Multimodality Imaging of Breast Augmentations: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024;27:Epub 14 November 2024
Multimodality Imaging of Breast Augmentations: A Pictorial Essay
HL Chan1, EHY Hung2, CWY Tam3, KF Tam1, HHL Chau2
1 Department of Radiology, North District Hospital, Hong Kong SAR, China
2 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
3 Department of Diagnostic Radiology, Alice Ho Miu Ling Nethersole Hospital, Hong Kong SAR, China
Correspondence: Dr HL Chan, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: hollischanrad@gmail.com
Submitted: 25 June 2023; Accepted: 19 October 2023. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. HLC and EHYH acquired the data. All authors analysed the data. HLC, EHYH and CWYT drafted the manuscript. KFT and HHLC critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: 2023.168). A waiver of patient consent was granted by the Committee due to the retrospective nature of the study.
INTRODUCTION
Breast augmentation is a procedure that has been
performed for over a century. The first breast augmentation
was performed in 1895 by Austrian-German surgeon
Vincent Czerny, who used autologous fat implantation
for breast reconstruction after partial mastectomy.[1]
Since then, numerous techniques have been developed,
whether used for cosmetic purpose, reconstruction after
mastectomy, or correction of congenital malformations.
Breast augmentation can be divided into implant and
injection types, which involve different materials and
anatomical locations. Depending on the material used
and the different methodologies, the corresponding
complications are also specific.
It is crucial for radiologists to be familiar with the
normal and abnormal appearance of breast augmentation
in different imaging modalities including mammography
(MG), ultrasonography (USG), and magnetic resonance
imaging (MRI). This pictorial essay illustrates the
imaging features of patients with breast augmentation
in our institution from 2010 to 2022, highlighting the
normal and abnormal radiological appearances.
IMPLANT AUGMENTATION
Implant Materials
Silicone and saline implants are the two most commonly used materials. Silicone implants are typically preferred
for their natural texture and appearance. Saline implants are
filled with sterile saline that, in case of implant rupture, is
absorbed by the body. Silicon is a semi-metallic element,
while silicone is an organic silicon polymer product with
a main chain of alternating silicon and oxygen atoms.[2]
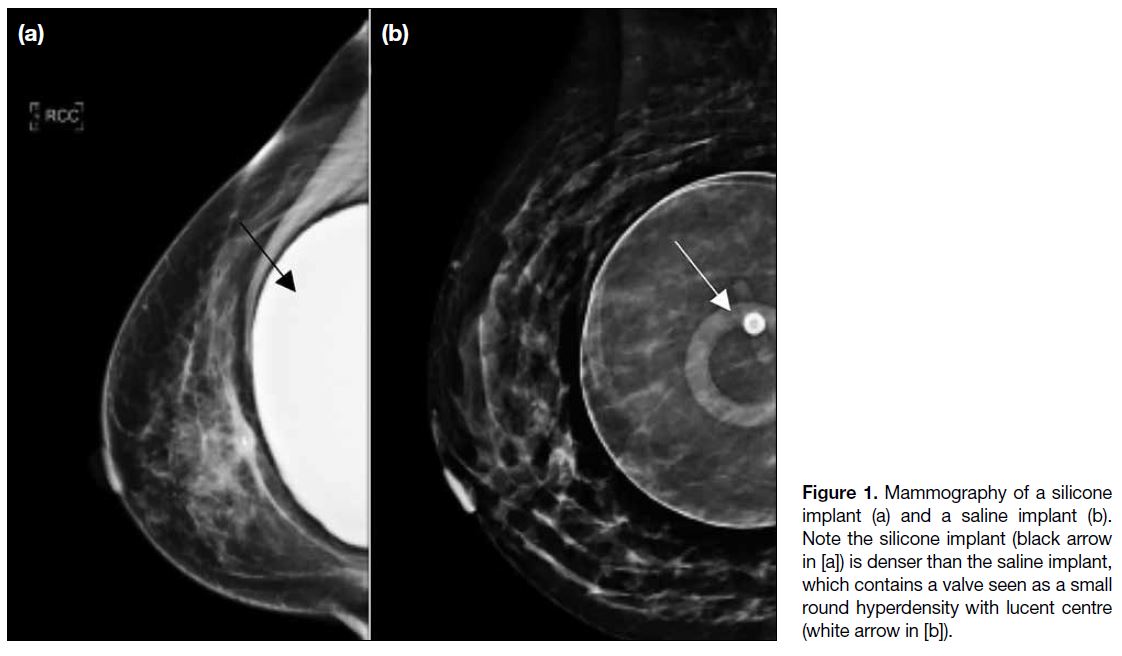
On MG, silicone appears denser than saline. Figure 1 demonstrates the difference on MG, which is easily
identifiable.
Figure 1. Mammography of a silicone
implant (a) and a saline implant (b).
Note the silicone implant (black arrow
in [a]) is denser than the saline implant,
which contains a valve seen as a small
round hyperdensity with lucent centre
(white arrow in [b]).
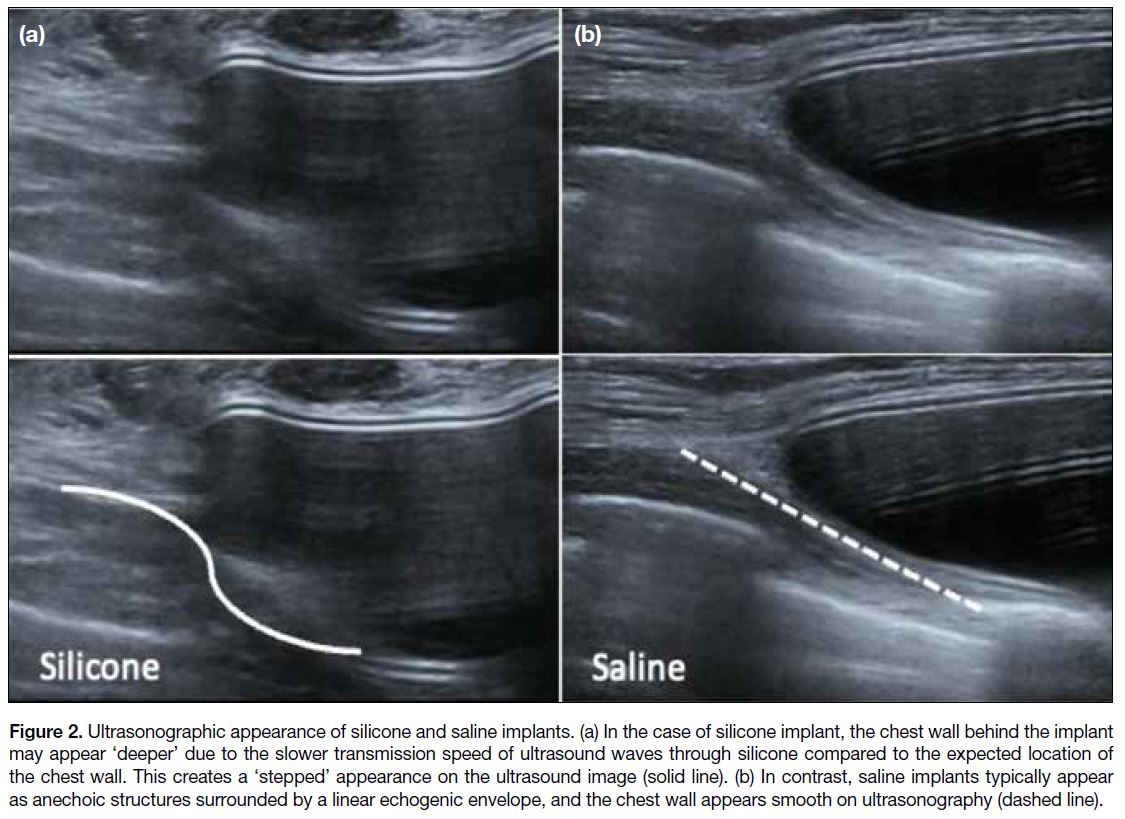
Distinguishing between silicone and saline breast
implants on USG can be challenging. One potential
differentiating factor is the appearance of the chest
wall, which may appear ‘deeper’ than the expected
position deep to the silicone implant due to the slower
transmission speed of ultrasound waves through
silicone.[3] This can result in a ‘stepped’ appearance at
the edge of the implant compared to the smooth chest
wall appearance seen with saline implants on USG
(Figure 2). It is often difficult to detect such subtle
differences in clinical practice.
Figure 2. Ultrasonographic appearance of silicone and saline implants. (a) In the case of silicone implant, the chest wall behind the implant
may appear ‘deeper’ due to the slower transmission speed of ultrasound waves through silicone compared to the expected location of the chest wall. This creates a ‘stepped’ appearance on the ultrasound image (solid line). (b) In contrast, saline implants typically appear
as anechoic structures surrounded by a linear echogenic envelope, and the chest wall appears smooth on ultrasonography (dashed line).
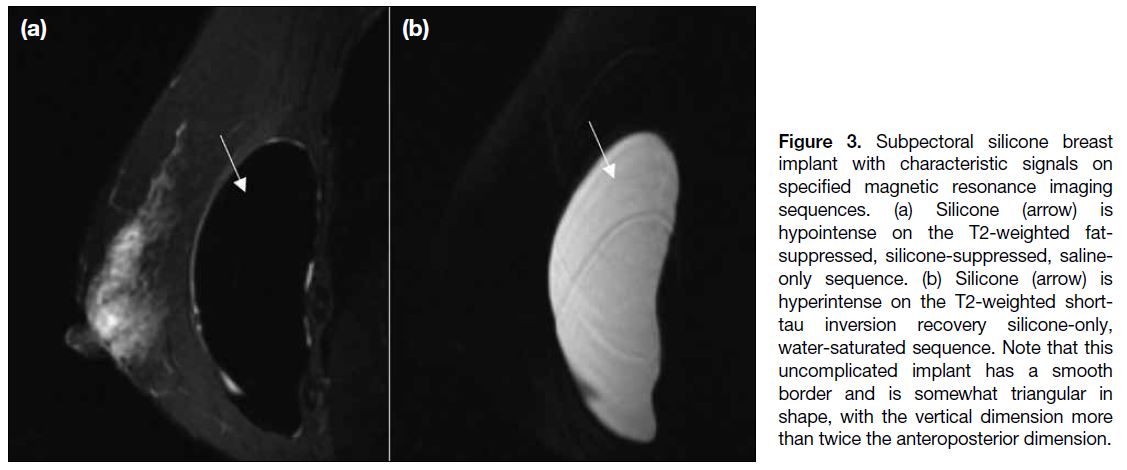
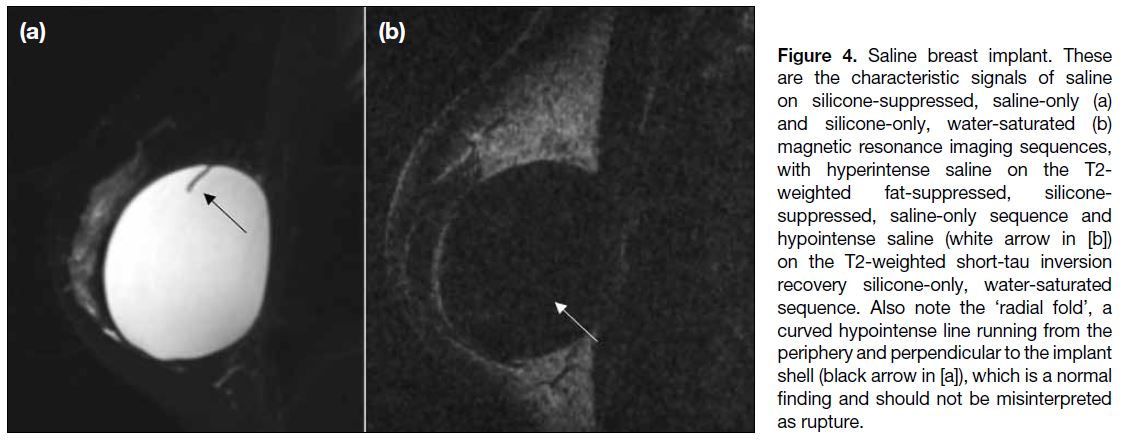
Silicone and saline implants also show different signal
intensities with the use of specific MRI sequences due
to their inherent material differences (Figures 3 and 4).
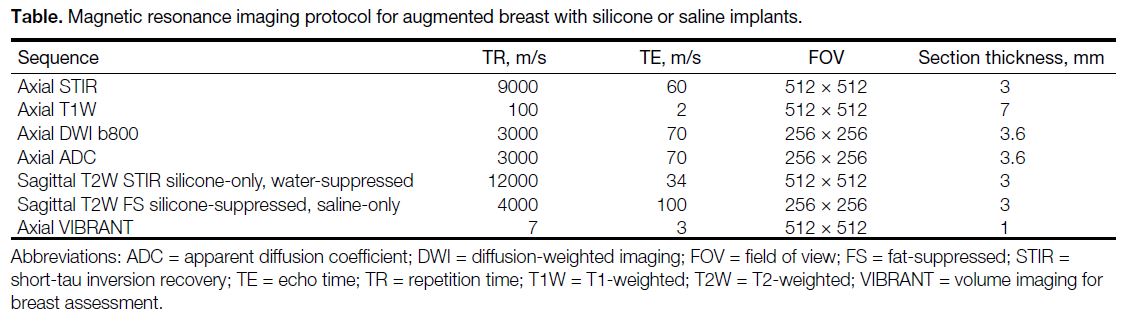
Our MRI protocol for post-augmentation breast imaging is
summarised in the Table. The silicone-only MRI sequence is a protocol that has been specifically designed to visualise
silicone gel–filled breast implants using a combination of
pulse sequences and specialised software to improve the
signal-to-noise ratio of silicone gel in the imaging data,
allowing for more accurate visualisation of the implants.
Figure 3. Subpectoral silicone breast
implant with characteristic signals on
specified magnetic resonance imaging
sequences. (a) Silicone (arrow) is
hypointense on the T2-weighted fat-suppressed,
silicone-suppressed, saline-only
sequence. (b) Silicone (arrow) is
hyperintense on the T2-weighted short-tau
inversion recovery silicone-only,
water-saturated sequence. Note that this
uncomplicated implant has a smooth
border and is somewhat triangular in
shape, with the vertical dimension more
than twice the anteroposterior dimension.
Figure 4. Saline breast implant. These
are the characteristic signals of saline
on silicone-suppressed, saline-only (a)
and silicone-only, water-saturated (b)
magnetic resonance imaging sequences,
with hyperintense saline on the T2-weighted fat-suppressed, silicone-suppressed,
saline-only sequence and
hypointense saline (white arrow in [b])
on the T2-weighted short-tau inversion
recovery silicone-only, water-saturated
sequence. Also note the ‘radial fold’, a
curved hypointense line running from the
periphery and perpendicular to the implant
shell (black arrow in [a]), which is a normal
finding and should not be misinterpreted
as rupture.
Table. Magnetic resonance imaging protocol for augmented breast with silicone or saline implants.
Location of Implant
Subpectoral and subglandular are two different placement
options for breast implants. Subpectoral placement refers
to placing the breast implant posterior to the pectoral
muscles, which can supply added support and stability to
the implant, therefore decreasing the chance of implant
exposure, skin necrosis, and capsular contracture.[4][5] It is
the standard technique of breast implant reconstruction.[6]
However, it may cause animation deformities and
relatively unnatural state.[7] Animation deformities occur
when the pectoral muscle’s contraction causes the breast
implant to move or appear distorted during physical
activity. Unnatural state refers to the aesthetic outcome
where the breasts may not exhibit natural movement,
resulting in an appearance that can seem artificial or
rigid.
Subglandular placement, also called prepectoral
placement, refers to placing the breast implant anterior to
the pectoral muscle and posterior to the glandular tissue
of the breast, and is considered less invasive. There are
several relative contraindications, including obesity,
poorly controlled diabetes, and previous radiation
treatment, which carry a higher risk of skin necrosis.[7]
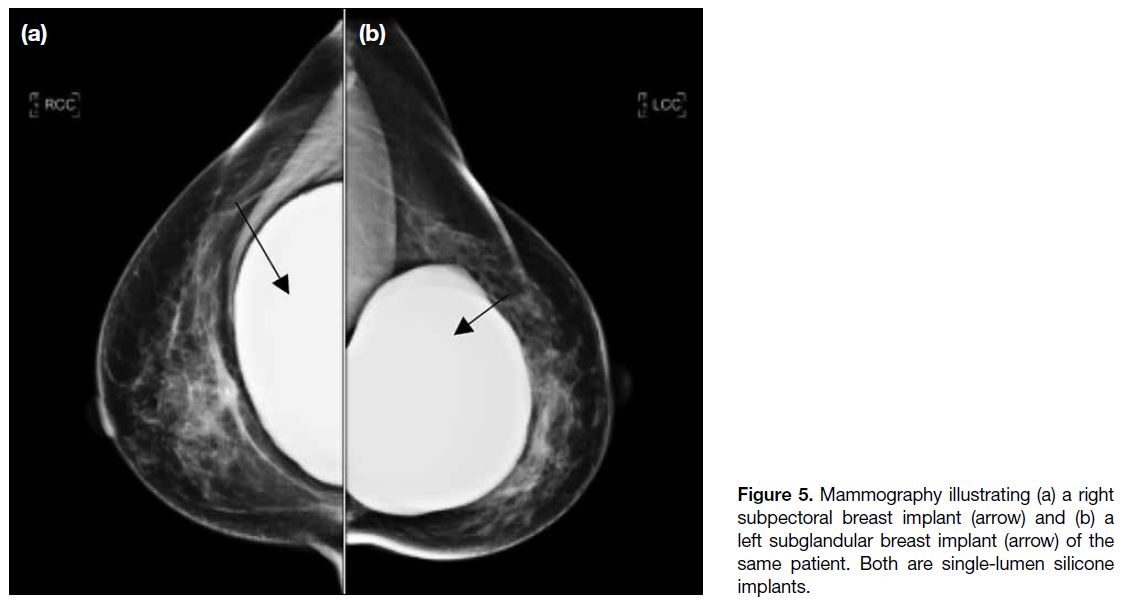
The different MG appearances of subglandular and
subpectoral implants are demonstrated in Figure 5.
Figure 5. Mammography illustrating (a) a right subpectoral breast implant (arrow) and (b) a left subglandular breast implant (arrow) of the same patient. Both are single-lumen silicone implants.
Type of Implant
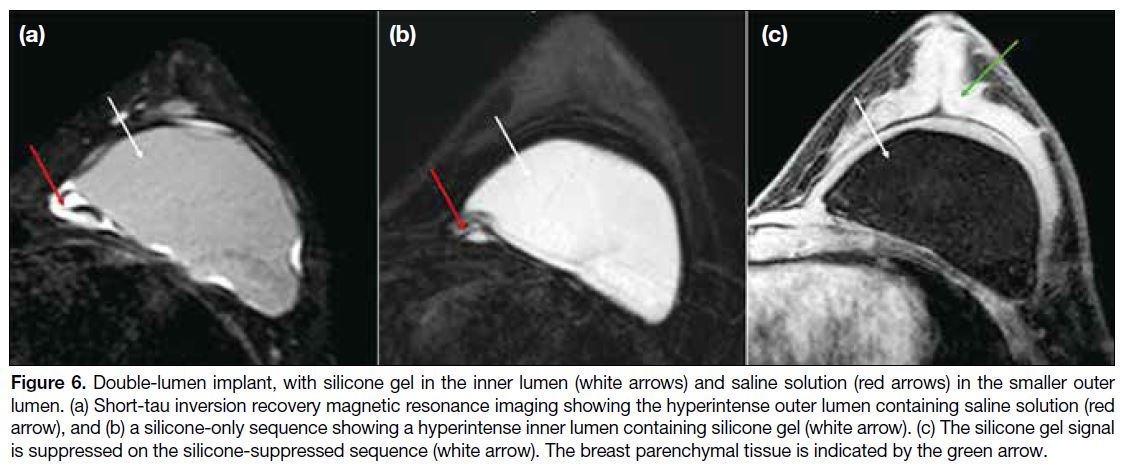
A single-lumen implant is a shell filled with silicone gel or saline solution. A standard double-lumen implant
is filled with silicone gel in the inner lumen and saline
solution in the smaller outer lumen (Figure 6). An inverse
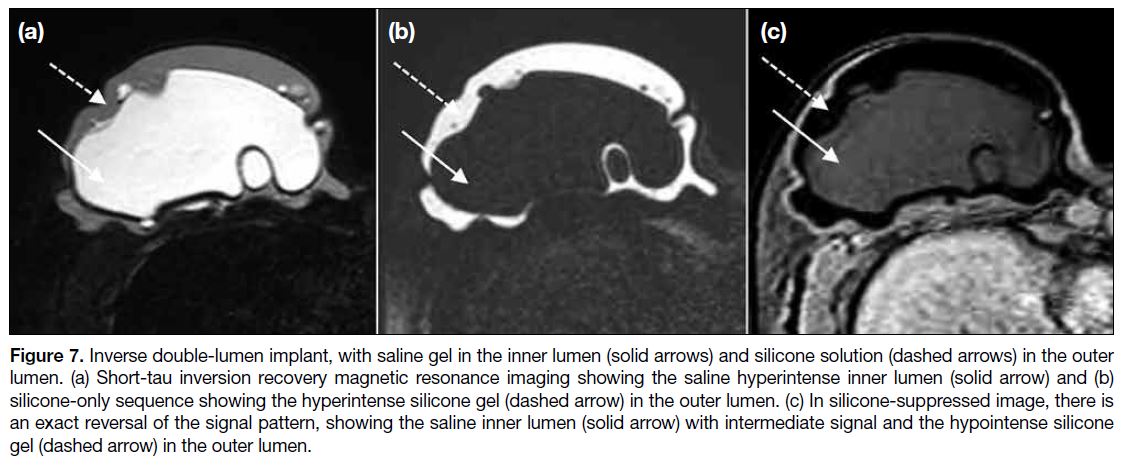
double-lumen implant is filled with saline solution in the
inner lumen, which can be expanded as necessary, and
with silicone gel in the outer lumen (Figure 7).
Figure 6. Double-lumen implant, with silicone gel in the inner lumen (white arrows) and saline solution (red arrows) in the smaller outer
lumen. (a) Short-tau inversion recovery magnetic resonance imaging showing the hyperintense outer lumen containing saline solution (red
arrow), and (b) a silicone-only sequence showing a hyperintense inner lumen containing silicone gel (white arrow). (c) The silicone gel signal
is suppressed on the silicone-suppressed sequence (white arrow). The breast parenchymal tissue is indicated by the green arrow.
Figure 7. Inverse double-lumen implant, with saline gel in the inner lumen (solid arrows) and silicone solution (dashed arrows) in the outer
lumen. (a) Short-tau inversion recovery magnetic resonance imaging showing the saline hyperintense inner lumen (solid arrow) and (b)
silicone-only sequence showing the hyperintense silicone gel (dashed arrow) in the outer lumen. (c) In silicone-suppressed image, there is
an exact reversal of the signal pattern, showing the saline inner lumen (solid arrow) with intermediate signal and the hypointense silicone gel (dashed arrow) in the outer lumen.
A double-lumen breast implant is designed to prevent massive deflation of the implant. In the event of inner
shell rupture, the ruptured material will be contained
by the outer chamber. Some designs allow volume
adjustment of the chamber during surgery, so that the
size and shape of the augmented breast can be adjusted
accordingly with a more personalised result. However, it
has been reported that there might be less natural result
in some patients due to the difference in consistence of
silicone and saline materials, and a potential complication
due to the reaction between inner and outer layer
implants.[8] The placement of a double-lumen implant
requires special expertise due to their special structure.[8]
Complications
Capsular Contracture
Capsular contracture is the most common complication
of breast augmentation, yet its reported rate is highly
variable depending on surgical technique and diagnostic
threshold, ranging from 0% to 45% in different cohorts.[9]
It occurs when there is excessive foreign body reaction, with collagen production contracting the capsule and
distorting the implants. Patients commonly present with
breast firmness, palpability of the implant, tenderness,
or distortion. Capsular contracture is diagnosed with the
Baker classification system, a subjective classification
system that is based on clinical findings[9] to categorise
the aesthetic outcomes and complications associated
with breast implants, particularly focusing on capsular
contracture.
Some radiological features can aid in the diagnosis of capsular contracture. Instead of the normal oval shape,
the implants appear more rounded in shape, with an
increase in anteroposterior diameter (Figure 8).[10] Also,
visible capsular calcifications might develop due to local
inflammation and fibrosis occurring as the implant ages
(Figure 9).[11] [12]
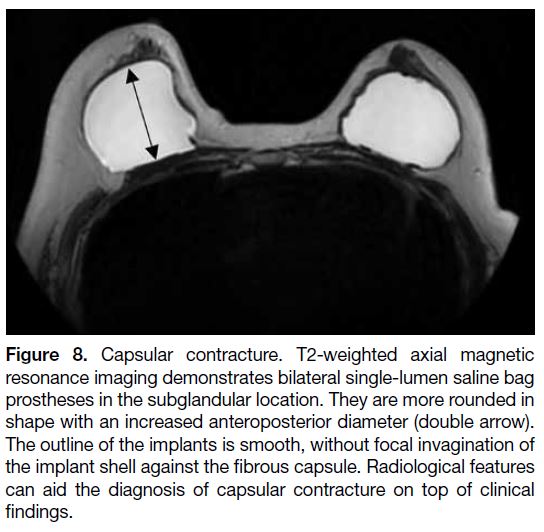
Figure 8. Capsular contracture. T2-weighted axial magnetic
resonance imaging demonstrates bilateral single-lumen saline bag
prostheses in the subglandular location. They are more rounded in
shape with an increased anteroposterior diameter (double arrow).
The outline of the implants is smooth, without focal invagination of
the implant shell against the fibrous capsule. Radiological features
can aid the diagnosis of capsular contracture on top of clinical
findings.
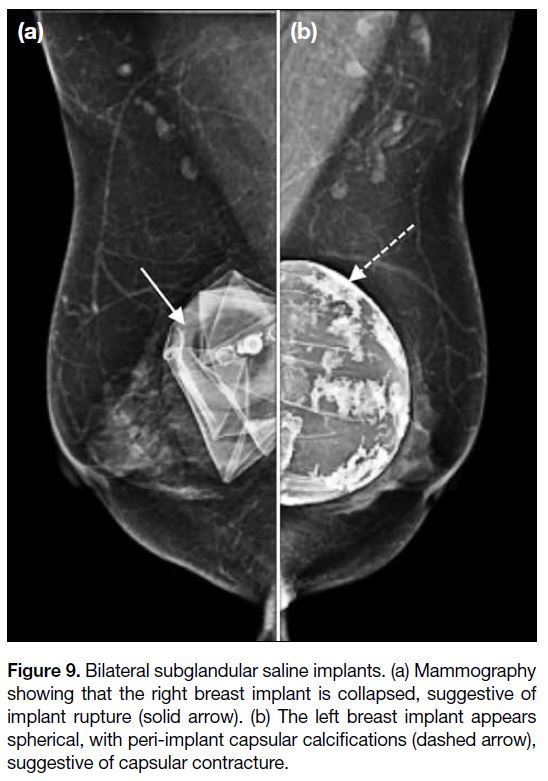
Figure 9. Bilateral subglandular saline implants. (a) Mammography
showing that the right breast implant is collapsed, suggestive of
implant rupture (solid arrow). (b) The left breast implant appears
spherical, with peri-implant capsular calcifications (dashed arrow),
suggestive of capsular contracture.
Implant Rupture
Implant rupture is one of the commonest reasons for
implant removal and can occur without an obvious
traumatic cause, frequently in asymptomatic patients.[13]
The clinical diagnosis of implant rupture can be challenging as it can present with nonspecific findings
such as palpable nodules, asymmetry, or tenderness.[14]
A slowly developing breast implant rupture without
loss of breast volume or shape can be difficult to detect
during clinical evaluation. Contour deformity is the
most frequent sign of implant rupture, followed by
displacement, mass formations, pain, and inflammation.[15]
Saline implant rupture can usually be clinically identified by a significant reduction in size, while the detection of silicone implant rupture may be challenging clinically. The body normally creates a fibrous capsule around a breast
implant. Intracapsular rupture indicates rupture via the
implant shell, but the fibrous capsule remains intact,
whereas extracapsular rupture means there is further
rupture through the fibrous capsule. MRI has a high
sensitivity and specificity of >90% for identification of
breast implant rupture and is considered the criterion
standard.[15]
Intracapsular Rupture
On MG, intracapsular rupture appears as progressive
contour deformity and undulation of implant shell
(Figure 9).[3] USG can also demonstrate a ‘stepladder
sign’, due to the collapsed and infolded elastomer shell
producing multiple thin echogenic lines parallel to the
probe surface, which is equivalent to the ‘linguine sign’
on MRI.[3]
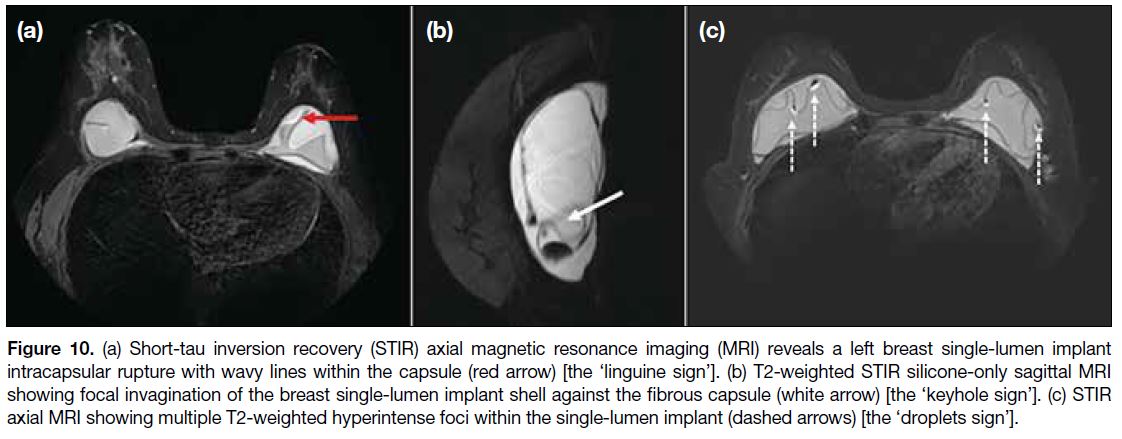
The ‘linguine sign’ on MRI is characterised by hypointense wavy lines inside the fibrous capsule
(Figure 10a).[16] The ‘keyhole sign’ shows silicone on
both sides of the implant (Figure 10b).[3] The ‘droplet sign’ is seen when there are saline drops in the silicone
gel as a result of intracapsular rupture, presenting as
small, hyperintense foci within the silicone gel on T2-weighted MRI (Figure 10c). The presence of the droplet
sign alone is not enough to confirm intracapsular rupture
but should alert the interpreter to that possibility.[12] [17]
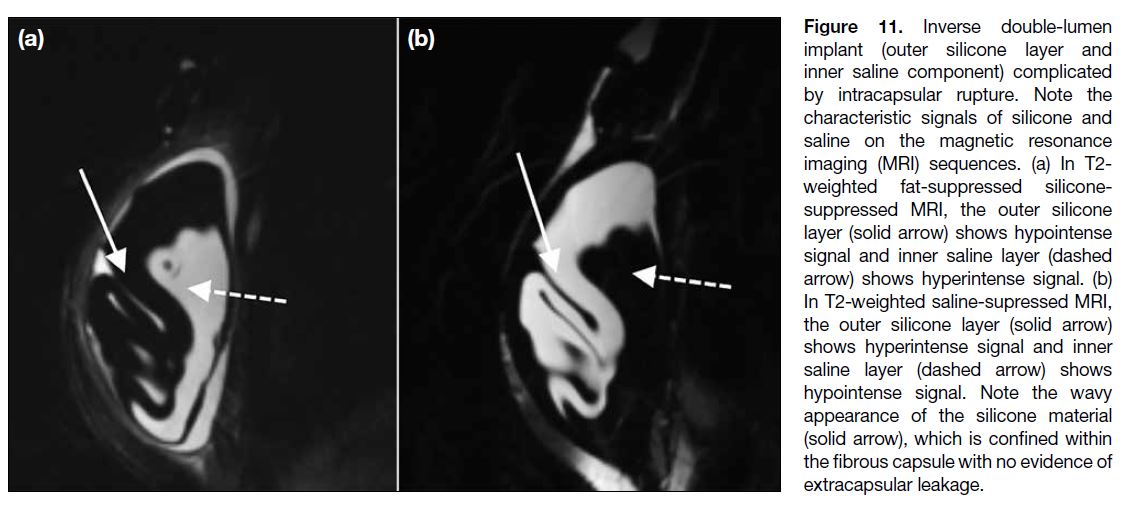
Double-lumen implants can also undergo intracapsular
rupture as demonstrated in Figure 11.
Figure 10. (a) Short-tau inversion recovery (STIR) axial magnetic resonance imaging (MRI) reveals a left breast single-lumen implant intracapsular rupture with wavy lines within the capsule (red arrow) [the ‘linguine sign’]. (b) T2-weighted STIR silicone-only sagittal MRI showing focal invagination of the breast single-lumen implant shell against the fibrous capsule (white arrow) [the ‘keyhole sign’]. (c) STIR axial MRI showing multiple T2-weighted hyperintense foci within the single-lumen implant (dashed arrows) [the ‘droplets sign’].
Figure 11. Inverse double-lumen
implant (outer silicone layer and
inner saline component) complicated
by intracapsular rupture. Note the
characteristic signals of silicone and
saline on the magnetic resonance
imaging (MRI) sequences. (a) In T2-weighted fat-suppressed silicone-suppressed
MRI, the outer silicone
layer (solid arrow) shows hypointense
signal and inner saline layer (dashed
arrow) shows hyperintense signal. (b)
In T2-weighted saline-supressed MRI,
the outer silicone layer (solid arrow)
shows hyperintense signal and inner
saline layer (dashed arrow) shows
hypointense signal. Note the wavy
appearance of the silicone material
(solid arrow), which is confined within
the fibrous capsule with no evidence of
extracapsular leakage.
Extracapsular Rupture
Extracapsular rupture means the implant material has migrated freely beyond the fibrous capsule into the
surrounding breast tissues via defect of the implant
shell and fibrous capsule.[17] It cannot occur without
intracapsular rupture. Therefore, on radiological
examination, features of extracapsular rupture are
usually found with the accompany sign of intracapsular
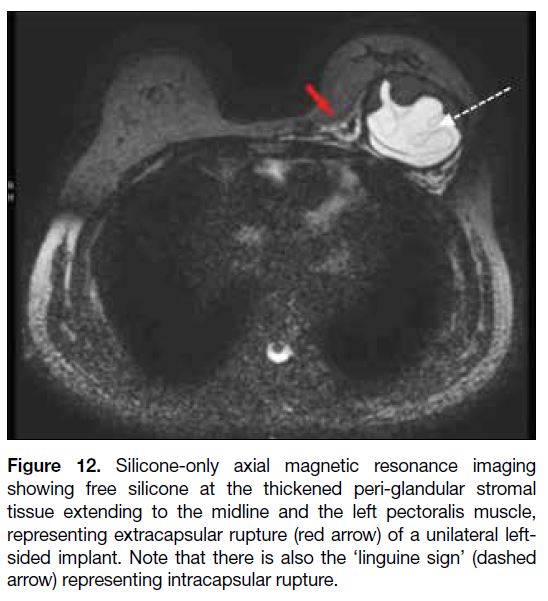
rupture.[18] This would be shown on images with free
silicone present outside the capsule as well as other
intracapsular rupture features (Figure 12). On USG, free
silicone would manifest as a moderately echogenic mass
with posterior echoic shadowing.
Figure 12. Silicone-only axial magnetic resonance imaging
showing free silicone at the thickened peri-glandular stromal tissue
extending to the midline and the left pectoralis muscle, representing
extracapsular rupture (red arrow) of a unilateral left-sided
implant. Note that there is also the ‘linguine sign’ (dashed arrow)
representing intracapsular rupture.
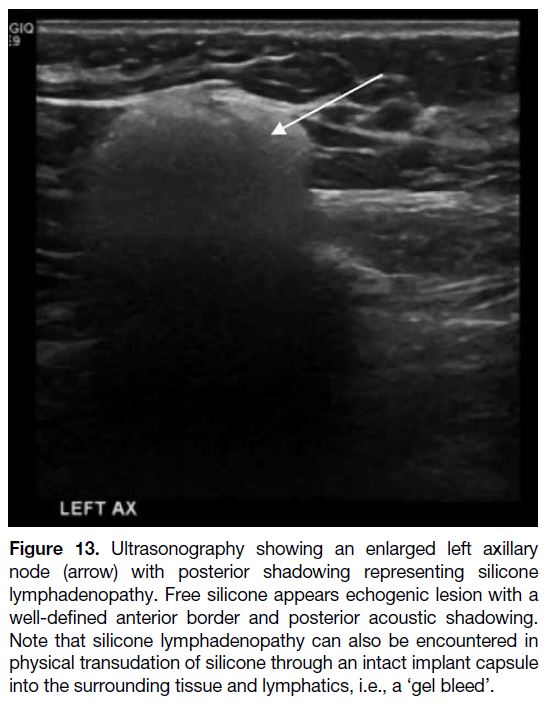
‘Gel Bleed’
A ‘gel bleed’ is defined as microscopic silicone
transudation through an intact implant shell. It is due to
the chemical affinity of the silicone gel for the silicone
elastomer of the implant shell.[19] [20] This would appear as extracapsular echogenic silicone (e.g., in the axilla or
more distant sites) on USG (Figure 13) and MRI with
posterior acoustic shadowing.[18]
Figure 13. Ultrasonography showing an enlarged left axillary
node (arrow) with posterior shadowing representing silicone
lymphadenopathy. Free silicone appears echogenic lesion with a
well-defined anterior border and posterior acoustic shadowing.
Note that silicone lymphadenopathy can also be encountered in
physical transudation of silicone through an intact implant capsule
into the surrounding tissue and lymphatics, i.e., a ‘gel bleed’.
Large Cell Lymphoma
Breast implant–associated anaplastic large cell
lymphoma is a rare complication of breast implant
augmentation, which would present as early as 3 months
to as late as 25 years after implantation.[21] Its incidence is
rare, estimated between 1:500,000 and 1:3,000,000.[22] Its
aetiology and pathogenesis remain poorly understood.[23]
While its clinical presentations are rather nonspecific, including pain, inflammation, breast asymmetry or
breast mass, up to 80% of cases present with peri-implant
effusion.[24] Should there any late-onset effusion (defined
as occurring >1 year of implantation) or breast mass
formation, further investigations should be performed,
including such as MRI and pathological analysis with flow cytometry.[25] [26] USG has a high sensitivity in
detecting the peri-implant effusion; however, it has
limited specificity. MG is not accurate for the diagnosis
of implant effusion or mass-forming breast implant—associated anaplastic large cell lymphoma. MRI can
detect peri-implant effusion and small peri-implant mass
lesions, which may not be visualised on USG.[26] Small amount (5-10 ml) of peri-implant fluid is considered normal, thus the presence of such amount is not representative of the overall condition.[26]
INJECTION AUGMENTATION
Injection augmentation is tissue filler injection into the breast tissue using a needle or cannula without a shell.
Various materials have been used as the fillers; the
three major ones include paraffin, liquid silicone, and
polyacrylamide hydrogel (PAAG).[27]
Paraffin
Paraffin is a purified mineral oil which was first used in vehicles for oil-soluble substances. Paraffin breast
augmentation was introduced in the early 20th century as
an alternative to other methods of breast augmentation.[27]
Despite its early promising cosmetic result, the
complications did not manifest until later stage. These
complications include paraffin migration, ‘paraffinoma’
formation, and foreign body reaction with fibrosis and
calcification and has been largely abandoned.[28] [29]
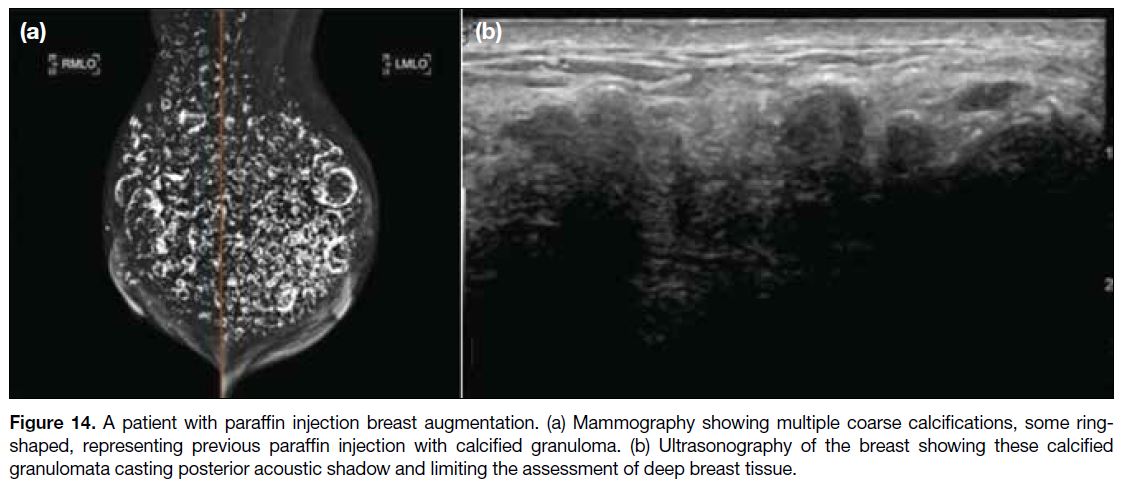
Within a few months after injection, the injected paraffin would be shown on MG as circumscribed and noncalcified
masses that were largely indistinguishable from
the surrounding glandular tissue. At later stages, rings
and other coarse calcifications usually develop and can
be manifested on MG and USG (Figure 14).[29]
Figure 14. A patient with paraffin injection breast augmentation. (a) Mammography showing multiple coarse calcifications, some ring-shaped,
representing previous paraffin injection with calcified granuloma. (b) Ultrasonography of the breast showing these calcified
granulomata casting posterior acoustic shadow and limiting the assessment of deep breast tissue.
Liquid Silicone
Free liquid silicone injection has been banned by the United States Food and Drug Administration since
1992 due to safety concerns.[30] Complications include
granulomatous reactions, nodule formation, and vascular
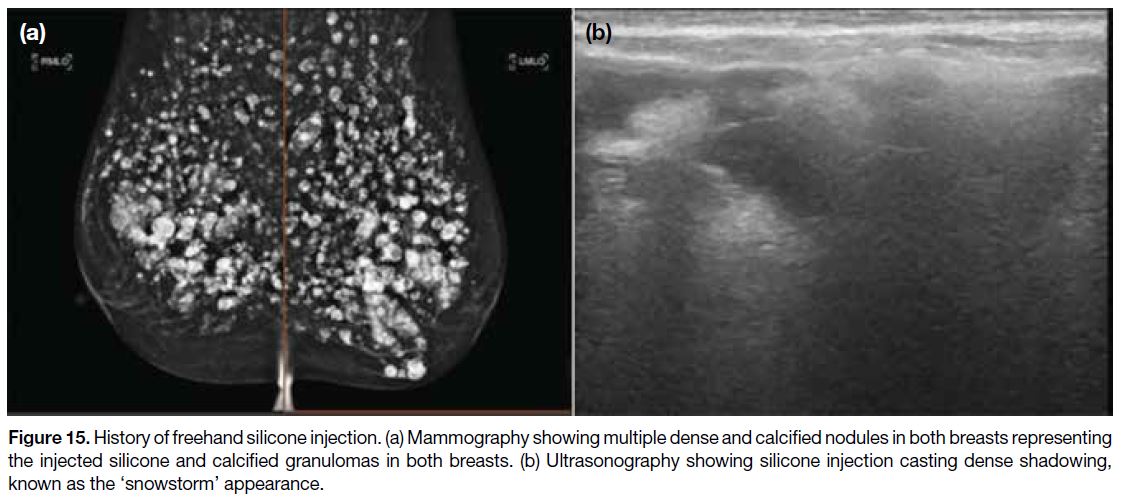
embolisation.[27] Free silicone manifests as multiple
extremely dense lobulated masses of various sizes
distributed over both breasts on MG, often accompanied
by calcified granulomas (Figure 15a). They also cast
dense shadowing known as the ‘snowstorm’ appearance
on USG (Figure 15b).[31]
Figure 15. History of freehand silicone injection. (a) Mammography showing multiple dense and calcified nodules in both breasts representing
the injected silicone and calcified granulomas in both breasts. (b) Ultrasonography showing silicone injection casting dense shadowing,
known as the ‘snowstorm’ appearance.
Polyacrylamide Hydrogel
PAAG is a non-resorbable sterile suspension made
with 2.5% acrylamide monomers and 97.5% water
that has been used for augmentation. It is injected
into the breast tissue, aiming to form a focal large
collection at subglandular layer to increase volume and
improve the shape of the breasts.[32] This would result in
loculated collection formation in subglandular breast
and may mimic saline bag implant augmentation on
MRI (Figure 16) in uncomplicated and non-displaced case.[32]
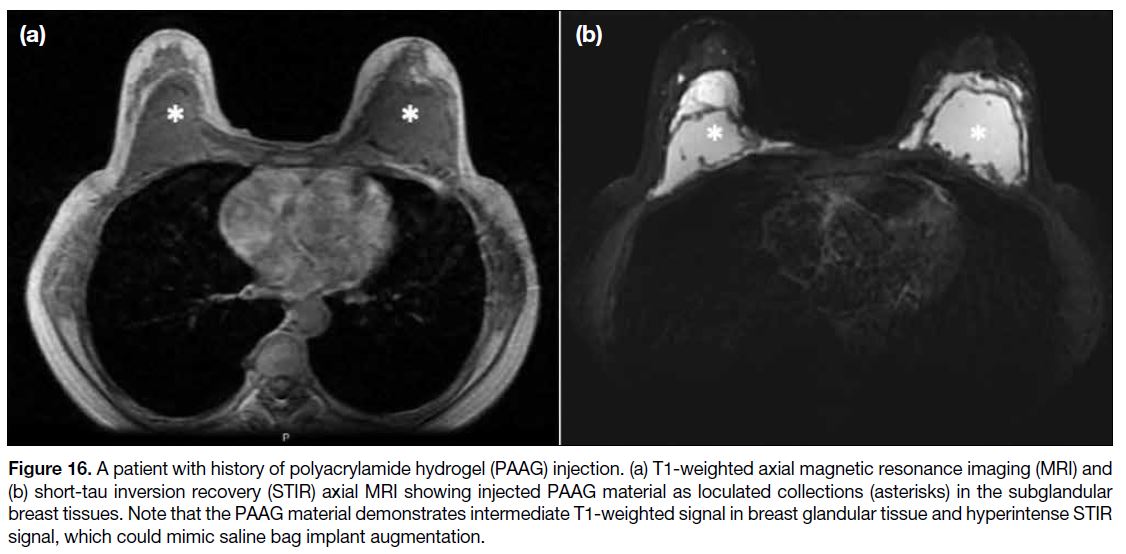
Figure 16. A patient with history of polyacrylamide hydrogel (PAAG) injection. (a) T1-weighted axial magnetic resonance imaging (MRI) and
(b) short-tau inversion recovery (STIR) axial MRI showing injected PAAG material as loculated collections (asterisks) in the subglandular breast tissues. Note that the PAAG material demonstrates intermediate T1-weighted signal in breast glandular tissue and hyperintense STIR signal, which could mimic saline bag implant augmentation.
However, the use of PAAG for breast augmentation has
not been approved by the United States Food and Drug
Administration and has been associated with several
complications including induration, lumps, haematoma,
infection, inflammation, persistent mastalgia, glandular
atrophy, gel migration, etc.[33] [34] There have also been
case reports of breast tumours being concealed by the
inflammatory reaction to PAAG, misdiagnosed as gel
collection.[35] [36] On radiological examination, the injected
PAAG material would appear as conglomerated, well-circumscribed
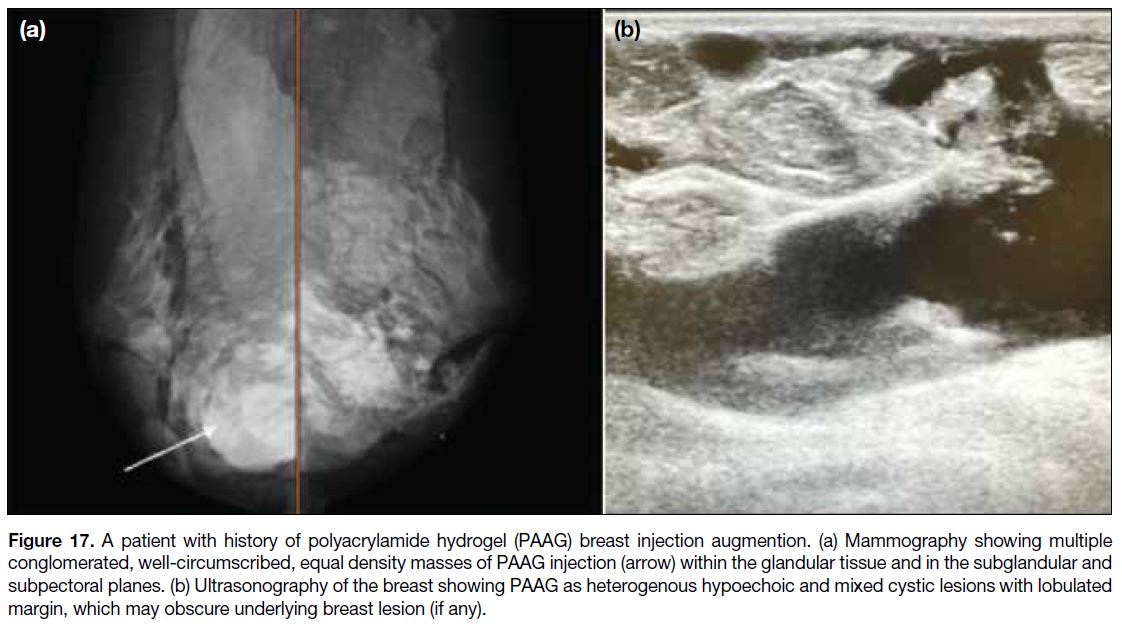
equal density masses on MG (Figure 17a), and variable-sized, complex solid and cystic or heterogeneous echoic masses on USG (Figure 17b).
Figure 17. A patient with history of polyacrylamide hydrogel (PAAG) breast injection augmention. (a) Mammography showing multiple
conglomerated, well-circumscribed, equal density masses of PAAG injection (arrow) within the glandular tissue and in the subglandular and subpectoral planes. (b) Ultrasonography of the breast showing PAAG as heterogenous hypoechoic and mixed cystic lesions with lobulated margin, which may obscure underlying breast lesion (if any).
Complication
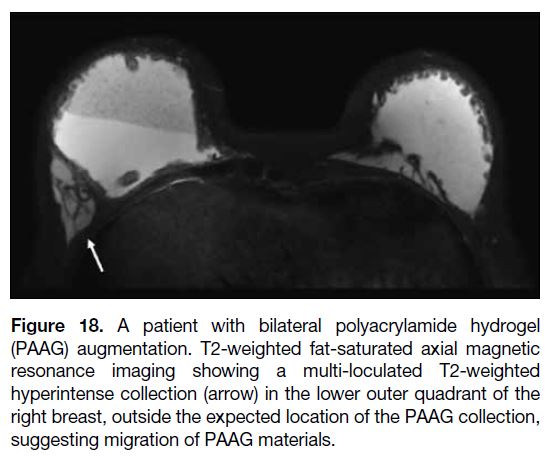
Injection material migration may be seen as an
asymmetrical appearance with the filling material
displaced from its normal position (Figure 18).
Figure 18. A patient with bilateral polyacrylamide hydrogel
(PAAG) augmentation. T2-weighted fat-saturated axial magnetic
resonance imaging showing a multi-loculated T2-weighted
hyperintense collection (arrow) in the lower outer quadrant of the
right breast, outside the expected location of the PAAG collection,
suggesting migration of PAAG materials.
GENERAL COMPLICATIONS
Infection
Infection is one of the general complications that can
occur after injection breast augmentation, with a reported
rate up to 2.9% in breast aesthetic augmentation or
even a higher rate from 1% to 53% in post-mastectomy
reconstruction. Patients present with mastalgia, fever,
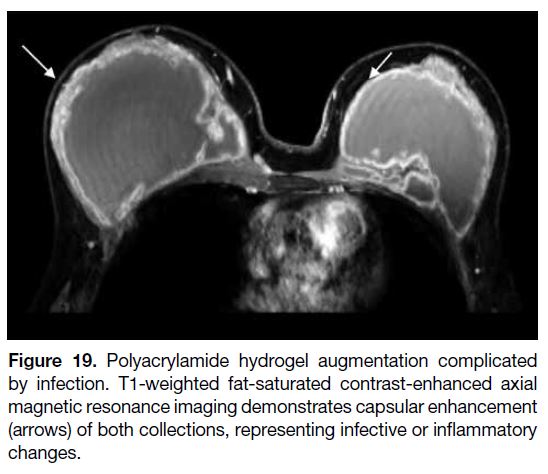
erythema, or discharge.[37] [38] [39] [40] Radiologically, infection can present as an irregular hypoechoic fluid collection
with internal debris on USG, while MRI features include
skin thickening, oedema, enhancement (Figure 19), and
complex fluid collections.[10]
Figure 19. Polyacrylamide hydrogel augmentation complicated
by infection. T1-weighted fat-saturated contrast-enhanced axial
magnetic resonance imaging demonstrates capsular enhancement
(arrows) of both collections, representing infective or inflammatory
changes.
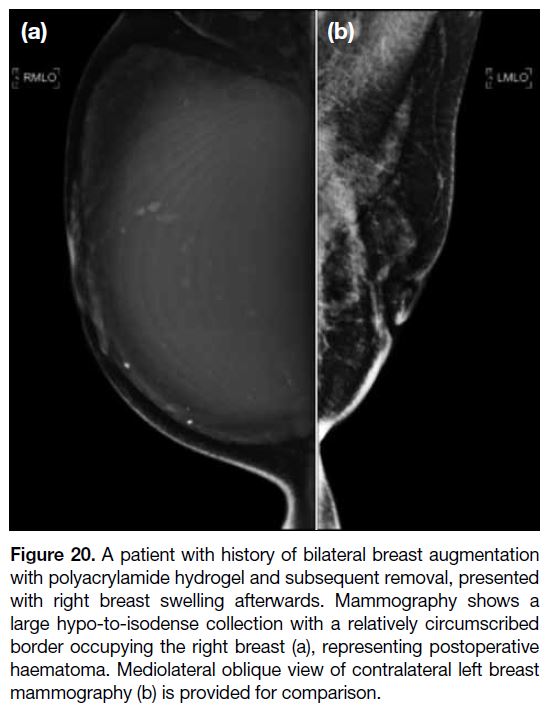
Haematoma Formation
Hematomas can be an early (perioperative period)
presentation or delayed presentation (rare, caused by
trauma, infection and coagulopathy).[41] This can be manifested as progressive breast swelling. On MG,
haematomas appear as collections of different density
depending on the age of the blood products (Figure 20).
USG and MRI show complex blood product collections.[10]
Figure 20. A patient with history of bilateral breast augmentation
with polyacrylamide hydrogel and subsequent removal, presented
with right breast swelling afterwards. Mammography shows a
large hypo-to-isodense collection with a relatively circumscribed
border occupying the right breast (a), representing postoperative haematoma. Mediolateral oblique view of contralateral left breast mammography (b)
is provided for comparison.
CONCLUSION
As breast augmentations are becoming more common,
it is crucial for radiologists familiarise themselves with the radiological appearance of various breast implants
and injection augmentations and their associated
complications on different imaging modalities.
REFERENCES
1. Kaoutzanis C, Winocour J, Unger J, Gabriel A, Maxwell GP. The
evolution of breast implants. Semin Plast Surg. 2019;33:217-23. Crossref
2. Perry R, Quinn C, Traver F, Murthy K, editors. Silicones. In: Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology. 3rd ed. CRC Press; 2020. p 235. Crossref
3. Seiler SJ, Sharma PB, Hayes JC, Ganti R, Mootz AR, Eads ED, et al.
Multimodality imaging–based evaluation of single-lumen silicone
breast implants for rupture. Radiographics. 2017;37:366-82. Crossref
4. Ching AH, Lim K, Sze PW, Ooi A. Quality of life, pain of prepectoral and subpectoral implant-based breast reconstruction with a discussion on cost: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2022;75:2550-60.
Crossref
5. Mégevand V, Scampa M, McEvoy H, Kalbermatten DF, Oranges CM. Comparison of outcomes following prepectoral and subpectoral implants for breast reconstruction: systematic review and meta-analysis. Cancers (Basel). 2022;14:4223.
Crossref
6. Li Y, Xu G, Yu N, Huang J, Long X. Prepectoral versus subpectoral
implant-based breast reconstruction: a meta-analysis. Ann Plast
Surg. 2020;85:437-47. Crossref
7. Sigalove S, Maxwell GP, Sigalove NM, Storm-Dickerson TL, Pope N, Rice J, et al. Prepectoral implant-based breast reconstruction and postmastectomy radiotherapy: short-term outcomes. Plast Reconstr Surg Glob Open. 2017;5:e1631. Crossref
8. Avendaño AL. Literature review on the different types of breast implants: advantages and disadvantages. Int J Med Sci Clin Res Stud. 2023;3:990-4. Crossref
9. Bachour Y, Bargon CA, de Blok CJ, Ket JC, Ritt MJ, Niessen FB. Risk factors for developing capsular contracture in women after breast implant surgery: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2018;71:e29-48. Crossref
10. Shah AT, Jankharia BB. Imaging of common breast implants and implant-related complications: a pictorial essay. Indian J Radiol Imaging. 2016;26:216-25. Crossref
11. Pagani A, Aitzetmüller MM, Larcher L. A forgotten entity following breast implant contracture: does baker need a change? Arch Plast Surg. 2022;49:360-4. Crossref
12. Juanpere S, Perez E, Huc O, Motos N, Pont J, Pedraza S. Imaging of breast implants—a pictorial review. Insights Imaging. 2011;2:653-70. Crossref
13. Oulharj S, Pauchot J, Tropet Y. PIP breast implant removal: a study of 828 cases. J Plast Reconstr Aesthet Surg. 2014;67:302-7. Crossref
14. Herborn CU, Marincek B, Erfmann D, Meuli-Simmen C, Wedler V, Bode-Lesniewska B, et al. Breast augmentation and reconstructive surgery: MR imaging of implant rupture and malignancy. Eur Radiol. 2002;12:2198-206. Crossref
15. Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg. 2017;6:163-8. Crossref
16. Safvi A. Linguine sign. Radiology. 2000;216:838-9. Crossref
17. Wong T, Lo LW, Fung PY, Lai HY, She HL, Ng WK, et al. Magnetic resonance imaging of breast augmentation: a pictorial review. Insights Imaging. 2016;7:399-410. Crossref
18. Swezey E, Shikhman R, Moufarrege R. Breast Implant Rupture. StatPearls. Treasure Island (FL); 2023.
19. Yang N, Muradali D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am J Roentgenol. 2011;196:W451-60. Crossref
20. Wiedenhoefer JF, Shahid H, Dornbluth C, Otto P, Kist K. MR imaging of breast implants: useful information for the interpreting radiologist. Appl Radiol. 2015;44:18-24. Crossref
21. Lazzeri D, Agostini T, Bocci G, Giannotti G, Fanelli G, Naccarato AG, et al. ALK-1–negative anaplastic large cell lymphoma associated with breast implants: a new clinical entity. Clin Breast Cancer. 2011;11:283-96. Crossref
22. Ducastel N, Cimpean IM, Theate I, Vanhooteghem O. Breast erythema and nodular skin metastasis as the first manifestation of breast implant–associated anaplastic large cell lymphoma. Rare Tumors. 2021;13:20363613211028498. Crossref
23. Hwang MJ, Brown H, Murrin R, Momtahan N, Sterne GD. Breast
implant–associated anaplastic large cell lymphoma: a case report
and literature review. Aesthetic Plast Surg. 2015;39:391-5. Crossref
24. Galván JR, Cordera F, Arrangoiz R, Paredes L, Pierzo JE. Breast
implant–associated anaplastic large cell lymphoma presenting as
a breast mass: a case report and literature review. Int J Surg Case
Rep. 2023;108:108482. Crossref
25. Adrada BE, Miranda RN, Rauch GM, Arribas E, Kanagal-Shamanna R, Clemens MW, et al. Breast implant–associated anaplastic large cell lymphoma: sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. 2014;147:1-14. Crossref
26. Sharma B, Jurgensen-Rauch A, Pace E, Attygalle AD, Sharma R, Bommier C, et al. Breast implant–associated anaplastic large cell lymphoma: review and multiparametric imaging paradigms. Radiographics. 2020;40:609-28. Crossref
27. Peters W, Fornasier V. Complications from injectable materials
used for breast augmentation. Can J Plast Surg. 2009;17:89-96. Crossref
28. Kim HJ, Lee SJ, Lee JH, Shin SH, Kim SH, Kim JH, et al.
Breast reconstruction after complications following breast
augmentation with massive filler injections. Medicine (Baltimore).
2020;99:e21516. Crossref
29. Erguvan-Dogan B, Yang WT. Direct injection of paraffin into the
breast: mammographic, sonographic, and MRI features of early
complications. AJR Am J Roentgenol. 2006;186:888-94. Crossref
30. Schenone GE, Riera DN, Fontbona M, Triana L.US FDA safety
communication on illegal use of injectable silicone for body
contouring and associated health risks. Aesthetic Plast Surg.
2023;47:1232-3. Crossref
31. Samreen N, Glazebrook KN, Bhatt A, Venkatesh SK, McMenomy BP, Chandra A, et al. Imaging findings of mammary and systemic silicone deposition secondary to breast implants. Br J Radiol. 2018;91:20180098. Crossref
32. Lui CY, Ho CM, Iu PP, Cheung WY, Lam HS, Cheng MS, et al.
Evaluation of MRI findings after polyacrylamide gel injection for
breast augmentation. AJR Am J Roentgenol. 2008;191:677-88. Crossref
33. Qian B, Xiong L, Guo K, Wang R, Yang J, Wang Z, et al.
Comprehensive management of breast augmentation with
polyacrylamide hydrogel injection based on 15 years of experience:
a report on 325 cases. Ann Transl Med. 2020;8:475. Crossref
34. Winter J, Shiga S, Islur A. The complications of polyacrylamide
hydrogel augmentation mammoplasty: a case report and review of
the literature. Plas Surg Case Stud. 2017;3:2513826X17693821. Crossref
35. Cheng NX, Liu LG, Hui L, Chen YL, Xu SL. Breast cancer
following augmentation mammaplasty with polyacrylamide
hydrogel (PAAG) injection. Aesthetic Plast Surg. 2009;33:563-9. Crossref
36. Zhao Y, Yuan NA, Li K, Geng YI, Zhou H, Wang H, et al.
Bilateral breast cancer following augmentation mammaplasty
with polyacrylamide hydrogel injection: a case report. Oncol Lett.
2015;9:2687-93. Crossref
37. Cohen JB, Carroll C, Tenenbaum MM, Myckatyn TM. Breast
implant–associated infections: the role of the national surgical
quality improvement program and the local microbiome. Plast
Reconstr Surg. 2015;136:921-9. Crossref
38. Washer LL, Gutowski K. Breast implant infections. Infect Dis Clin
North Am. 2012;26:111-25. Crossref
39. Mesa F, Cataño S, Tuberquia O. Study of infections in breast
augmentation surgery with implants in 9,691 patients over 5 years.
Plast Reconstr Surg Glob Open. 2021;9:e3752. Crossref
40. Rubino C, Brongo S, Pagliara D, Cuomo R, Abbinante G,
Campitiello N, et al. Infections in breast implants: a review with a
focus on developing countries. J Infect Dev Ctries. 2014;8:1089-95. Crossref
41. Lee JH, Hong HK, Kim WH, Kim HJ, Lee J, Park HY, et al.
Delayed unilateral hematoma after reconstructive and aesthetic
breast surgery with implants in Asian patients: two case reports.
Gland Surg. 2021;10:1515-22. Crossref