Tenosynovial Giant Cell Tumour of the Temporomandibular Joint with Initial Suspicion of Nodal Metastases: A Case Report
CASE REPORT
Hong Kong J Radiol 2024;27:Epub 18 November 2024
Tenosynovial Giant Cell Tumour of the Temporomandibular Joint with Initial Suspicion of Nodal Metastases: A Case Report
LY Lam1, WL Wong1, KWS Ko1, ZCS Tsang2, KM Chu1
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Pathology, Kwong Wah Hospital, Hong Kong SAR, China
Correspondence: Dr LY Lam, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: lly858@ha.org.hk
Submitted: 19 September 2023; Accepted: 10 January 2024. This version may differ from the final version when published in an issue.
Contributors: LYL and WLW designed the study. All authors acquired and analysed the data. LYL and WLW drafted the manuscript. All
authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Kowloon Central Cluster and Kowloon East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: KC/KE-28-0056/ER-1). The requirement for patient consent was waived by the Committee due to loss to
follow-up of the patient and the use of de-identified information of the patient in the study.
CASE PRESENTATION
A 26-year-old female with good past health presented
with 1-month history of progressive swelling at the left
preauricular region. Physical examination revealed a
4-cm mass over the left preauricular region, not fixed to
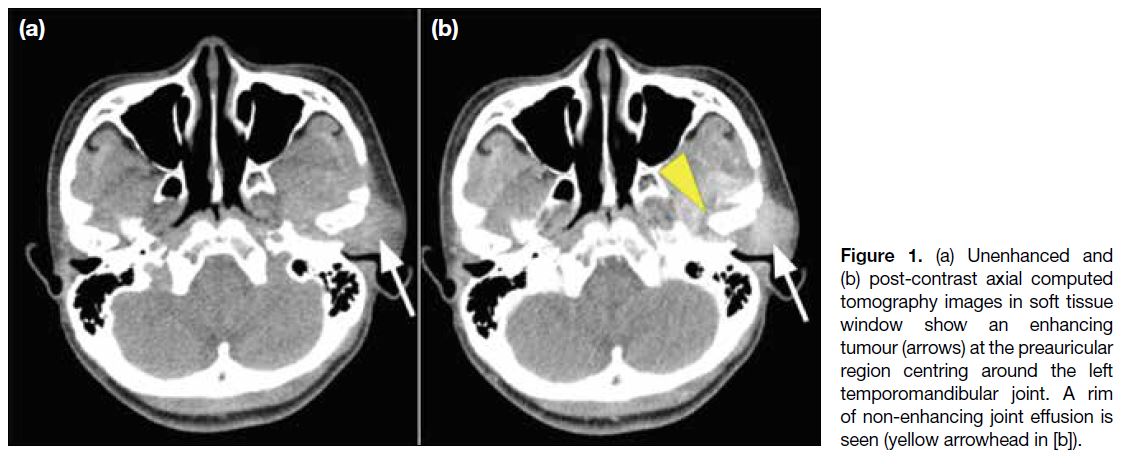
the underlying structures. Computed tomography (CT)
demonstrated a 5-cm hyperdense and heterogeneously
enhancing soft tissue mass at the left preauricular region
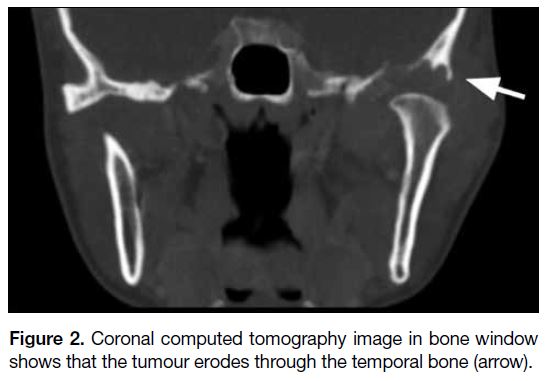
(Figure 1). There was bony erosion of the left mandibular
head and articular tubercle of the temporal bone with
extension to the base of the middle cranial fossa (Figure 2). No parenchymal invasion of the left temporal lobe
was observed. Differential diagnoses included an
aggressive parotid tumour with bony extension or an
aggressive bone condition arising from the temporal
bone such as osteomyelitis or metastasis or primary bone
tumours such as aneurysmal bone cyst.
Figure 1. (a) Unenhanced and
(b) post-contrast axial computed tomography images in soft tissue window show an enhancing tumour (arrows) at the preauricular region centring around the left temporomandibular joint. A rim of non-enhancing joint effusion is seen (arrowhead).
Figure 2. Coronal computed tomography image in bone window shows that the tumour erodes through the temporal bone (arrow).
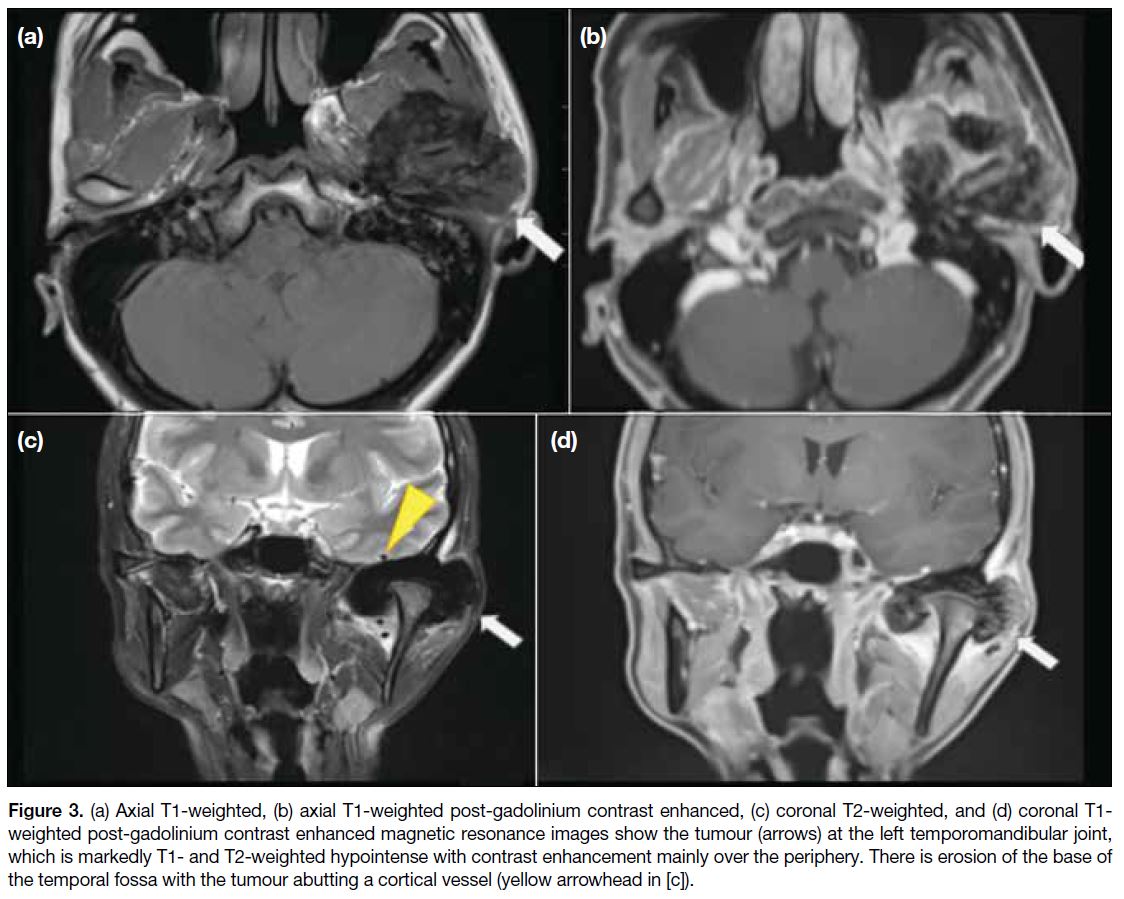
Magnetic resonance imaging (MRI) showed an
expansile and lobulated mass with T1- and T2-weighted
hypointense signals and moderate contrast enhancement centring at the left temporomandibular joint (TMJ)
[Figure 3] and indenting the left middle cranial fossa dura
superiorly. There was involvement of the left mandibular
condyle and superficial lobe of the left parotid gland.
Several nodules showing similar signal characteristics to
the index lesion were seen within the left parotid gland.
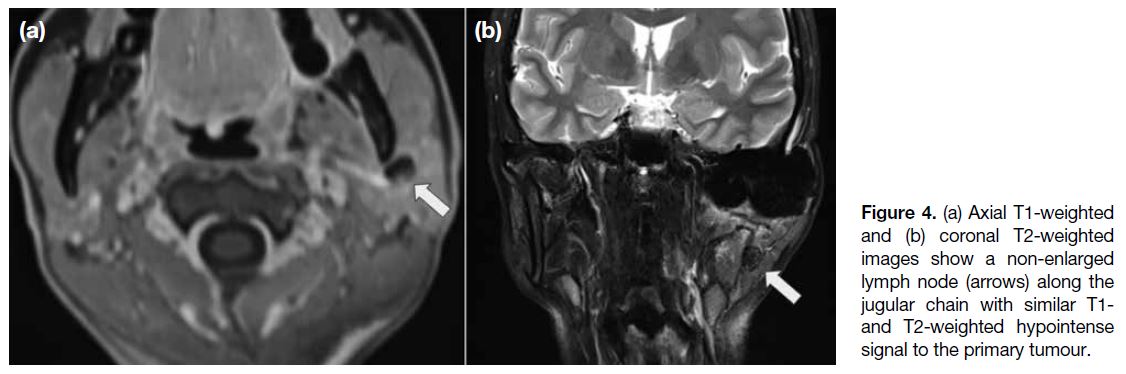
A few lymph nodes were seen along the left jugular
chain (Figure 4), with similar signal characteristics.
These were suspicious of nodal metastases. Biopsy of
the mass revealed mononuclear cells with osteoclast-type
giant cells, presence of hemosiderin pigment and
expression of clusterin and D2-40 via immunostaining.
Features were compatible with diffuse-type tenosynovial
giant cell tumour (TGCT).
Figure 3. (a) Axial T1-weighted, (b) axial T1-weighted post-gadolinium contrast enhanced, (c) coronal T2-weighted, and (d) coronal T1-weighted post-gadolinium contrast enhanced magnetic resonance images show the tumour (arrows) at the left temporomandibular joint, which is markedly T1- and T2-weighted hypointense with contrast enhancement mainly over the periphery. There is erosion of the base of the temporal fossa with the tumour abutting a cortical vessel (yellow arrowhead in [c]).
Figure 4. (a) Axial T1-weighted
and (b) coronal T2-weighted images show a non-enlarged lymph node (arrows) along the jugular chain with similar T1-and T2-weighted hypointense signal to the primary tumour.
Wide local excision and cervical lymph node dissection
were performed. Final pathology of the specimen
confirmed local involvement including the mandibular
fossa of the temporal bone (Figure 5), mandibular
condyle, zygoma, left parotid gland (Figure 6), and dura
at the left temporal fossa. No malignant cells were seen. Cervical lymph nodes from the jugular chain showed
hemosiderin laden macrophages without giant cells or
malignant cells.
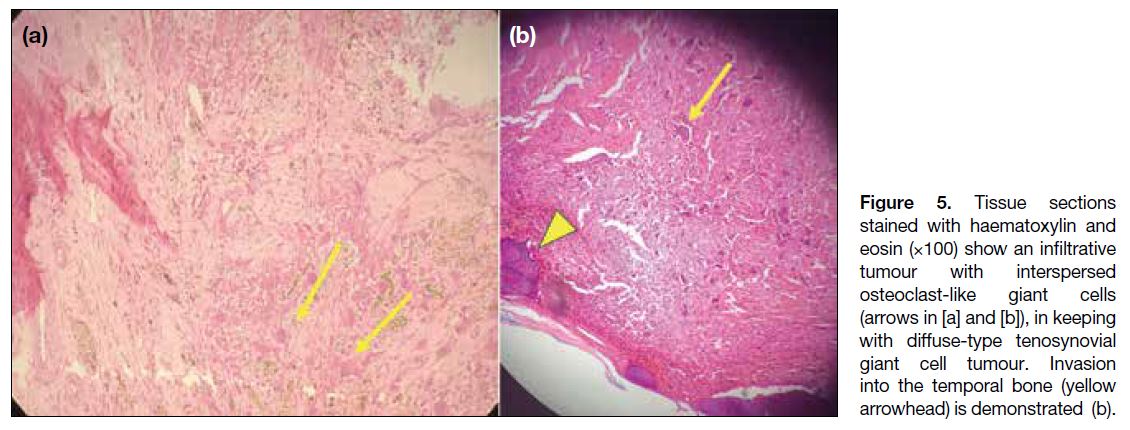
Figure 5. Tissue sections
stained with haematoxylin and eosin (×100) show an infiltrative tumour with interspersed osteoclast-like giant cells (arrows in [a] and [b]), in keeping with diffuse-type tenosynovial giant cell tumour. Invasion into the temporal bone (arrowhead) is demonstrated (b).
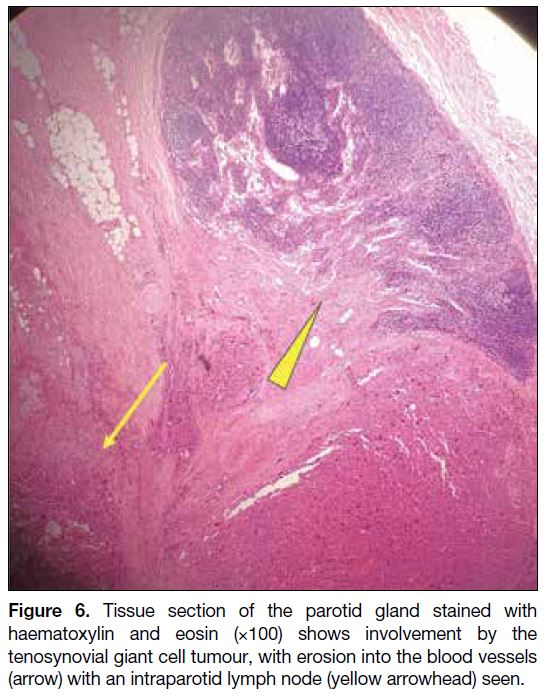
Figure 6. Tissue section of the parotid gland stained with haematoxylin and eosin (×100) shows involvement by the tenosynovial giant cell tumour, with erosion into the blood vessels
(arrow) with an intraparotid lymph node (arrowhead) seen.
In view of the positive surgical margins, the patient
underwent postoperative adjuvant radiotherapy of 40 Gy
in 20 fractions. There have been no signs of recurrence
on clinical and imaging follow-up by MRI 6 months
post-operation.
DISCUSSION
TGCT encompasses a group of neoplasms arising
from the synovial membranes of the joints, bursae, or
tendons. Previously diffuse TGCTs were well known
as pigmented villonodular synovitis. This term is no
longer recommended by the World Health Organization3
as the suffix -itis may wrongly imply an inflammatory
condition.
Most TGCTs are benign. Malignant TGCT is exceedingly
rare, with only 50 reported cases worldwide, typically
affecting the lower extremities and most affected adults
aged 50 to 60 years.[1]
The aetiology of TGCTs is largely unknown. Some
studies attribute the process to repeated intra-articular
haemorrhage following trauma, whereas some proposed
that it is due to disturbed lipid metabolism.[2] The patient
in our case had good past health with no history of facial
trauma.
TGCTs are classified according to their location
(intra-articular vs. extra-articular) and growth pattern
(localised vs. diffuse). There is no sex predilection for
intra-articular disease, while there is a slight female
predilection for extra-articular disease.[3] The clinical
presentation and affected joints differ between localised
and diffuse subtypes. Localised TGCTs involve part of
the synovium and are commonly found in the fingers.3
Patients typically present in their 3rd to 5th decade with a
female predilection. The second most common location
is the hand and wrist regions. They often present as
painless and slow growing masses.[4] Diffuse TGCTs are
less common with an annual incidence of 4 per million
population. These are monoarticular diseases affecting
large joints, typically the knees and hips, accounting
for 66% to 80% and 4% to 16% of cases, respectively.[1]
Other joints such as the ankles, shoulders, and elbows
are affected in descending order of frequency.[1] [3] Patients
are commonly in their early middle age. Patients with
diffuse TGCTs present with painful joint swelling with
reduced range of movement. Haemarthrosis is commonly encountered. This is a locally aggressive lesion that tends
to have local recurrence following complete excision,
with a reported recurrence rate of 35%.[1] [3]
TGCT of the TMJ is rare and is typically the diffuse
form. The first case of TGCT involving the TMJ was
reported in 1973.[5] To date, around 100 cases have been reported worldwide.[6] The most common presentation
is of a preauricular mass. Some cases may present with
limited range of movement at the TMJ. Because of
their close proximity to the parotid gland, they are often
mistaken for parotid lesions.[7]
Microscopically, TGCTs have a variable appearance,
consisting of variable proportions of multinucleated giant cells, foamy macrophages, haemosiderin, and stromal collagenisation.
Radiographs of diffuse intra-articular TGCTs commonly
demonstrate joint effusion, soft tissue swelling and
extrinsic pressure erosion of bone, usually involving
both sides of a joint. Radiographs may appear normal in
21% of cases.[3]
On CT and MRI, other than erosion and joint effusion,
extensive synovial thickening with villous or nodular
projections extending into the joint can be seen. These
synovial thickening and masses are hyperdense on CT
due to their high iron content. On MRI, they appear
hypointense on T1- and T2-weighted sequences due to
hemosiderin deposition.[1] [7] These were well illustrated
in our case. Blooming artefacts are pathognomonic
for TGCTs. These tumours show predominantly high
signals in short-tau inversion recovery sequence.[7]
Contrast enhancement is observed due to their significant
vascularity.[3]
Sonographic findings are less specific for the diffuse
intra-articular subtype of TGCTs and include complex
heterogeneous echogenic masses along the thickened
hypoechoic synovium and joint effusion.[3] Doppler
imaging commonly reveals increased blood flow.
TGCTs show hypermetabolism on fluorine-18
fluorodeoxyglucose positron emission tomography with
an average standard uptake value of 5.9.[3] This may be a
potential pitfall for a misdiagnosis of malignant tumour.
In our case, the CT and MRI demonstrated significant involvement of both sides of the TMJ by the TGCTs,
with more erosive effect on the mandibular fossa side
than that of the mandibular condyle. This leads to the
false impression that the disease is bony in origin.
The characteristic magnetic resonance features of
hemosiderin deposition were helpful in making the
correct diagnosis.
Both malignant TGCTs and TGCTs of the TMJ are very
rare conditions; only a few cases of malignant TGCTs
of the TMJs have been reported in the literature.[6] By
imaging alone, it is difficult to differentiate benign
from malignant TGCTs. Features such as rapid growth,
aggressive bone destruction, and evidence of distant
metastases should raise suspicion of a malignant nature.[5]
Common metastatic sites of malignant TGCTs are the
regional lymph nodes, lungs, and spine.
In our case, multiple lymph nodes with similar
hypointense signals to the index lesion were found, raising
an initial suspicion of nodal metastases. Nonetheless the
pathology of the index tumour and lymph nodes showed
no malignant cells.
A few cases of metastatic spread of benign TGCTs
have been described.[8] The presentation of metastases
occurred in longer time spans after the initial diagnosis,
from years to decades. In our case, the discovery of
suspicious lymph nodes was within 1 month of the
initial symptoms. The histological findings of the lymph
nodes did not meet the criteria for metastases from
TGCTs described in the case report by Malik et al.[9]
These rendered the possibility of true nodal metastases
less likely. The histological findings of haemosiderin
laden macrophages and the imaging findings of the
lymph nodes can be due to lymphatic drainage of
degraded blood products from the haemorrhagic tumour,
which has been reported in chronic cases of tumoural
haemorrhage.[10] Our findings suggest that imaging alone
is not definitive for the diagnosis of nodal metastases
and pathology remains the gold standard. Nevertheless
clinicians should be vigilant for metastasis in patients
with known diffuse TGCTs who present with palpable
lymph nodes since benign TGCTs can metastasise and
recur after definitive treatment.[8]
The mainstay of treatment for TGCTs is surgical excision
with wide margin. Aggressive cases with a high chance
of recurrence will benefit from postoperative radiation
therapy.[11] In view of the positive surgical margins in the surgical specimen, postoperative radiation and long-term
follow-up will be beneficial to our patient to reduce the
chance of recurrence and metastases.
CONCLUSION
We report a case of TGCT of the TMJ. Such tumours
should be considered in the differential diagnoses of
preauricular aggressive swellings. Clinicians should
consider nodal metastasis in patients with diffuse TGCTs
who present with palpable lymph nodes.
REFERENCES
1. De Saint Aubain Somerhausen N, van de Rijn M; The WHO Classification of Tumours Editorial Board. Tenosynovial giant cell tumour. WHO Classification of Tumours Soft Tissue and Bone
Tumours, 5th ed. Lyon: IARC Press; 2020: 133-6.
2. Bemporad JA, Chaloupka JC, Putman CM, Roth TC, Tarro J, Mitra S, et al. Pigmented villonodular synovitis of the
temporomandibular joint: diagnostic imaging and endovascular
therapeutic embolization of a rare head and neck tumor. AJNR
Am J Neuroradiol. 1999;20:159-62.
3. Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis:
radiologic-pathologic correlation. Radiographics. 2008;28:1493-518. Crossref
4. Peh WC, Shek TW, Ip WY. Growing wrist mass. Ann Rheum Dis. 2001;60:550-3. Crossref
5. Yoon HJ, Cho YA, Lee JI, Hong SP, Hong SD. Malignant
pigmented villonodular synovitis of the temporomandibular joint
with lung metastasis: a case report and review of the literature. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e30-6. Crossref
6. Verspoor FG, Mastboom MJ, Weijs WL, Koetsveld AC, Schreuder HW, Flucke U. Treatments of tenosynovial giant cell
tumours of the temperomandibular joint: a report of three cases and
a review of literature. Int J Oral Maxillofac Surg. 2018;47:1288-94. Crossref
7. Le WJ, Li MH, Yu Q, Shi HM. Pigmented villonodular synovitis of
the temporomandibular joint: CT imaging findings. Clin Imaging.
2014;38:6-10. Crossref
8. Chen EL, de Castro CM 4th, Hendzel KD, Iwaz S, Kim MA, Valeshabad AK, et al. Histologically benign metastasizing
tenosynovial giant cell tumor mimicking metastatic malignancy: a
case report and review of literature. Radiol Case Rep. 2019;14:934-40. Crossref
9. Malik K, Raja A, Shirley S. Isolated regional nodal metastasis in giant cell tumor of the bone: case report and review of literature. South Asian J Cancer. 2020;9:58. Crossref
10. Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 2006;34:425-54. Crossref
11. Baniel C, Yoo CH, Jiang A, von Eyben R, Mohler DG, Ganjoo K,
et al. Long-term outcomes of diffuse or recurrent tenosynovial
giant cell tumor treated with postoperative external beam radiation
therapy. Pract Radiat Oncol. 2023;13:e301-7. Crossref