Adrenal Venous Sampling for Recognising the Many Variants of Adrenal Veins: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024;27:Epub 18 December 2024
Adrenal Venous Sampling for Recognising the Many Variants of Adrenal Veins: A Pictorial Essay
CCY Chan1, KKF Fung2, JC Ng1, BKH Lee1, KK Cheng1, DHY Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr CCY Chan, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR,
China. Email: chancherrycy@gmail.com
Submitted: 30 July 2023; Accepted: 14 November 2023. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. CCYC acquired the data. All authors analysed the data. CCYC drafted the manuscript and critically
revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As editors of the journal, CCYC and KKFF were not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: IRB-2024-625). The requirement for patient consent was waived by the Board due to the retrospective nature of the study.
INTRODUCTION
Primary aldosteronism is a leading cause of secondary
hypertension. Its management lies in the determination
of subtype, i.e., whether aldosterone overproduction is
unilateral or bilateral. In unilateral autonomous secretion,
the commonest cause is an aldosterone-producing
adenoma; other causes include unilateral nodular or
diffuse adrenal hyperplasia, or, rarely, carcinoma.[1]
Unilateral adrenalectomy offers a definitive cure in such
cases with successful normalisation of blood pressure
achieved in 50% to 80% of patients and improvement
in the rest.[1] In bilateral aldosterone hypersecretion,
pharmacotherapy (i.e., mineralocorticoid receptor
antagonists such as spironolactone) and eplerenone are
the preferred treatment.
Adrenal venous sampling (AVS) is the gold standard
to distinguish between unilateral and bilateral adrenal
disease in primary aldosteronism. It is a challenging
procedure with success rates ranging from 30% to 96%.[2]
Cannulation of the right adrenal vein is the most difficult
part; knowing its anatomy is key. We aimed to review the normal anatomy and variations of the right and left
adrenal veins, and the techniques to achieve success
based on experience in our centre.
The adrenals are highly vascularised. Arterial supply to
the adrenal glands is via three adrenal arteries, namely,
the superior adrenal artery arising from the inferior
phrenic artery, the middle adrenal artery arising from the
abdominal aorta, and the inferior adrenal artery arising
from the ipsilateral renal artery.[3] They branch into
several smaller arteries penetrating the adrenal capsule
and supply the cortex and medulla. The adrenal veins
eventually drain into the inferior vena cava (IVC), and
their anatomy is described in the following section.
ANATOMY AND VENOGRAPHIC APPEARANCE OF THE ADRENAL VEINS
AVS requires accurate identification of the adrenal veins
prior to catheterisation. The right adrenal vein typically
drains directly into the IVC, while the left adrenal vein
usually joins the inferior phrenic vein, which drains into the left renal vein.[4] There are typically three tributary
veins to the central adrenal vein of each adrenal gland:
the superior, lateral, and inferior tributary veins on the
right; and the superior-median, superior-lateral, and
lateral tributary veins on the left.[5] Anatomical variations
of the drainage patterns have been reported in 13% of
patients.[6]
Right Adrenal Vein
Failure to identify or cannulate the right adrenal vein is the most common cause of an unsuccessful procedure.[7]
The right adrenal vein is a short, straight vein which
originates from the medial gland and drains directly into
the IVC, usually entering posterolaterally at the T11-T12
or T12-L1 level.[8] It is shorter than the left adrenal vein,
measuring 1 to 2 cm in length, and 3 to 5 mm in calibre.[8]
Different venographic patterns of the right adrenal vein
have been described by Daunt[7]:
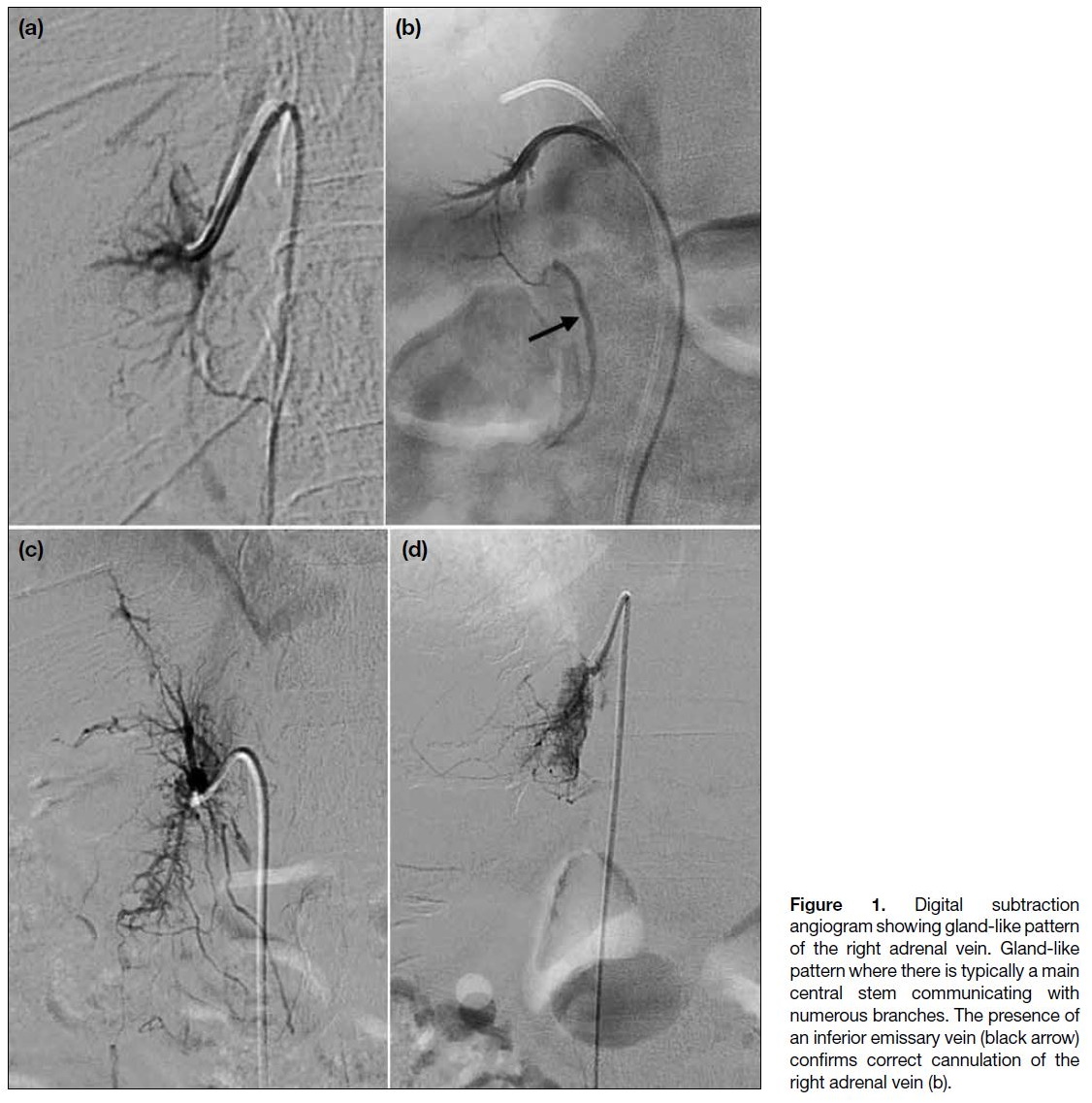
- A characteristic gland-like pattern formed by a main central vein and numerous branches (Figure 1);
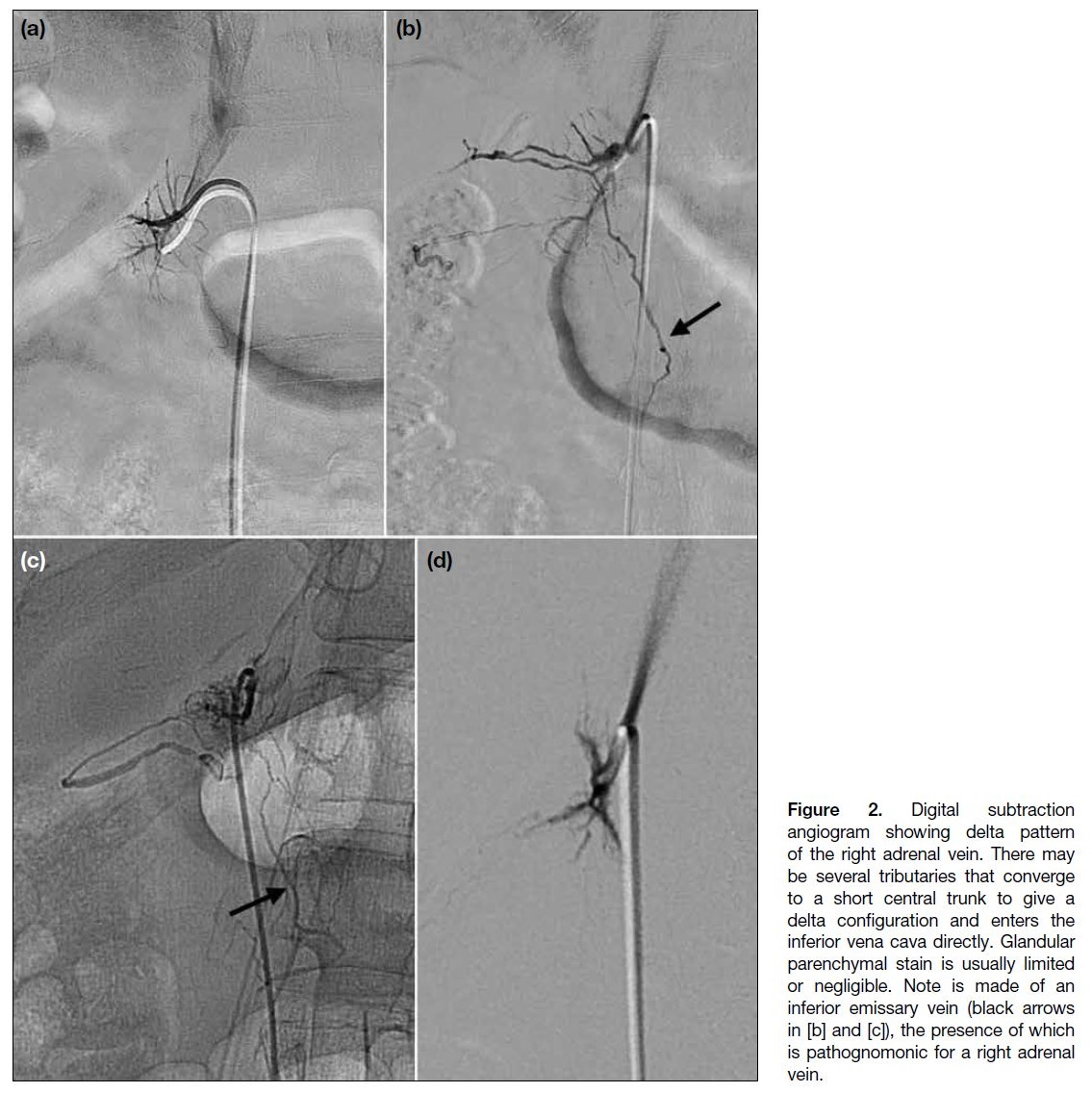
- A delta pattern with little filling of internal structures (Figure 2);
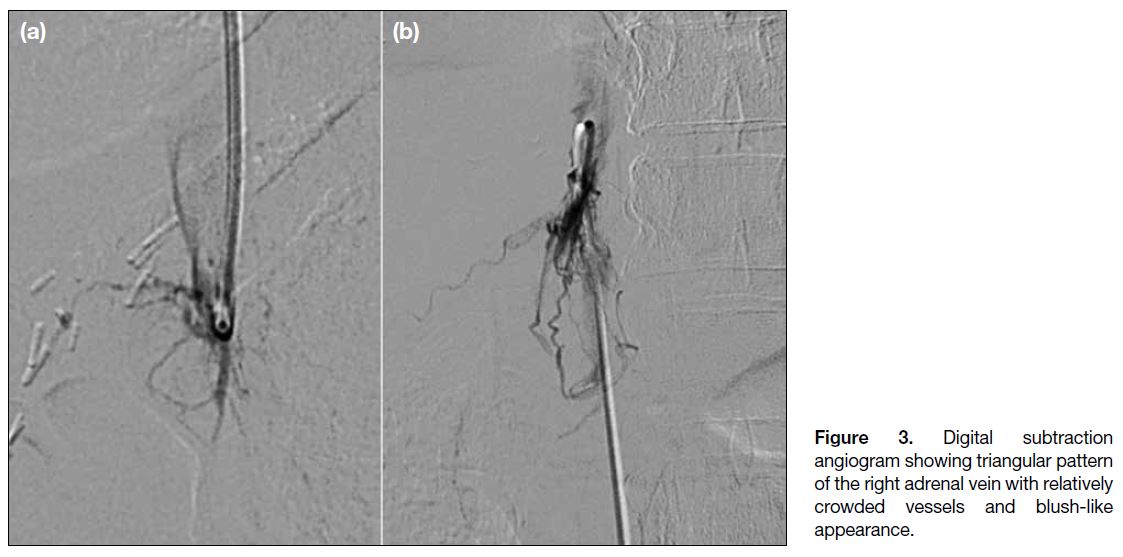
- A triangular pattern with crowded vessels and a blush-like appearance (Figure 3);
- No discernible adrenal veins, but the main vessel position is characteristic and fits with the position estimated at computed tomography; and
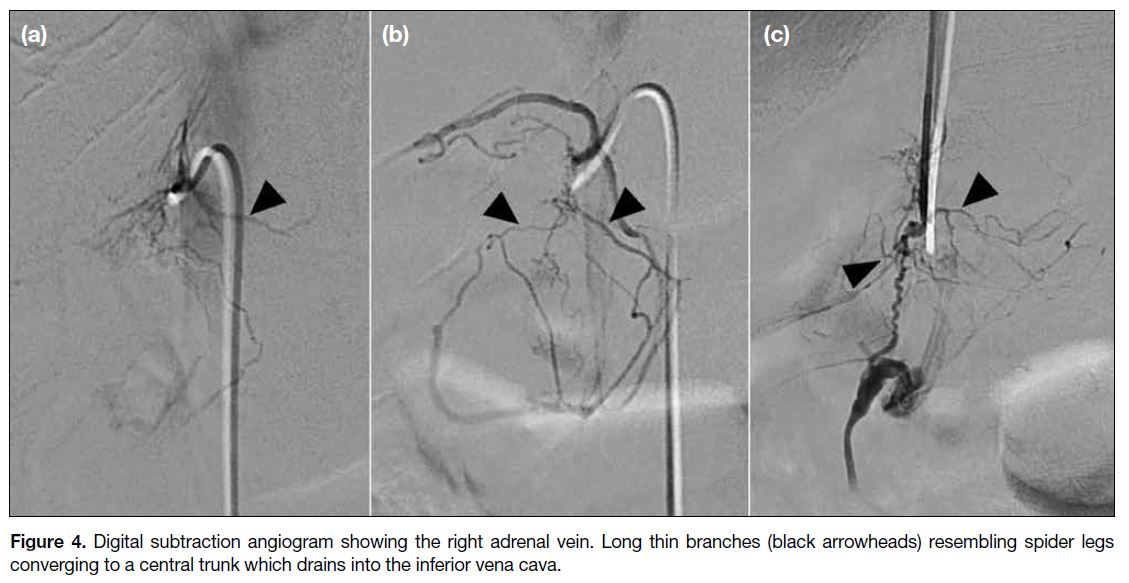
- Spider-like (Figure 4) or stellate (Figure 5) branches communicating with a central vein.
Figure 1. Digital subtraction
angiogram showing gland-like pattern
of the right adrenal vein. Gland-like
pattern where there is typically a main
central stem communicating with
numerous branches. The presence of
an inferior emissary vein (black arrow)
confirms correct cannulation of the
right adrenal vein (b).
Figure 2. Digital subtraction
angiogram showing delta pattern
of the right adrenal vein. There may
be several tributaries that converge
to a short central trunk to give a
delta configuration and enters the
inferior vena cava directly. Glandular
parenchymal stain is usually limited
or negligible. Note is made of an
inferior emissary vein (black arrows
in [b] and [c]), the presence of which
is pathognomonic for a right adrenal
vein.
Figure 3. Digital subtraction
angiogram showing triangular pattern
of the right adrenal vein with relatively
crowded vessels and blush-like
appearance.
Figure 4. Digital subtraction angiogram showing the right adrenal vein. Long thin branches (black arrowheads) resembling spider legs
converging to a central trunk which drains into the inferior vena cava.
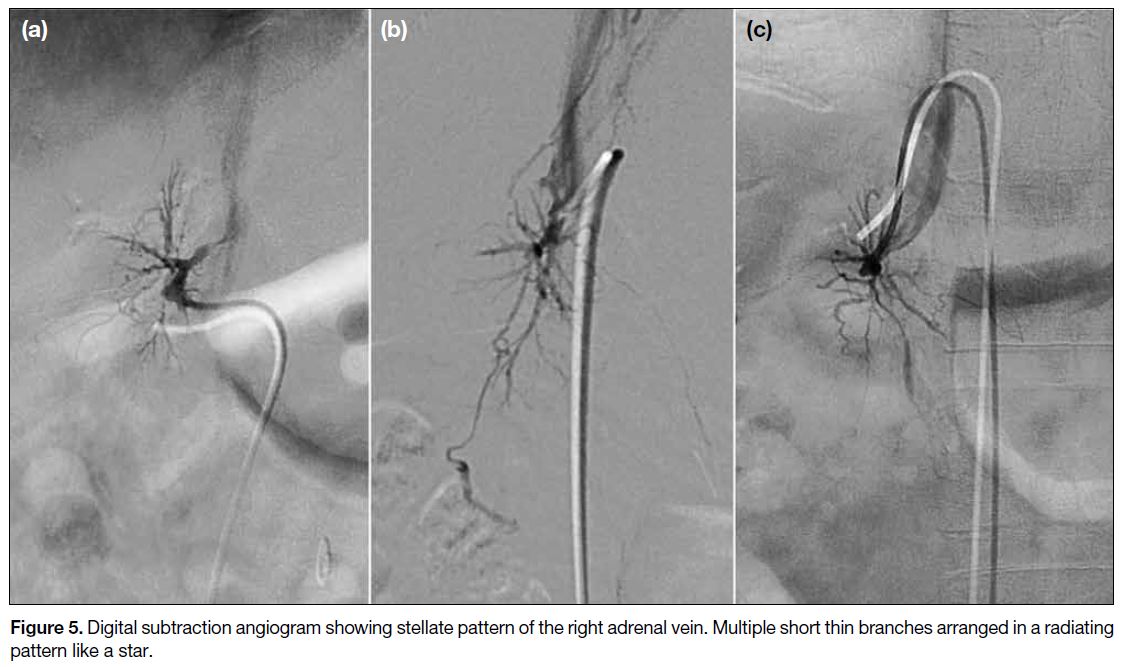
Figure 5. Digital subtraction angiogram showing stellate pattern of the right adrenal vein. Multiple short thin branches arranged in a radiating pattern like a star.
Communication with renal capsular, inferior phrenic,
and intercostal veins is common. Cases of connection
with superficial hepatic veins have also been reported.[7]
Small accessory hepatic veins are common mimics of the right adrenal vein (Figure 6). Distinguishing features
include: (1) communication with a larger hepatic vein;
(2) the presence of hepatic parenchymal staining, which
is uncommon in adrenal venography; and (3) reports of
ipsilateral flank or abdominal discomfort by the patient
upon contrast injection with pressure into the adrenal
vein (particularly on the right side), which is typically
absent in injections into hepatic veins.[7] Adrenal venous
drainage into hepatic veins instead of direct drainage into
the IVC is also reported (Figure 7).[8] In addition, the right
adrenal vein may also take a more upward and straight
course in some cases (Figure 8).
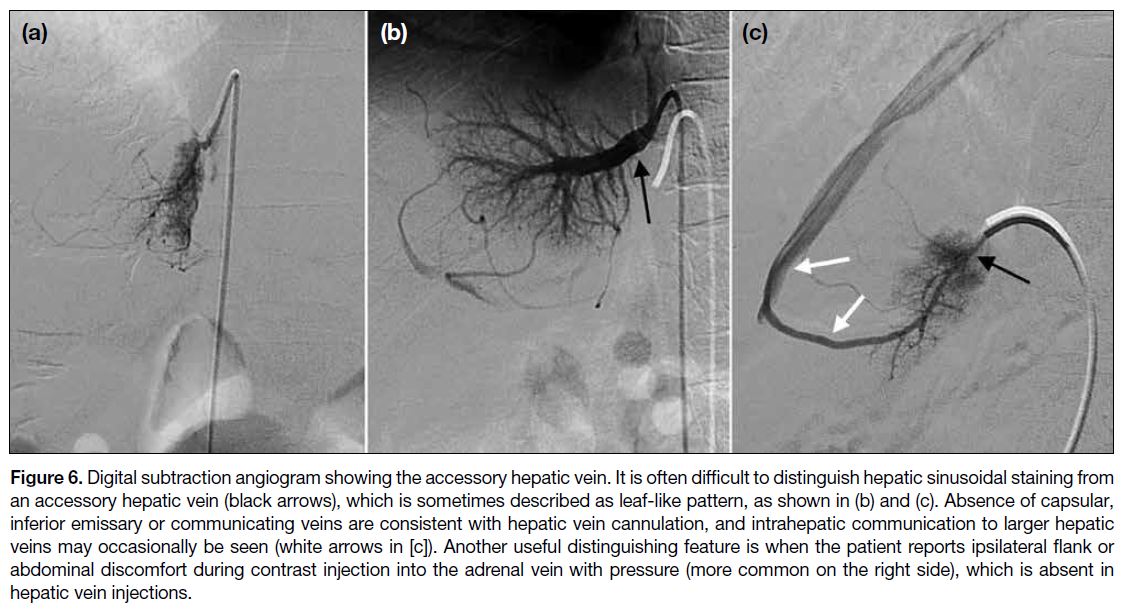
Figure 6. Digital subtraction angiogram showing the accessory hepatic vein. It is often difficult to distinguish hepatic sinusoidal staining from
an accessory hepatic vein (black arrows), which is sometimes described as leaf-like pattern, as shown in (b) and (c). Absence of capsular,
inferior emissary or communicating veins are consistent with hepatic vein cannulation, and intrahepatic communication to larger hepatic
veins may occasionally be seen (white arrows in [c]). Another useful distinguishing feature is when the patient reports ipsilateral flank or
abdominal discomfort during contrast injection into the adrenal vein with pressure (more common on the right side), which is absent in
hepatic vein injections.
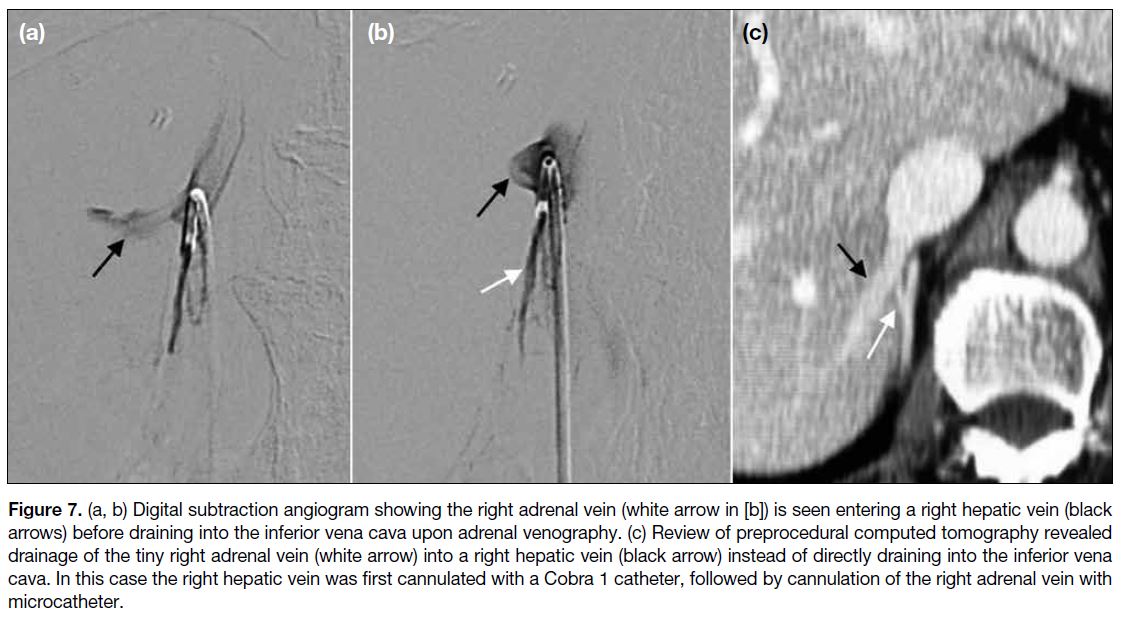
Figure 7. a, b) Digital subtraction angiogram showing the right adrenal vein (white arrow in [b]) is seen entering a right hepatic vein (black
arrows) before draining into the inferior vena cava upon adrenal venography. (c) Review of preprocedural computed tomography revealed
drainage of the tiny right adrenal vein (white arrow) into a right hepatic vein (black arrow) instead of directly draining into the inferior vena
cava. In this case the right hepatic vein was first cannulated with a Cobra 1 catheter, followed by cannulation of the right adrenal vein with
microcatheter.
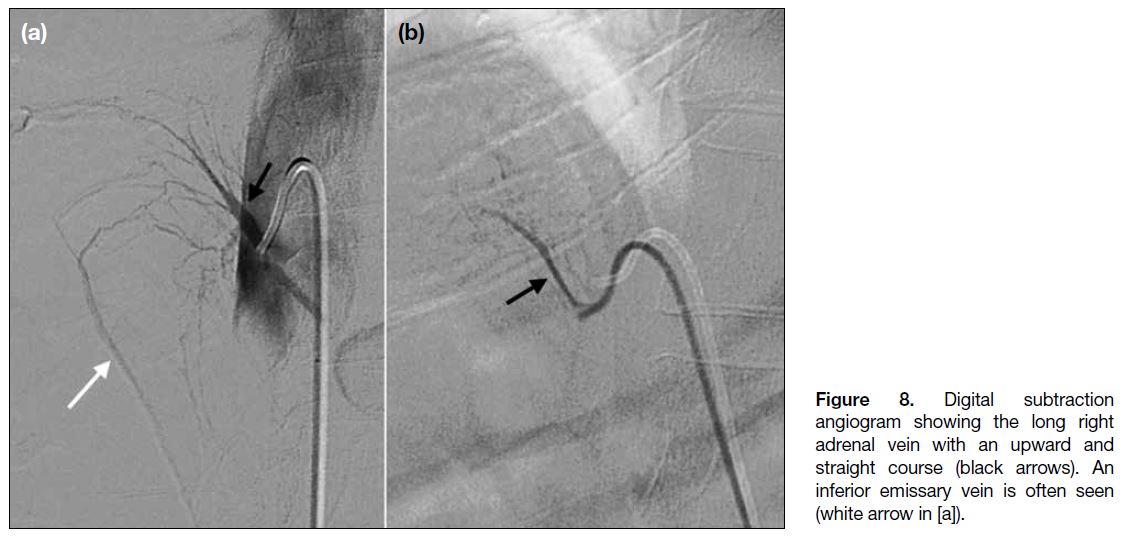
Figure 8. Digital subtraction
angiogram showing the long right
adrenal vein with an upward and
straight course (black arrows). An
inferior emissary vein is often seen
(white arrow in [a]).
With its varied venographic appearance, the only certain
finding of a right adrenal vein is the presence of an
inferior emissary vein, which was identified in up to 86%
of successful AVS cases in a study reported by Kohi
et al.[9] The Table summarises the venographic features
suggesting correct cannulation of the right adrenal vein.
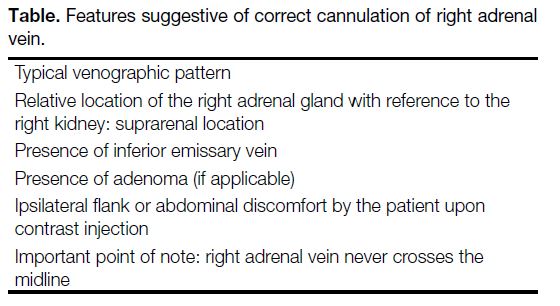
Table. Features suggestive of correct cannulation of right adrenal vein.
Left Adrenal Vein
The left adrenal vein usually joins the inferior phrenic vein to form a common phrenicoadrenal trunk, of
varying length, before taking a caudal path to drain
into the superior aspect of the left renal vein[8] (Figure 9). The left adrenal vein typically measures 1 to 4 cm to its confluence with the inferior phrenic vein,
then approximately 1 to 3 cm from there to the left
renal vein, and measures approximately 4 to 5 mm in
calibre.[8] Anatomical variations include separate
drainage of the left adrenal vein and inferior phrenic vein into the left renal vein. There may also be
superficial, emissary, or capsular veins extending
from the surface of the adrenal gland into the perirenal
fat, and occasional penetration of the renal capsule.
These can communicate with the inferior phrenic vein, intercostal veins, left renal vein, as well as the azygous
or hemiazygos vein.[7]
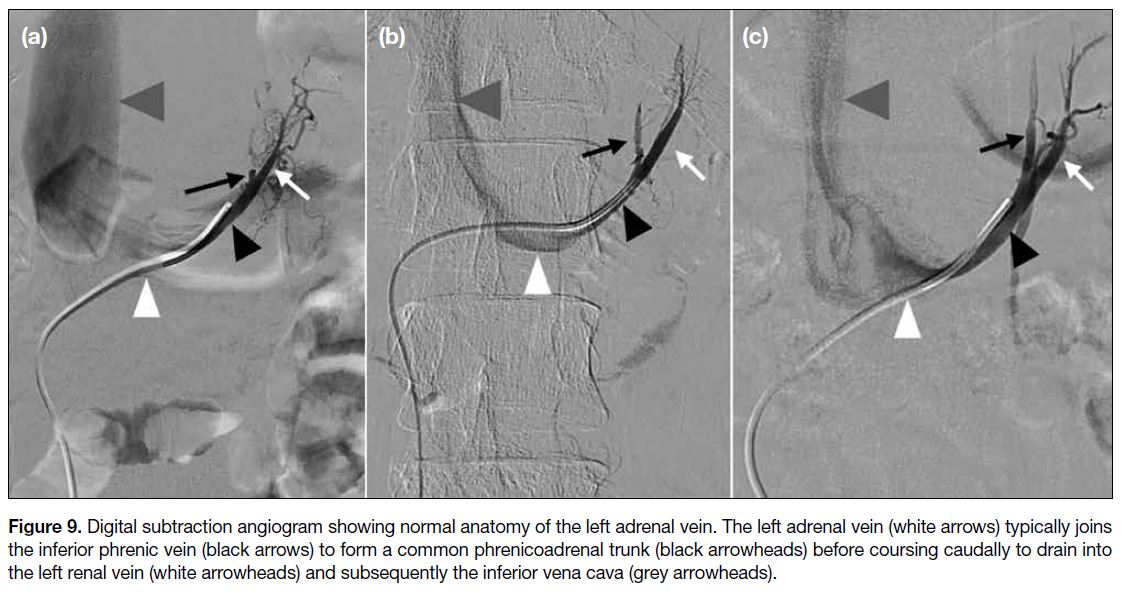
Figure 9. Digital subtraction angiogram showing normal anatomy of the left adrenal vein. The left adrenal vein (white arrows) typically joins
the inferior phrenic vein (black arrows) to form a common phrenicoadrenal trunk (black arrowheads) before coursing caudally to drain into
the left renal vein (white arrowheads) and subsequently the inferior vena cava (grey arrowheads).
There are anatomical variations of the left adrenal vein
that operators should be aware of. Examples include direct drainage of the left adrenal vein into the left renal
vein without forming a common phrenicoadrenal trunk
with left inferior phrenic vein, or direct drainage into the
IVC[8] [10] (Figure 10). There are also reported cases where
the central vein may be absent or very short, with multiple
adrenal tributaries draining into the phrenicoadrenal
trunk directly or into the inferior phrenic vein without a
central vein.[8]
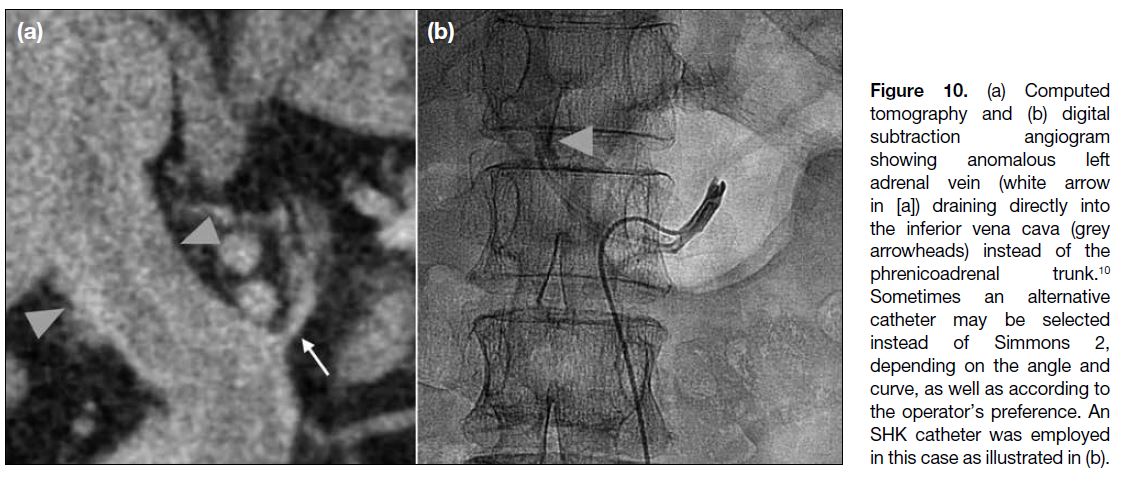
Figure 10. (a) Computed
tomography and (b) digital
subtraction angiogram
showing anomalous left
adrenal vein (white arrow
in [a]) draining directly into
the inferior vena cava (grey
arrowheads) instead of the
phrenicoadrenal trunk.[10]
Sometimes an alternative
catheter may be selected
instead of Simmons 2,
depending on the angle and
curve, as well as according to
the operator’s preference. An
SHK catheter was employed
in this case as illustrated in (b).
PROCEDURE AND TECHNIQUE
Preprocedural computed tomography is helpful to
evaluate the adrenal glands for any surgically amenable
lesions which may account for the patient’s clinical
presentation, e.g., adrenal adenoma (Figure 11). It also
allows evaluation of the anatomy of the adrenal veins and
any anatomic variations, which is crucial in procedure
planning.
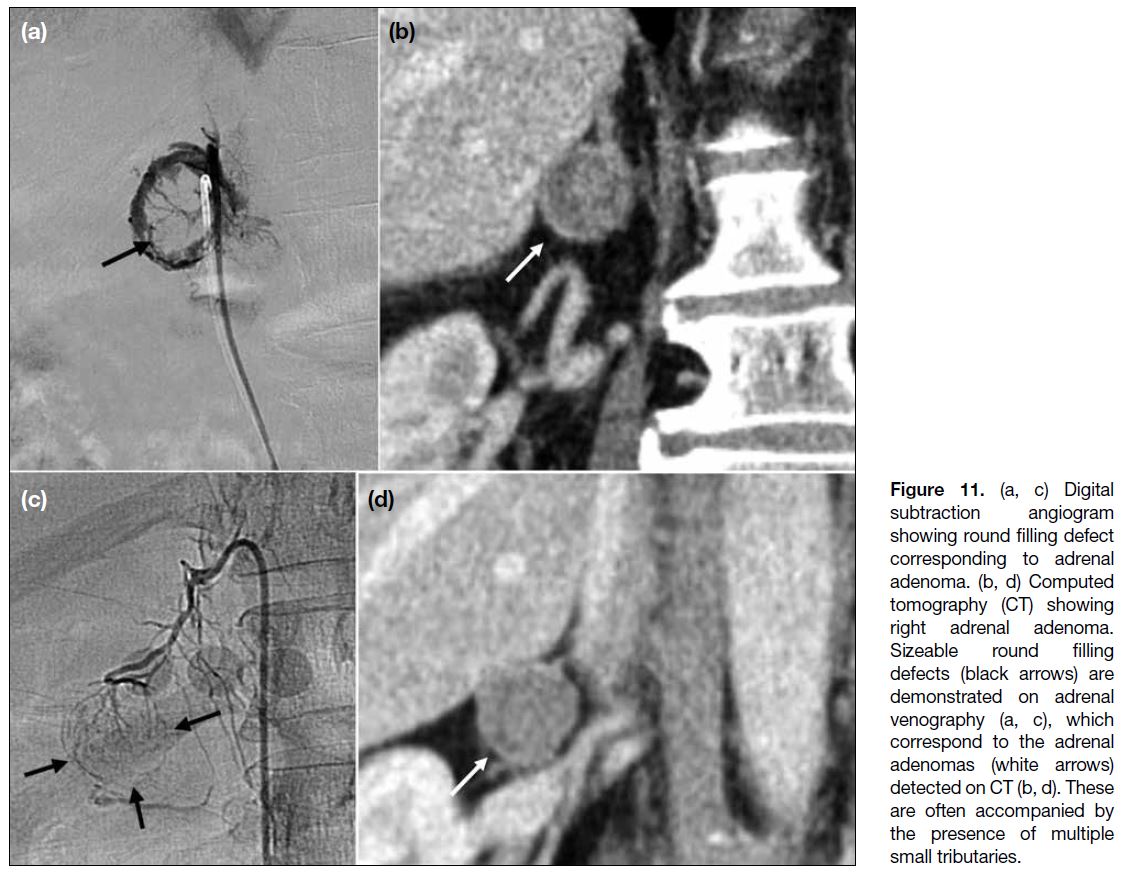
Figure 11. (a, c) Digital
subtraction angiogram
showing round filling defect
corresponding to adrenal
adenoma. (b, d) Computed
tomography (CT) showing
right adrenal adenoma.
Sizeable round filling
defects (black arrows) are
demonstrated on adrenal
venography (a, c), which
correspond to the adrenal
adenomas (white arrows)
detected on CT (b, d). These
are often accompanied by
the presence of multiple
small tributaries.
AVS may be performed with or without pharmacological
stimulation with synthetic adrenocorticotropic hormone
(ACTH), known as cosyntropin. Cortisol levels may
fluctuate throughout the procedure due to a number
of factors, including diurnal variation, pulsatile
pattern of secretion, and the effect of physiological
stress, under which its concentration in the blood will
surge.[2] These interfere with the indices used in the
evaluation of acquired samples (see the next section).
ACTH stimulation overcomes fluctuations in cortisol
secretion influenced by the aforementioned factors and
maximises the gradient between cortisol concentrations
in the adrenal veins and that in the peripheral veins, thus
increasing the confidence of successful sampling.[11] It
also stimulates aldosterone secretion from aldosterone-producing
adenomas that overexpress ACTH receptors, thereby increasing the chance of lateralisation.[11] A study
has suggested that pharmacological stimulation with
ACTH may increase blood flow to the adrenal glands,
enlarging them and increasing the cannulation success
rate.[11]
Vascular access is typically acquired through puncture
of the right or both common femoral veins. Access via
an upper limb vein such as the basilic vein[12] has been
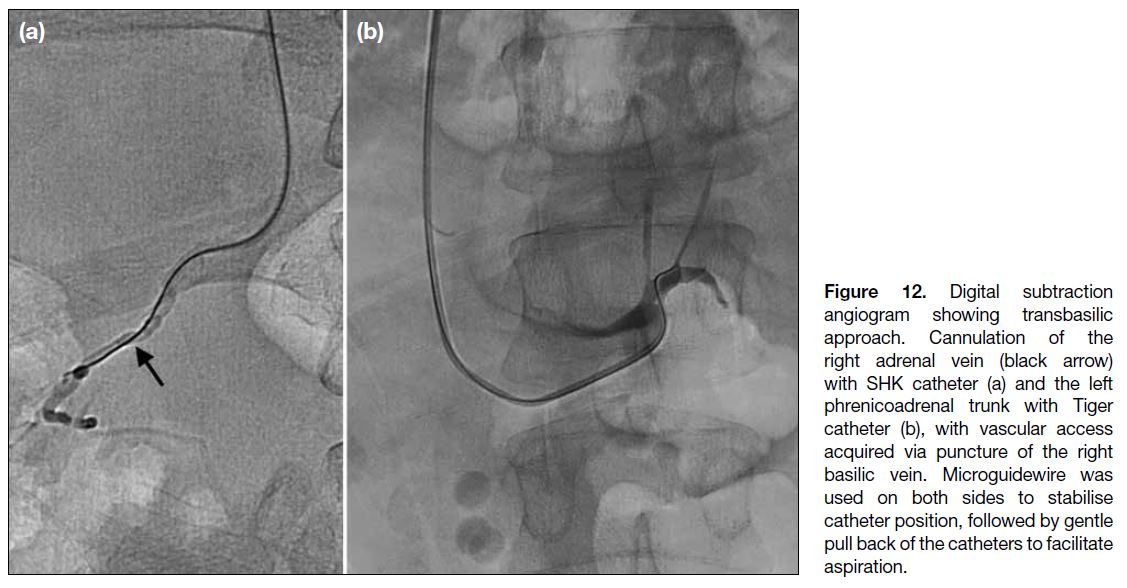
reported (Figure 12).
Figure 12. Digital subtraction
angiogram showing transbasilic
approach. Cannulation of the
right adrenal vein (black arrow)
with SHK catheter (a) and the left
phrenicoadrenal trunk with Tiger
catheter (b), with vascular access
acquired via puncture of the right
basilic vein. Microguidewire was
used on both sides to stabilise
catheter position, followed by gentle
pull back of the catheters to facilitate
aspiration.
The operator may opt for either sequential or
simultaneous sampling methods. In sequential sampling,
one adrenal vein is sampled before cannulation of the
contralateral side, creating a time gap between acquisition
of bilateral samples. In such cases, the right adrenal vein
should be cannulated first to reduce the time gap as it is
usually more time-consuming owing to its anatomy.[13] In
simultaneous sampling, the catheter is placed in the first
engaged adrenal vein and sampling is withheld until the
contralateral side is also successfully cannulated, after
which sampling is performed in a synchronous manner.
Simultaneous sampling is thought to reduce the chance
of creating artificial gradients between the glands due to
the pulsatility of aldosterone secretion. However, this is
possibly at the expense of a theoretical slight increase
in risk of adrenal vein thrombosis due to lengthened
duration of catheter positioning in the adrenal vein.[13]
Intermittent flushing with saline may reduce the risk of adrenal vein thrombosis, though this technique should be
carefully practised as it in turn poses an increased risk
of catheter dislodgement from the target vein as well
as sample dilution. These can be prevented by gentle
injection pressure upon flushing and ensuring that initial
blood samples are discarded.
Superselective AVS, also called segmental AVS,
is a method whereby sampling is performed via the
adrenal tributary veins in place of central adrenal veins
and is thought to allow identification of aldosterone
hypersecretion in specific segment(s) of the glands,
thereby sparing lesion-free segments in cases where
bilateral adrenalectomy is planned.[5]
Catheter selection is important to ensure effective
cannulation and sampling. For the right adrenal vein,
4.1-Fr SHK catheter (Cook Medical, Bloomington
[IN], US) is often the catheter of choice in our centre
due to its specific curve with a soft tapered end which
favours the venous anatomy in the majority of cases.
The use of other catheter shapes such as Tiger[14] (Terumo
Corporation, Tokyo, Japan) and specifically designed
catheters such as the MK-adrenal catheter[5] (Hanaco
Medical, Tokyo, Japan) have also been reported. Other
options for the right side include the Simmons 1 (Cook
Medical, Bloomington [IN], US), Cobra 1 and Cobra 2
(Terumo Corporation, Tokyo, Japan), and, in some cases,
Mikaelsson (Boston Scientific, Marlborough [MA], US). Of particular note, catheters with large reverse curves
must be used with caution, as they may result in too deep
of a cannulation beyond an aldosterone-rich tributary,
as well as increasing the risk of venous rupture and
thrombosis.[15] The catheter is first advanced to a level
from T11 to L1 and the catheter tip is rotated to face the
posterior wall of the IVC. It is gently withdrawn with
a slight probing motion until the catheter ‘drops into’
the right adrenal vein. Other veins, for example, the
accessory hepatic veins, phrenic veins, or other small
retroperitoneal veins, may be inadvertently cannulated
throughout the search for the right adrenal vein. If no
vessel is engaged, the catheter may be rotated to the right
by a few degrees and advanced in the cranial direction to
repeat the above manoeuvre until the right adrenal vein
is engaged.[15]
For the left phrenicoadrenal trunk, with its more
consistent anatomy, a Simmons 2 catheter (Cook
Medical, Bloomington [IN], US) is usually selected.
Once reaching the ostium of the left renal vein, the
catheter is gently pulled back, causing its tip to advance
further. Continued retraction will result in the catheter
flicking superiorly to engage the phrenicoadrenal trunk.[7]
Selective cannulation of the left adrenal vein is generally
not recommended in order to avoid missing any
tributaries contributing to sources of aldosterone excess,
given the potential presence of the aforementioned
anatomic variations.[15] One study reported paradoxically lower aldosterone concentrations from selective central
vein samples compared to phrenicoadrenal trunk samples
in 17% of the cases.[16]
During AVS, owing to the small calibre of the target
veins, the catheter tip commonly wedges against the
venous wall, resulting in difficulty in aspiration of blood
samples. Creation of side holes allows easier aspiration
of blood samples when the catheter tip is wedged. In
our centre, one pair of side holes is usually created by
piercing the distal limb of the catheter (approximately
3 mm from the end hole) using a 21-gauge needle. We
always test the integrity of the catheter before in vivo use.
While the theoretical risk of catheter fracture is possible,
this has not been encountered during our past 10 years
of practice. In challenging cases such as small target
veins or unstable cannulation, coaxial technique with
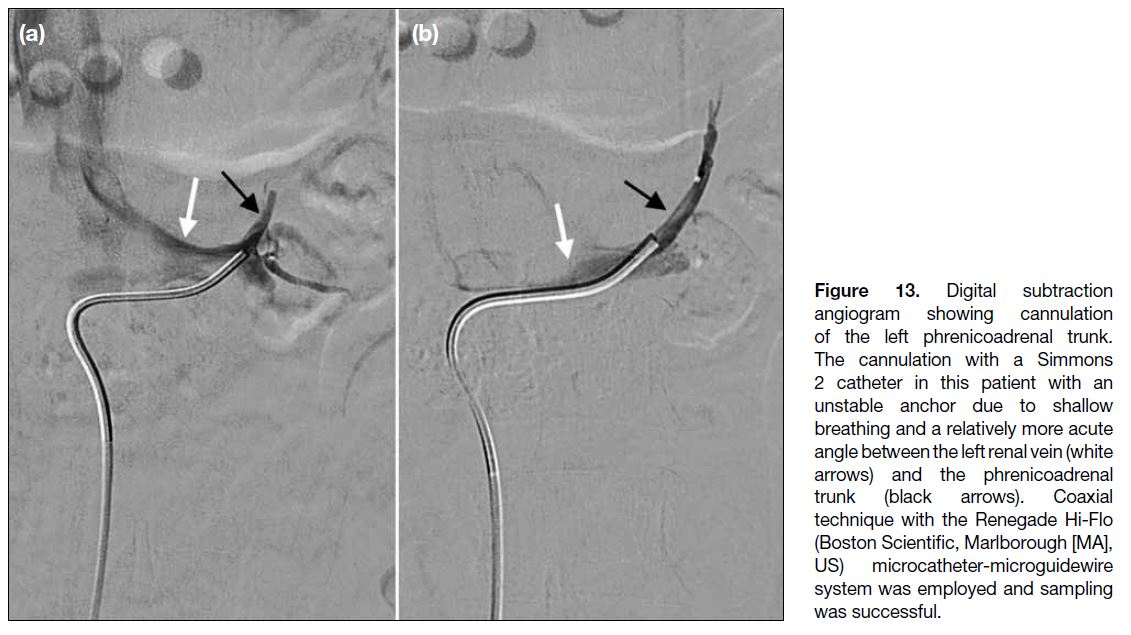
microcatheters and microguidewires may be employed[17] (Figure 13).
Figure 13. Digital subtraction
angiogram showing cannulation
of the left phrenicoadrenal trunk.
The cannulation with a Simmons
2 catheter in this patient with an
unstable anchor due to shallow
breathing and a relatively more acute
angle between the left renal vein (white
arrows) and the phrenicoadrenal
trunk (black arrows). Coaxial
technique with the Renegade Hi-Flo
(Boston Scientific, Marlborough [MA],
US) microcatheter-microguidewire
system was employed and sampling
was successful.
Adrenal venograms should be performed with gentle,
slow injection of a low volume of iodinated contrast to
confirm correct cannulation of the target vein, and to
avoid rupture of the fragile adrenal veins and resulting
venous haemorrhage. Loin or back pain may be reported
by the patient during contrast injection, especially into
the right adrenal vein; this is absent during injection into mimics such as accessory hepatic veins.[7]
Gentle intermittent aspiration is recommended during
venous sampling, as forceful suction may result in
collapse of the venous wall onto the catheter tip, which
hinders sampling.[15] The first 5 mL of the aspirated
sample is discarded as it lowers the accuracy of serum
aldosterone measurements due to contamination by
iodinated contrast. Subsequently, another 10 mL is
aspirated and a venogram is repeated to confirm the
catheter is still within the target vein to validate the
acquired sample. After sampling both the right adrenal
vein and the left phrenicoadrenal trunk, 10 mL of
peripheral blood is drawn from the femoral vascular
sheath for biochemical confirmation of sampling success
and calculations of appropriate indices for results
analysis as detailed in the section below.
INDICES FOR ADRENAL VENOUS
SAMPLING
The spectrum of venographic appearances of the right
adrenal vein precludes reliance on venography alone
to determine accurate cannulation. The most common
technique to confirm the success of AVS entails
measurements of hormonal concentrations in the adrenal
and peripheral venous samples and calculations of the
following indices.[18]
The selectivity index is defined as the ratio of cortisol
concentration in the adrenal veins to that in the peripheral
veins. According to the Adrenal Venous Sampling
International Study,[19] the majority of centres use a
cutoff of 2 under non-stimulated conditions and 3 to 5
under ACTH stimulation. In our centre, where ACTH
stimulation is performed, a selectivity index of 5 is taken
as a cut-off for successful cannulation in accordance
with the protocol established with our endocrinologists.
Lateralisation index (LI) is defined as the aldosterone-to-cortisol ratio in the dominant adrenal vein (i.e.,
the side with the higher aldosterone level) over the
aldosterone-to- cortisol ratio in the non-dominant
adrenal vein. It is used to establish whether a lateralised
aldosterone excess exists. Most centres use an LI of 2
to 4 under non-stimulated conditions and 2.6 to 4 under
ACTH stimulation.[19] In our centre, an LI of ≥4 suggests
lateralised aldosterone excess, and an LI of <3 implies
absence of lateralisation.
The contralateral suppression index is calculated by
dividing the aldosterone-to-cortisol ratio in the non-dominant
adrenal vein by that in the IVC. If LI is ≥3 but
<4, a contralateral suppression index of <1.0 is predictive
of good surgical outcome and is considered lateralised.[19]
An intraoperative rapid automated cortisol assay may
also be performed to expedite confirmation of procedural
success without having to render the patient at risk of a
repeated invasive examination.
CONCLUSION
AVS is a technically challenging procedure with variable
success rates, which is highly operator dependent. Proper
recognition of normal and variant adrenal venographic
findings, especially that of the right adrenal vein, is
necessary for success. Variant adrenal venous anatomy
influences catheter selection and sampling techniques.
This pictorial review showcases different venographic
patterns and anatomical variations of the adrenal gland
veins in hopes of facilitating future operators to achieve
safe and successful AVS.
REFERENCES
1. Benham JL, Eldoma M, Khokhar B, Roberts DJ, Rabi DM,
Kline GA. Proportion of patients with hypertension resolution
following adrenalectomy for primary aldosteronism: a systematic
review and meta-analysis. J Clin Hypertens (Greenwich). 2016;18:1205-12. Crossref
2. Quencer KB, Singh A, Sharma A. Best practices: indications and
procedural controversies of adrenal vein sampling for primary
aldosteronism. AJR Am J Roentgenol. 2023;220:190-200. Crossref
3. Avisse C, Marcus C, Patey M, Ladam-Marcus L, Delattre JF,
Flament JB. Surgical anatomy and embryology of the adrenal
glands. Surg Clin North Am. 2000;80:403-15. Crossref
4. Cesmebasi A, Du Plessis M, Iannatuono M, Shah S, Tubbs RS,
Loukas M. A review of the anatomy and clinical significance of
adrenal veins. Clin Anat. 2014;27:1253-63. Crossref
5. Makira K, Nishimoto K, Kiriyama-Kitamoto K, Karashima S,
Seki T, Yasuda M, et al. A novel method: super-selective adrenal
venous sampling. J Vis Exp. 2017;(127):55716. Crossref
6. Scholten A, Cisco RM, Vriens MR, Shen WT, Duh QY. Variant
adrenal venous anatomy in 546 laparoscopic adrenalectomies.
JAMA Surg. 2013;148:378-83. Crossref
7. Daunt N. Adrenal vein sampling: how to make it quick, easy, and
successful. Radiographics. 2005;25 Suppl 1:S143-58. Crossref
8. Monroe EJ, Carney BW, Ingraham CR, Johnson GE, Valji K. An
interventionist’s guide to endocrine consultations. Radiographics.
2017;37:1246-67. Crossref
9. Kohi MP, Agarwal VK, Naeger DM, Taylor AG, Kolli KP,
Fidelman N, et al. The inferior emissary vein: a reliable landmark
for right adrenal vein sampling. Acta Radiol. 2015;56:454-7. Crossref
10. Fung KK, Cheng KK, Lee BK, Cho DH. Anomalous direct drainage
of left adrenal vein into left-sided inferior vena cava encountered
during adrenal venous sampling: a case report. Hong Kong J Radiol.
2019;22:254-7. Crossref
11. Kline GA, So B, Dias VC, Harvey A, Pasieka JL. Catheterization
during adrenal vein sampling for primary aldosteronism: failure
to use (1-24) ACTH may increase apparent failure rate. J Clin
Hypertens (Greenwich). 2013;15:480-4. Crossref
12. Jiang X, Dong H, Peng M, Che W, Zou Y, Song L, et al. A novel
method of adrenal venous sampling via an antecubital approach.
Cardiovasc Intervent Radiol. 2017;40:388-93. Crossref
13. Almarzooqi MK, Chagnon M, Soulez G, Giroux MF, Gilbert P,
Oliva VL, et al. Adrenal vein sampling in primary aldosteronism:
concordance of simultaneous vs sequential sampling. Eur J
Endrocrinol. 2017;176:159-67. Crossref
14. Wan J, Ran F, Xia S, Hou J, Wang D, Liu S, et al. Feasibility
and effectiveness of a single-catheter approach for adrenal vein
sampling in patients with primary aldosteronism. BMC Endocr
Disord. 2021;21:22. Crossref
15. Quencer KB. Adrenal vein sampling: technique and protocol, a
systematic review. CVIR Endovasc. 2021;4:38. Crossref
16. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M,
Plouin PF, et al. An expert consensus statement on use of adrenal
vein sampling for the subtyping of primary aldosteronism.
Hypertension. 2014;63:151-60. Crossref
17. Andrews JC, Thompson SM, Young WF. A coaxial guide
wire-catheter technique to facilitate right adrenal vein sampling:
evaluation in 76 patients. J Vasc Interv Radiol. 2015;26:1871-3. Crossref
18. Naruse M, Tanabe A, Yamamoto K, Rakugi H, Kometani M,
Yoneda T, et al. Adrenal venous sampling for subtype diagnosis
of primary hyperaldosteronism. Endocrinol Metab (Seoul).
2021;36:965-73. Crossref
19. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, et al.
The Adrenal Vein Sampling International Study (AVIS) for
identifying the major subtypes of primary aldosteronism. J Clin
Endocrinol Metab. 2012;97:1606-14. Crossref