks to every 6 weeks regimen for easier logistics
and less frequent hospital visits.
Twenty-one patients received concurrent chemotherapy
with the choice of agents depending on histological
subtypes, where 18 of them received concurrent
pemetrexed and carboplatin (16 had adenocarcinoma,
one had large cell carcinoma, and one had NSCLC
not otherwise specified) with a median number of five
cycles (range, 1-10). The remaining three patients
received concurrent paclitaxel and carboplatin (one had
squamous cell carcinoma, one had lymphoepithelial-like
carcinoma, and one had NSCLC not otherwise specified)
with a median number of five cycles (range, 3-5).
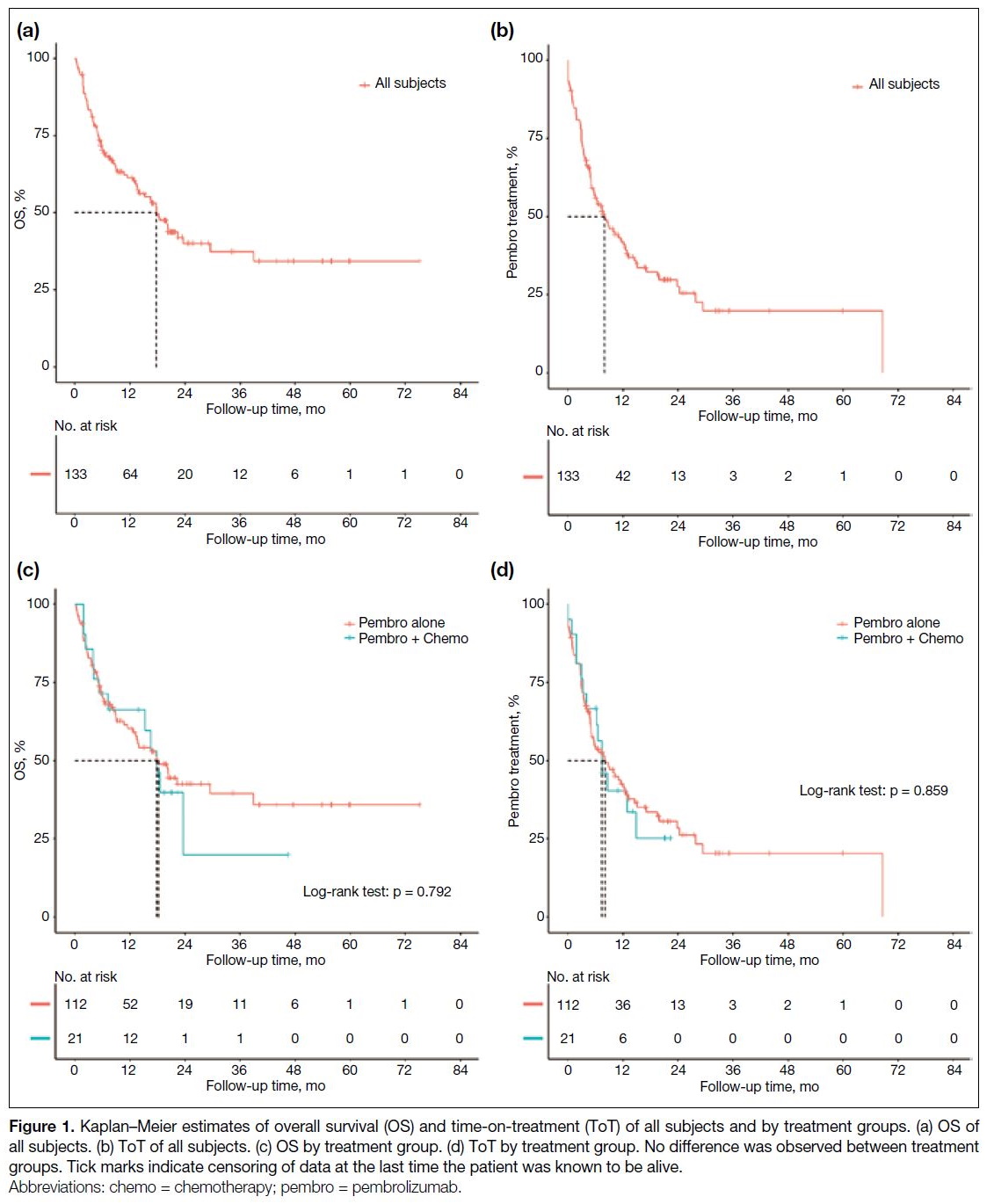
Clinical Outcomes
The median follow-up time was 10.2 months (range,
0.2-75). One patient was lost to follow-up. At the time
of analysis, 69 deaths had occurred, and 88 patients had
discontinued pembrolizumab. Median OS and ToT of
the entire cohort were 17.8 months (95% CI = 13.4-23.5)
[Figure 1a] and 8.0 months (95% CI = 5.5-12.0) [Figure 1b], respectively. No significant difference was observed
between IO-alone and IO-combination groups (median
OS = 18.2 months [95% CI = 12.6-38.9] vs. 17.8 months
[95% CI = 5.2 to not applicable], p = 0.792; median ToT
= 8.1 months [95% CI = 5.0-12.4] vs. 7.4 months [95% CI = 3.2-14.8], p = 0.863) [Figure 1c and 1d].
Figure 1. Kaplan–Meier estimates of overall survival (OS) and time-on-treatment (ToT) of all subjects and by treatment groups. (a) OS of all subjects. (b) ToT of all subjects. (c) OS by treatment group. (d) ToT by treatment group. No difference was observed between treatment groups. Tick marks indicate censoring of data at the last time the patient was known to be alive.
Reasons for discontinuation of pembrolizumab included death (n = 30); disease progression (n = 45); disease
remission (n = 2); severe immune-related adverse
events (n = 9) including four with pneumonitis, two
with hepatitis, two with skin reactions, and one with
flareup of stable autoimmune disease; deteriorated
performance status (n = 2); second malignancies (n = 2; one hepatocellular carcinoma and one liposarcoma);
and one case switching to capmatinib after receipt of
additional molecular results. Seven patients stopped
pembrolizumab due to financial reasons.
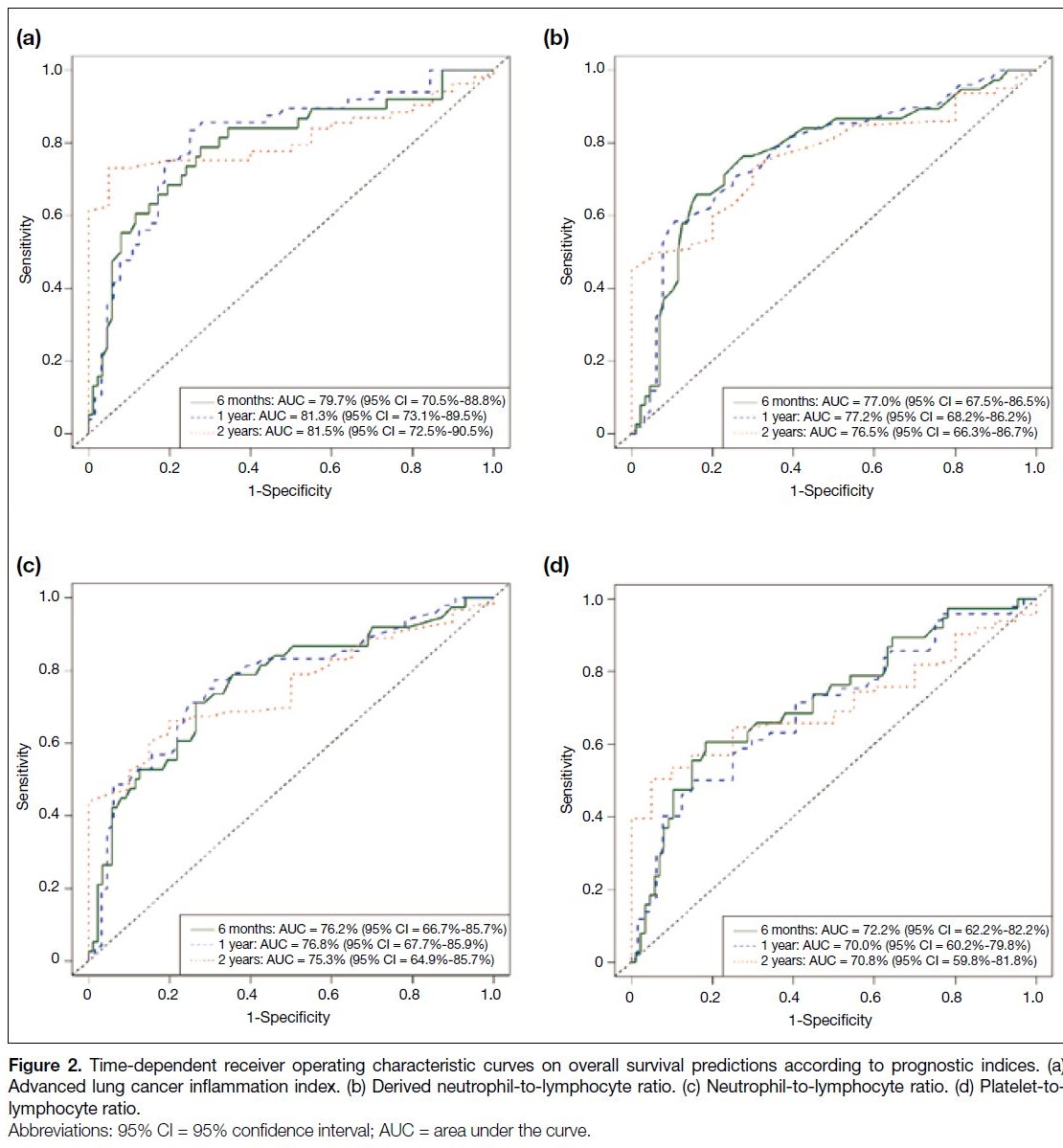
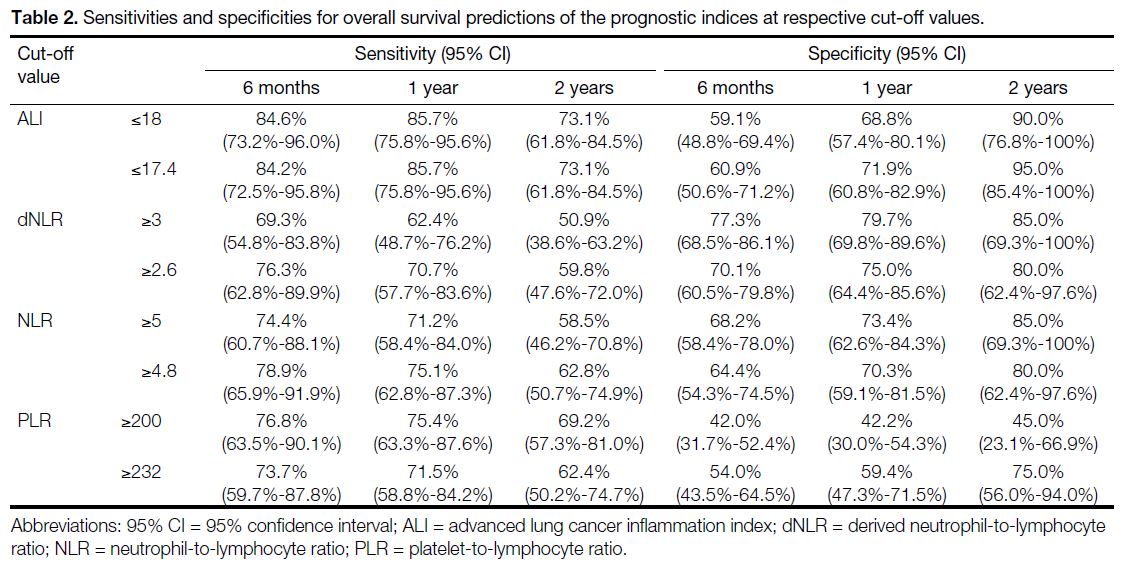
Performance of Clinical-Biochemical Prognostic Indices
The time-dependent ROC curves, sensitivities, and
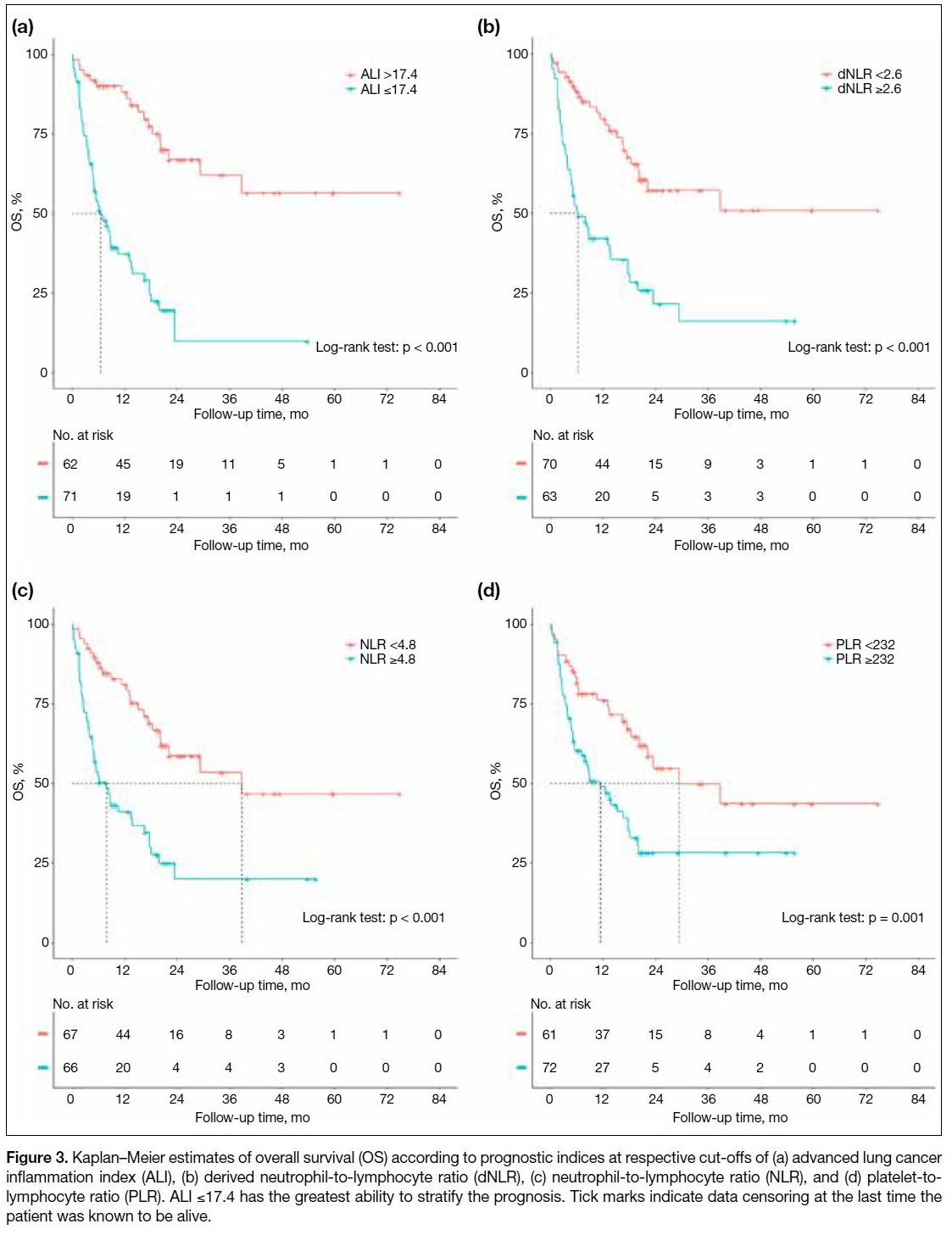
specificities of various prognostic indices are shown in Figure 2. The ALI outperformed the other prognostic
indices, with the highest AUCs of 0.797, 0.813 and
0.815 for 6-month, 1-year and 2-year OS predictions, respectively. An ALI ≤17.4 had sensitivities and
specificities in 6-month (84.2% and 60.9%), 1-year
(85.7% and 71.9%), and 2-year (73.1% and 95.0%) OS predictions. Cut-off values of ALI (≤17.4), dNLR (≥2.6),
NLR (≥4.8), and PLR (≥232) were comparable to those
identified in the literature (Table 2).[13] [14] [15] [16] [17] [18] [19] [20]
Figure 2. Time-dependent receiver operating characteristic curves on overall survival (OS) predictions according to prognostic indices. (a)
Advanced lung cancer inflammation index. (b) Derived neutrophil-to-lymphocyte ratio. (c) Neutrophil-to-lymphocyte ratio. (d) Platelet-tolymphocyte
ratio.
Table 2. Sensitivities and specificities for overall survival predictions of the prognostic indices at respective cut-off values.
Other Prognostic Factors Compared with the Advanced Lung Cancer Inflammation Index
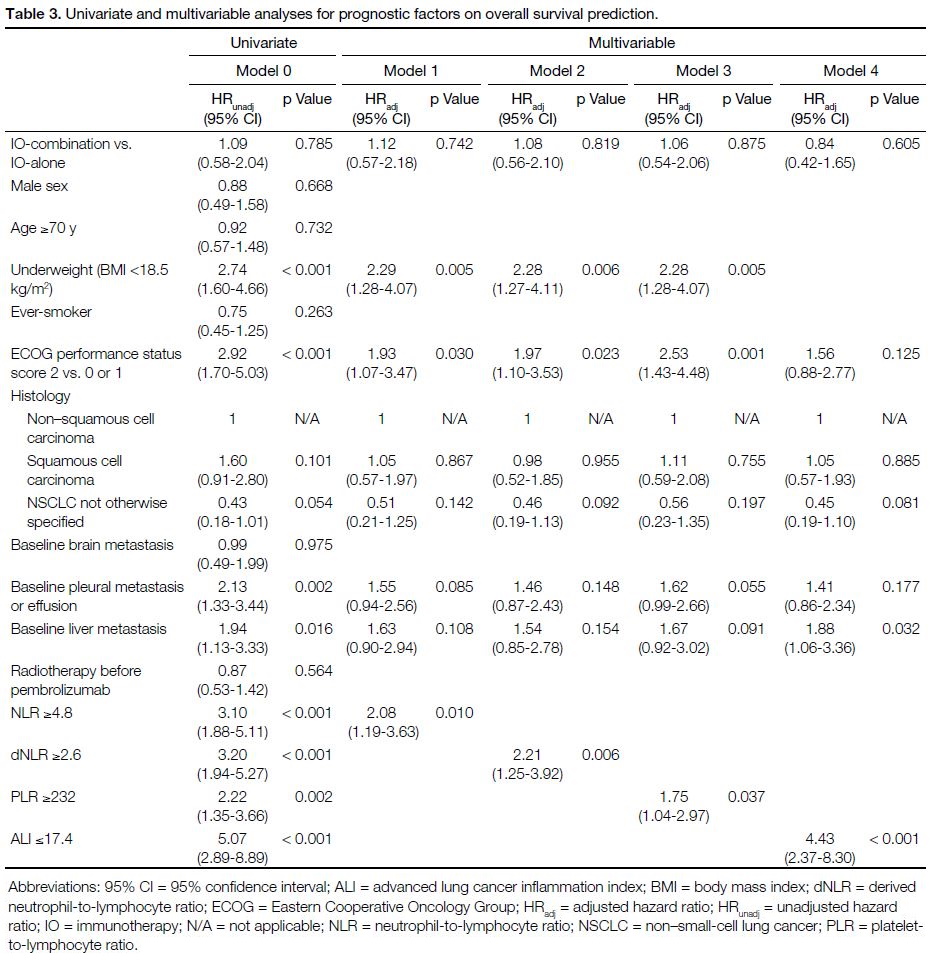
The variables included in the univariate and multivariable regression analyses are shown in Table 3. Low body
weight (body mass index [BMI] <18.5 kg/m2), ECOG
performance status score of 2, baseline liver metastasis,
and all prognostic indices at their respective cut-offs
were identified as independent poor prognostic factors
in multivariable regression analyses. Adjusted hazard
ratios were 4.43 for ALI ≤17.4 (p < 0.001), 2.21 for
dNLR ≥2.6 (p = 0.006), 2.08 for NLR ≥4.8 (p = 0.010), and 1.75 for PLR ≥232 (p = 0.037). OS curves showed
clear separation with each prognostic index in which
ALI performed the best (Figure 3).
Table 3. Univariate and multivariable analyses for prognostic factors on overall survival prediction.
Figure 3. Kaplan–Meier estimates of overall survival (OS) according to prognostic indices at respective cut-offs of (a) advanced lung cancer inflammation index (ALI), (b) derived neutrophil-to-lymphocyte ratio (dNLR), (c) neutrophil-to-lymphocyte ratio (NLR), and (d) platelet-to-lymphocyte ratio (PLR). ALI ≤17.4 has the greatest ability to stratify the prognosis. Tick marks indicate data censoring at the last time the patient was known to be alive.
DISCUSSION
The choice of the optimal first-line treatment for
metastatic NSCLC with PD-L1 expression of ≥50%
remains challenging. The level of PD-L1 expression
appears to be indicative and was suggested as part of
routine testing in non–oncogene-addicted metastatic
NSCLC by international guidelines such as those
from the European Society for Medical Oncology[21]
and the National Comprehensive Cancer Network.[22]
However, high PD-L1 expression alone was known
to have limited predictive value of IO benefit, given a
significant proportion of patients remained resistant to
pembrolizumab treatment. Emerging biomarkers such as
tumour mutation burden and tumour microenvironment
appeared promising, but the best clinical criteria and
biomarkers for patient selection and outcome prediction
remain unsettled.[23] To our knowledge, this is the largest
review in our locality of pembrolizumab with or without
chemotherapy for metastatic NSCLC with high PD-L1
expression along with the comparison of four prognostic
indices for OS prediction.
OS is a less ambiguous endpoint in the real-world
setting where the timing imaging is variable, and response
evaluation is not standardised. Yet, the exact treatment effectiveness could still be difficult to interpret due
to susceptibility to post-baseline events. Conversely,
treatment-based endpoints such as ToT are considered
an approximation of progression-free survival. It can
serve as a pragmatic endpoint for the evaluation of
treatment benefit, especially in the context of IO or
targeted therapy, by taking into consideration some other
clinically relevant reasons for treatment discontinuation
(such as worsened performance status, patient preference,
and immune-related toxicities) or treatment continuation
(such as for pseudo-progression during the early
treatment phase, treatment beyond progression in the
context of continued clinical benefits, and limited further
treatment choices).[24] However, its interpretation should
be cautioned as reasons including financial constraints
and second malignancy were also included. Response rate
analysis was not performed due to the lack of protocol
and inconsistency in response evaluation and follow-up
imaging.
We observed that a higher proportion of individuals in
the IO-combination group were younger, required less
radiotherapy, had more non-squamous histology, fewer
underweight, and fewer brain metastases compared
to the IO-alone group. However, only the former two
factors were statistically significant (Table 1). These
characteristics signified better prognosis at baseline and
were compatible with the usual clinical selection criteria
for combination treatment in which these patients were deemed fit enough to endure intensive combination
treatment. However, despite treatment escalation, both
the IO-alone and IO-combination groups showed similar
median OS. Slightly longer ToT was noted in the IO-combination
group, although the difference was not
statistically significant. This illustrates the unmet need
for optimal selection to receive IO-alone versus IO-combination
treatment in metastatic NSCLC with high
PD-L1 expression. There is no reason to expose patients
to chemotherapy early if there is no potential benefit.
There is no trial result directly comparing pembrolizumab alone and combination treatment in first-line setting
for patients with metastatic NSCLC with high PD-L1
expression.
Unsurprisingly, our real-world OS and ToT were shorter
than those in the trial setting, which is consistent with
prior real-world study.[25] Inclusion of patients with ECOG
performance status score of ≥2 for treatment in real-world
practice (19.6% in the IO-alone group and 19.0% in the
IO-combination group) [Table 1] compared with none
in the three landmark trials (KN-024,[5] KN-189,[8] and KN-407[10]) is one of the explanations. Poor performance
status is a well-established factor associated with poor
survival and increased toxicities in the chemotherapy
era, which also holds true for IO, as demonstrated in
prior retrospective studies.[26] [27] [28]
Prior studies have shown that various prognostic indices
were effective at their respective cut-off values.[13] [14] [15] [16] [17] [18] [19] [20] Still,
there were no local data to compare and demonstrate their
applicability in our locality. In our study, we performed
ROC analyses to determine the optimal cut-off values
based on local survival data. Given the prognostic nature
of these indices, preference was given to sensitivity
rather than specificity. We emphasised their ability
to identify patients with poor prognoses and at higher
risk of deterioration, in which clinicians could intensify
treatment at an early stage for a suitable population or
maintain closer monitoring of the patient’s condition
during treatment.
All four prognostic indices, namely ALI, dNLR, NLR,
and PLR in descending order of their prognostic abilities,
effectively stratified patients’ prognoses at respective
cut-off values. The identified cut-off values in our study
were comparable to the results from the literature.[14] [15] [16] [17] [18] [19] [20]
This served as a validation of these prognostic indices
and acted as the indicative reference for local practice.
ALI ≤17.4 had the largest AUCs, highest sensitivities,
and specificities (Table 2). This is likely explained by the
composition of ALI, which included the host’s general
well-being as reflected by nutritional status (BMI and
serum albumin level) and an inflammation-based marker
alone (dNLR) that might have a synergistic relationship
with IO.[19] Furthermore, our multivariable regression
analyses also demonstrated that BMI <18.5 kg/m2,
ECOG performance status score of 2, and baseline liver
metastases were statistically significant poor prognostic
factors (Table 3). This is consistent with prior studies
showing liver metastasis being an indicator of poor
prognosis and poor response to IO, which may favour
the use of combination treatment.[7] [29]
Given its prognostic value and easily collected variables
in daily practice, ALI is an appealing marker to be
incorporated into the treatment algorithm. However, the
interpretation and application of ALI in a clinical context
are important. Poor prognosis, despite pembrolizumab
treatment, may result from poor baseline condition,
limited treatment response, or both. For patients with
high disease load and turnover rate, intensifying the
treatment with an early chemotherapy combination allows a rapid cytotoxic effect on fast-growing cells and
provides a synergistic immune-modulating effect, hence
improving the efficacy of pembrolizumab.[30] [31] However,
patients who are deemed to be poor candidates for any form of anti-cancer
treatment, early introduction of best supportive
care instead of proceeding with pembrolizumab and/or chemotherapy would be more appropriate. ALI can
predict a patient’s prognosis with pembrolizumab, but
the exercise of clinical judgement is needed to formulate
a patient’s management plan.
Limitations
Several limitations were identified in our study. First,
our study was retrospective and therefore prone to
biases. Other commonly utilised prognostic indices,
including inflammatory markers (such as the C-reactive
protein/erythrocyte sedimentation rate) and lactate
dehydrogenase (LDH)–related markers (such as LDH
level and the lung immune prognostic index), were
unavailable in our study because they are not routine
baseline blood tests. It is believed that the C-reactive
protein/erythrocyte sedimentation rate may indicate host
inflammation status, and LDH level may indicate tumour
metabolism.[32]
Secondly, our sample size was relatively small, and
patients receiving IO-combination represent only a
minority within our cohort. This was explained by the
different approval timelines of pembrolizumab alone
in 2016[33] and combination treatment in 2017 (for non–squamous cell carcinoma)[34] and 2018 (for squamous
cell carcinoma)[35] by the United States Food and Drug
Administration and the time needed for the local working
group in Hong Kong to adopt their regular uses in public
health sectors. It was difficult to objectively compare
disease burden and tumour turnover among these two
groups, although the proportion of patients with baseline
brain, pleural, and liver metastases appeared comparable
among the two cohorts.
Thirdly, there is limited generalisability of our result to
NSCLC with driver mutations other than common ones
of EGFR and ALK due to small patient numbers. Variable
responses to IO had been observed in different studies,
especially concerning those harbouring rarer driver
mutations.[36] [37] However, the exact interplay between
biology and immunology is yet to be determined.
Lastly, other emerging biomarkers related to tumour
genome (e.g., tumour mutation burden, microsatellite
instability, and DNA repair gene) and immune microenvironment were not available for current
analysis. However, these newer biomarkers are much
more technology-demanding and research-based, and are
not readily available in day-to-day practice for clinicians
to quickly identify patients who are expected to have
poor prognoses and limited benefit from pembrolizumab.
Despite these limitations, our results validated and
suggested that patients with ALI ≤17.4 and the presence
of baseline liver metastasis are at higher risk of shorter
OS and earlier disease progression. Evaluation of ALI
and detection of liver metastasis may guide clinicians’
decisions in formulating management plans with patients.
CONCLUSION
Judicious evaluation of the reasons for low ALI
attributed to poor baseline general condition where
little gain from any anti-cancer treatment is expected, or
aggressive disease with high tumour burden and rapid
tumour turnover where treatment intensification with
combination treatment may be considered as essential.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority. Leading cancer
sites in Hong Kong in 2020. 2022. Available from: https://www3.ha.org.hk/cancereg/pdf/top10/rank_2020.pdf. Accessed 10 Oct 2023.
2. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT,
et al. A prospective, molecular epidemiology study of EGFR
mutations in Asian patients with advanced non–small-cell lung
cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol.
2014;9:154-62. Crossref
3. Pan Y, Zhang Y, Li Y, Hu H, Wang L, Li H, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive
study of common and fusion pattern-specific clinicopathologic,
histologic and cytologic features. Lung Cancer. 2014;84:121-6. Crossref
4. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823-
33. Crossref
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus
chemotherapy for metastatic non–small-cell lung cancer with PD-1
tumor proportion score ≥50. J Clin Oncol. 2021;39:2339-49. Crossref
6. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078-92. Crossref
7. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip
E, Dómine M, et al. Updated analysis from KEYNOTE-189:
pembrolizumab or placebo plus pemetrexed and platinum for
previously untreated metastatic nonsquamous non–small-cell lung
cancer. J Clin Oncol. 2020;38:1505-17. Crossref
8. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E,
Dómine M, et al. Pembrolizumab plus pemetrexed and platinum
in nonsquamous non–small-cell lung cancer: 5-year outcomes from
the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41:1992-8. Crossref
9. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040-51. Crossref
10. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J,
et al. Pembrolizumab plus chemotherapy in squamous non–small-cell
lung cancer: 5-year update of the phase III KEYNOTE-407
study. J Clin Oncol. 2023;41;1999-2006. Crossref
11. Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G,
Zitvogel L. Targeting the tumor microenvironment: removing
obstruction to anticancer immune responses and immunotherapy.
Ann Oncol. 2016;27:1482-92. Crossref
12. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N.
Involvement of PD-L1 on tumor cells in the escape from host
immune system and tumor immunotherapy by PD-L1 blockade.
Proc Natl Acad Sci U S A. 2002;99:12293-7. Crossref
13. Banna GL, Friedlaender A, Tagliamento M, Mollica V, Cortellini A,
Rebuzzi SE, et al. Biological rationale for peripheral blood cell–derived inflammatory indices and related prognostic scores in
patients with advanced non–small-cell lung cancer. Curr Oncol
Rep. 2022;24:1851-62. Crossref
14. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of
baseline neutrophil-to-lymphocyte ratio in patients receiving
immune checkpoint inhibitors: a review and meta-analysis. Onco
Targets Ther. 2018;11:955-65. Crossref
15. Russo A, Russano M, Franchina T, Migliorino MR, Aprile G,
Mansueto G, et al. Neutrophil-to-lymphocyte ratio (NLR),
platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab
in pretreated non–small cell lung cancer (NSCLC): a large
retrospective multicenter study. Adv Ther. 2020;37:1145-55. Crossref
16. Platini H, Ferdinand E, Kohar K, Prayogo SA, Amirah S, Komariah M,
et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte
ratio as prognostic markers for advanced non–small-cell lung
cancer treated with immunotherapy: a systematic review and meta-analysis.
Medicina (Kaunas). 2022;58:1069. Crossref
17. Zhang Q, Gong X, Sun L, Miao L, Zhou Y. The predictive value
of pretreatment lactate dehydrogenase and derived neutrophil-to-lymphocyte
ratio in advanced non–small cell lung cancer patients
treated with PD-1/PD-L1 inhibitors: a meta-analysis. Front Oncol.
2022;12:791496. Crossref
18. Zhou K, Cao J, Lin H, Liang L, Shen Z, Wang L, et al. Prognostic
role of the platelet to lymphocyte ratio (PLR) in the clinical
outcomes of patients with advanced lung cancer receiving
immunotherapy: a systematic review and meta-analysis. Front
Oncol. 2022;12:962173. Crossref
19. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index
(ALI) at diagnosis is a prognostic marker in patients with metastatic
non–small cell lung cancer (NSCLC): a retrospective review. BMC
Cancer. 2013;13:158. Crossref
20. Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J,
Samitas K, et al. Association of the advanced lung cancer
inflammation index (ALI) with immune checkpoint inhibitor
efficacy in patients with advanced non–small-cell lung cancer.
ESMO Open. 2021;6:100254. Crossref
21. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A,
et al. Non–oncogene-addicted metastatic non–small-cell lung
cancer: ESMO Clinical Practice Guideline for diagnosis, treatment
and follow-up. Ann Oncol. 2023;34:358-76. Crossref
22. National Comprehensive Cancer Network. NCCN Guidelines. Non–Small Cell Lung Cancer. 2023. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed
10 Oct 2023.
23. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. Crossref
24. Blumenthal GM, Gong Y, Kehl K, Mishra-Kalyani P, Goldberg KB, Khozin S, et al. Analysis of time-to-treatment discontinuation of
targeted therapy, immunotherapy, and chemotherapy in clinical
trials of patients with non–small-cell lung cancer. Ann Oncol.
2019;30:830-8. Crossref
25. Waterhouse D, Lam J, Betts KA, Yin L, Gao S, Yuan Y, et al. Real-world
outcomes of immunotherapy-based regimens in first-line
advanced non–small cell lung cancer. Lung Cancer. 2021;156:41-9. Crossref
26. Sehgal K, Gill RR, Widick P, Bindal P, McDonald DC, Shea M,
et al. Association of performance status with survival in patients with
advanced non–small cell lung cancer treated with pembrolizumab
monotherapy. JAMA Netw Open. 2021;4:e2037120. Crossref
27. Middleton G, Brock K, Savage J, Mant R, Summers Y, Connibear J, et al. Pembrolizumab in patients with non–small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet
Respir Med. 2020;8:895-904. Crossref
28. Billingham LJ, Cullen MH. The benefits of chemotherapy in patient
subgroups with unresectable non–small-cell lung cancer. Ann
Oncol. 2001;12:1671-5. Crossref
29. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with
anti–PD-1 monoclonal antibody in patients with melanoma and
NSCLC. Cancer Immunol Res. 2017;5:417-24. Crossref
30. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G.
Immunostimulation with chemotherapy in the era of immune
checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725-41. Crossref
31. Liu W, Zhang L, Xiu Z, Guo J, Wang L, Zhou Y, et al. Combination
of immune checkpoint inhibitors with chemotherapy in lung cancer. Onco Targets Ther. 2020;13:7229-41. Crossref
32. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic
index with immune checkpoint inhibitor outcomes in patients with
advanced non–small cell lung cancer. JAMA Oncol. 2018;4:351-7. Crossref
33. United States Food and Drug Administration. Pembrolizumab
(Keytruda) checkpoint inhibitor. 2016 Oct 24. Available from:
https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-checkpoint-inhibitor. Accessed 25 Oct 2024.
34. United States Food and Drug Administration. Pembrolizumab
(Keytruda) 5-10-2017. 2017 May 10. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-5-10-2017. Accessed 25 Oct 2024.
35. United States Food and Drug Administration. FDA approves
pembrolizumab in combination with chemotherapy for first-line
treatment of metastatic squamous NSCLC. 2018 Oct 30. Available
from: https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc. Accessed 25 Oct 2024.
36. Addeo A, Passaro A, Malapelle U, Banna GL, Subbiah V, Friedlaender A. Immunotherapy in non–small cell lung cancer
harbouring driver mutations. Cancer Treat Rev. 2021;96:102179. Crossref
37. Vokes NI, Pan K, Le X. Efficacy of immunotherapy in oncogene-driven non–small-cell lung cancer. Ther Adv Med Oncol. 2023;15:17588359231161409. Crossref