Transcatheter Arterial Embolisation of Renal Angiomyolipomas Using an Ethanol-Lipiodol Mixture
ORIGINAL ARTICLE
Hong Kong J Radiol 2024;27:Epub 15 November 2024
Transcatheter Arterial Embolisation of Renal Angiomyolipomas Using an Ethanol-Lipiodol Mixture
HL Chan, KH Wong, KS Tam, YY Man, SW Sim, PSF Lee
Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr HL Chan, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: hollischanrad@gmail.com
Submitted: 19 June 2023; Accepted: 18 January 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. HLC, KST and YYM acquired the data. HLC, KHW, SWS and PSFL analysed the data. HLC, KHW, KST and YYM drafted the manuscript. SWS and PSFL critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: 2023.207). The requirement for informed patient consent was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
This study aimed to evaluate the outcomes of transcatheter arterial embolisation (TAE) of renal
angiomyolipoma (AML) with a mixture of ethanol and Lipiodol, and to identify the factors predicting treatment response.
Methods
We performed a retrospective review of all patients who underwent elective TAE of renal AML using
ethanol and Lipiodol over a 7-year period at our institution. Patient demographics, the presence or absence of the
tuberous sclerosis complex, renal AML tissue components, pre- and post-procedure renal AML volumes, procedure
details, and clinical course were documented.
Results
We identified 32 patients (25 females and 7 males, mean age = 55.2 years) who underwent elective TAE for
renal AMLs. All cases showed technical success; one major complication without the need for escalated management
(3.1%) was identified. The mean volume reduction of renal AML after TAE was 59.8% (standard deviation = 25%)
with a mean imaging follow-up duration of 23.8 months. A predominance of angiomyogenic components of the lesion
was significantly associated with good treatment response (p = 0.002).
Conclusion
TAE of renal AML using a mixture of ethanol and Lipiodol is an effective and safe treatment option
that significantly reduces AML volume. Predominance of angiomyogenic components of an AML predicts significant
AML volume reduction after the procedure.
Key Words: Angiomyolipoma; Embolization, therapeutic; Kidney; Tuberous sclerosis
中文摘要
使用乙醇─碘化油混合物經導管動脈栓塞腎臟血管平滑肌脂肪瘤
陳凱玲、黃健開、譚健成、文欣欣、沈兆華、李醒芬
引言
本研究旨在評估使用乙醇和碘化油混合物進行腎臟血管平滑肌脂肪瘤(AML)經導管動脈栓塞(TAE)的效果,並了解預測治療反應的因素。
方法
我們對本機構7年來使用乙醇和碘化油接受選擇性TAE治療腎臟AML的所有患者進行回顧性分析。我們記錄了患者的基本資料、是否有結節性硬化症、腎臟AML組織成分、術前和術後腎臟AML體積、手術細節和臨床病程。
結果
本研究共有32名因腎臟AML接受選擇性 TAE 的患者(25名女性和7名男性,平均年齡 = 55.2歲)。所有案例均顯示技術成功,有一例無需升級處理的重要併發症(3.1%)。TAE後腎臟AML的平均體積減少為59.8%(標準差 = 25%),平均影像學追蹤時間 23.8 個月後。病變的血管肌生成成分佔主導與良好治療反應有顯著相關(p = 0.002)。
結論
使用乙醇和碘化油混合物治療腎臟AML是一種有效且安全的治療選擇,可顯著減少AML體積。 AML 的血管肌生成成分佔主導地位預示手術後AML體積顯著減少。
INTRODUCTION
Renal angiomyolipoma (AML) is a benign mesenchymal
neoplasm of the kidney. It is broadly classified into two
types, either sporadic (80%) or associated with tuberous
sclerosis complex (TSC) [20%] which is an autosomal
dominant disease with multisystem involvement.[1] [2]
AMLs are composed of an angiomyogenic component
(blood vessels and smooth muscle) and a lipomatous
component (fat).[3] The diagnosis of renal AML is based
on the presence of macroscopic fat.[4] [5] The differential
diagnosis includes fat- and calcification-containing
renal cell carcinomas, which are unusual.[6] [7] Therefore,
a fat-containing renal lesion without calcifications or
other atypical features can usually be diagnosed as AML
based on its radiological features.
The major complication of AML is spontaneous
tumoural rupture leading to retroperitoneal haemorrhage
into the subcapsular and perirenal space (Wunderlich
syndrome), which can be life-threatening.[8] Risk factors
for tumour rupture include large size, multifocality,
and aneurysm formation.[9] [10] Once the greatest tumour
dimension is >4 cm, there is a greater incidence of
symptoms which include bleeding and flank pain.[9]
In our institution, transcatheter arterial embolisation
(TAE) is employed for the treatment of AML in acute
haemorrhage due to spontaneous rupture and high-risk
AMLs that are considered suitable for prophylactic treatment (i.e., lesion dimension >4 cm and/or tumoural
aneurysm ≥5 mm) with multidisciplinary agreement
for selected cases. There is increasing use of TAE as
prophylactic treatment for non-ruptured AMLs, giving
its advantages of a low complication rate, avoiding
general anaesthesia, less invasiveness, renal function
preservation, and satisfactory outcome,[11] [12] [13] defined as
absence of residual tumoural stain on digital subtraction
angiography (DSA).[14] [15]
There are multiple embolisation agent options. In our institute, we use a 2:1 mixture of ethanol and Lipiodol.
Our study aimed to identify the outcome of TAE of renal
AML using this mixture, by documentation of the lesion
volume reduction and any complications. We also aimed
to identify any tumoural factors associated with volume
reduction after embolisation.
METHODS
We retrospectively identified all consecutive cases of renal AML TAE at North District Hospital in Hong Kong
from January 2016 to December 2022 by reviewing
the electronic records of the radiology department.
Cases which ethanol and Lipiodol were not used as the
embolic agent were excluded. Cases of urgent TAE
due to presentation with acute haemorrhage were also
excluded as the primary aim for this group of patients is
haemostasis instead of volume reduction of the lesion.
Patient demographics and clinical data were reviewed
in the electronic health records, including sex, age,
presence or absence of TSC, presenting symptom, renal
function tests (before and after TAE), procedure details,
and hospitalisation record.
AML volume and percentage reduction were assessed
from the latest computed tomography (CT) or
ultrasound (US) before and after embolisation. The
tissue components of each AML were determined on
the latest pre-embolisation CT by consensus from three
radiologists (with 12, 5, and 4 years of experience,
respectively), who were blinded to clinical details and
classified each AML as predominantly angiomyogenic
(>50% soft tissue and/or blood vessel components) or
predominantly lipomatous (>50% fat components). AML
volume was calculated based on the x-, y-, and z-axis
diameters (i.e., width, height, and length, respectively)
on CT or US examination, with the equation to calculate
the ellipsoid volume of v = xyzπ/6.
Embolisation Technique
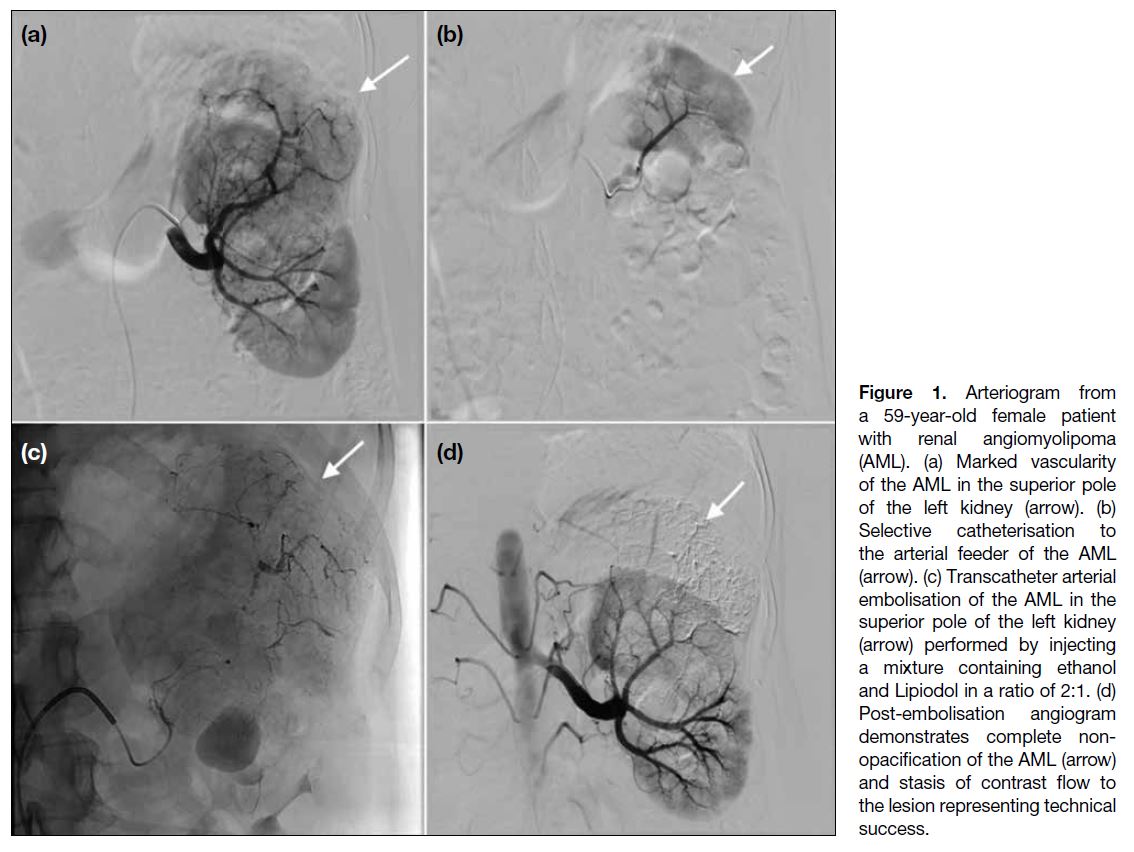
Figure 1 illustrates the embolisation procedure. TAE
was performed through the common femoral artery with
a 5-Fr angiographic catheter. Flush aortography and
renal arteriography were first performed using DSA to
identify the location and number of renal arteries and
the arterial supply to the AML (Figure 1a). The arterial
feeder to the AML was selectively catheterised (Figure 1b). TAE was performed (Figure 1c) by injecting the
ethanol-Lipiodol mixture into the arterial feeder with a
1-mL syringe intermittently. Then, renal arteriography
was performed to confirm that there were no residual
arterial feeders to the lesion. Angiographic success
was achieved when vascular stasis and the absence
of visible arterial feeding vessels resulted (Figure 1d). The total volume of mixture injected depended
on the endpoint of angiographic stasis and absence or
occlusion of other feeders, and should not reach the
maximum volume allowed according to the patient’s
weight.
Figure 1. Arteriogram from
a 59-year-old female patient
with renal angiomyolipoma
(AML). (a) Marked vascularity
of the AML in the superior pole
of the left kidney (arrow). (b)
Selective catheterisation to
the arterial feeder of the AML
(arrow). (c) Transcatheter arterial
embolisation of the AML in the
superior pole of the left kidney
(arrow) performed by injecting
a mixture containing ethanol
and Lipiodol in a ratio of 2:1. (d)
Post-embolisation angiogram
demonstrates complete non-opacification
of the AML (arrow)
and stasis of contrast flow to
the lesion representing technical
success.
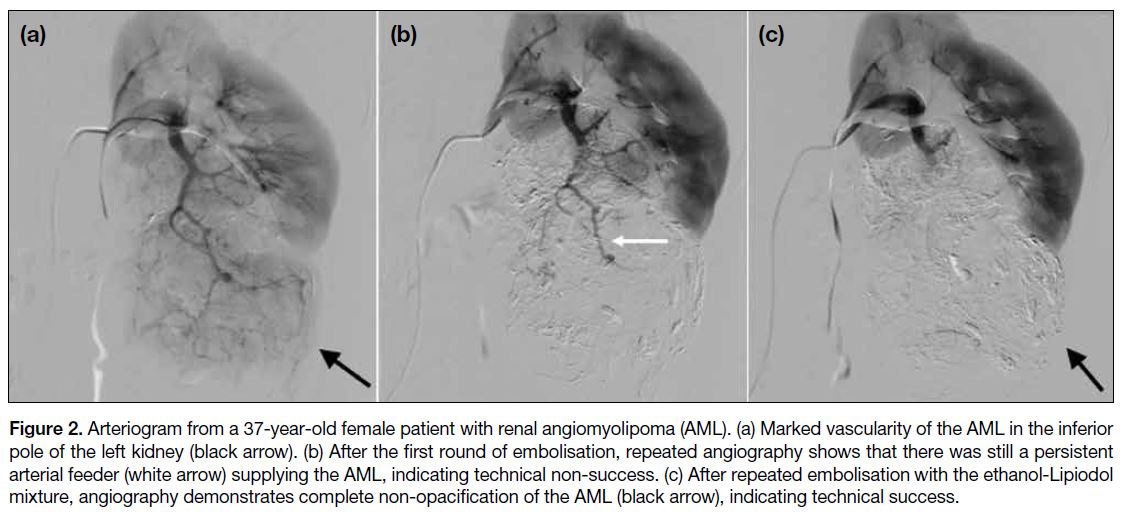
Figure 2 illustrates technical non-success in the first
round of TAE, with technical success after repeated
embolisations. The angiographic catheter was then
removed, and haemostasis was achieved by manual
compression. After haemostasis at the puncture site was
achieved, patients were transferred to the urology ward
for observation and were usually discharged the day after
the procedure if no major complications occurred.
Figure 2. Arteriogram from a 37-year-old female patient with renal angiomyolipoma (AML). (a) Marked vascularity of the AML in the inferior
pole of the left kidney (black arrow). (b) After the first round of embolisation, repeated angiography shows that there was still a persistent
arterial feeder (white arrow) supplying the AML, indicating technical non-success. (c) After repeated embolisation with the ethanol-Lipiodol
mixture, angiography demonstrates complete non-opacification of the AML (black arrow), indicating technical success.
Technical success, non-target embolisations, and major and minor complications were defined according to the
quality improvement guidelines for TAE by the Society
of Interventional Radiology (SIR) Standards of Practice
Committee.[15] Technical success was defined as absence
of residual tumoural stain on complete DSA. Major
complications were defined as those events that resulted
in prolonged hospitalisation, permanent renal damage,
transfusion, or death. Minor complications included
events that may have caused patient discomfort or some
morbidity but did not meet the criteria for major adverse
events.
Follow-up
Patients were followed up in the urology outpatient clinic
of our hospital, with follow-up radiological examination
including CT or US. Recurrence was defined as an
increase in tumour volume on follow-up imaging and/or recurrent symptoms that required repeated TAE of the
previously embolised tumour after 6 months of follow-up.
Tumoural volume reduction of >50% after TAE was considered a good response, whereas reduction of ≤50%
after TAE was considered a poor response.
Statistical Analysis
Statistical analysis was performed using commercial
software SPSS (Windows version 26.0; IBM Corp,
Armonk [NY], United States). Categorical variables
were expressed as frequencies and percentages, and
quantitative data were expressed as mean ± standard
deviation (SD). Fisher’s exact test was used to compare
the categorical variables, and an independent sample
t test was used to compare the continuous variables
between groups. Pre- and post-procedure AML size and
patient’s creatinine levels were compared using a paired
t test. A p value of < 0.05 were considered to indicate
statistical significance.
RESULTS
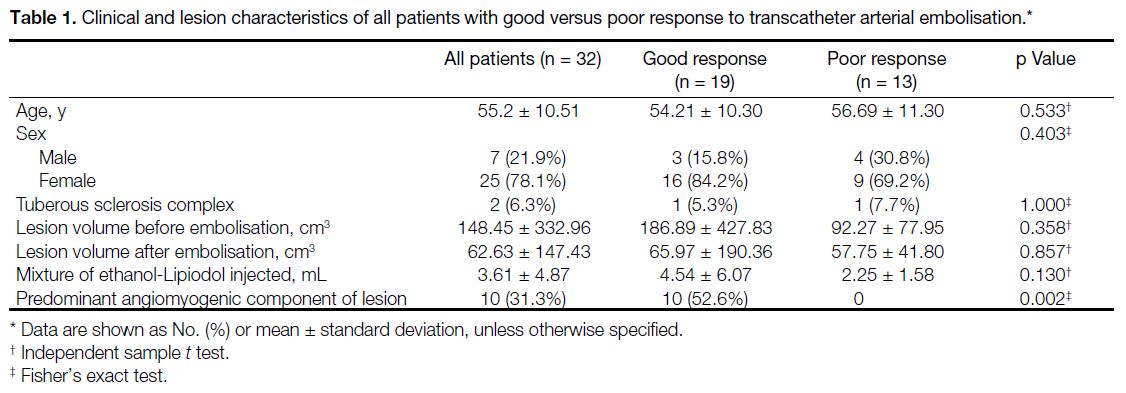
We identified 32 patients eligible for the study, with a mean age of 55.2 years (range, 30-73). Among these
patients, 25 (78.1%) were female and seven (21.9%)
were male. Two (6.3%) patients were diagnosed with
TSC (Table 1).
Table 1. Clinical and lesion characteristics of all patients with good versus poor response to transcatheter arterial embolisation.
A mean volume of 3.61 mL (SD = 4.87) of the ethanol-Lipiodol mixture was injected for each AML. The average
procedure time was 53.8 minutes (range, 22-143), with
the starting time and ending time defined by the first and
last fluoroscopy images. Clinical characteristics of the
patients are summarised in Table 1.
All the cases (100%) were technically successful,
which was greater than the suggested threshold of 90%
according to the SIR reporting standards.[15] A paired t test
was conducted to determine the effect of TAE on each
patient’s serum creatinine level. The results indicated
no significant difference between the creatinine level
before and after TAE (mean ± SD = 63.67 ± 12.56
μmol/L vs. 64.8 ± 13.34 μmol/L; p = 0.342), suggesting
no significant renal function impairment attributable to
embolisation in this series.
Complications were classified as major and minor.[15]
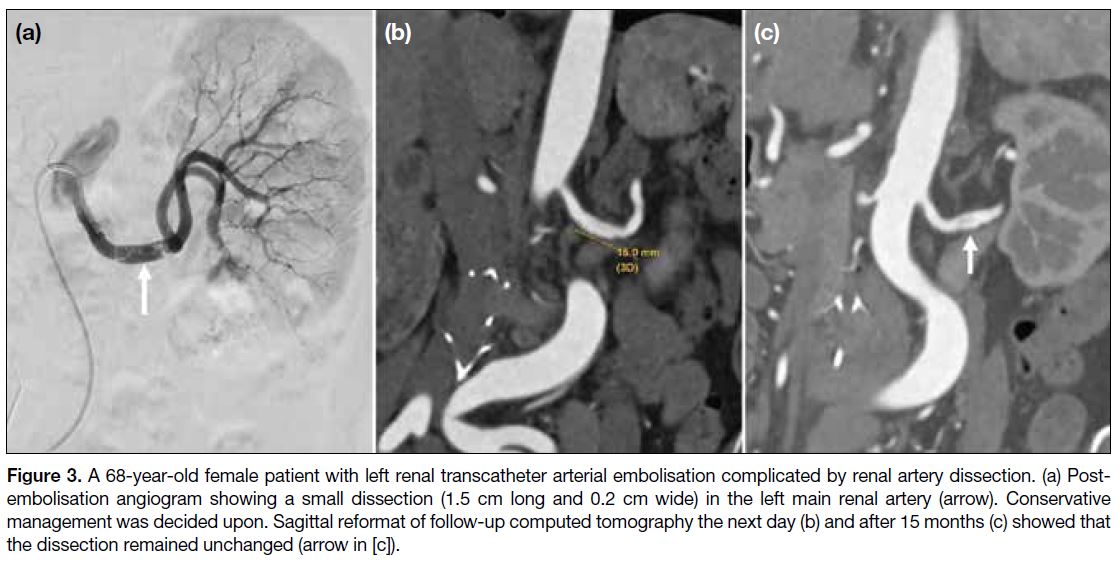
Only one patient had a major complication, with a small
dissection (1.5 cm in length and 0.2 cm in thickness) at the middle part of the left renal artery identified on postprocedure
angiogram (Figure 3a). A total of 4000 IU
intra-arterial heparin was injected immediately. Follow-up
CT showed the small dissection of the left renal artery
(Figure 3b). After discussion with the urologist and in
view of the small size of the dissection, conservative
management was decided upon. On repeated follow-up
CT 15 months after the procedure, the dissection remained
static (Figure 3c). The overall major complication rate
was 3.1%, which was lower than the suggested threshold
of 6% according to the SIR reporting standards.[15] Four
(12.5%) patients had minor complications which were
all cases of post-embolisation syndrome[16] characterised
by fever and pain.
Figure 3. A 68-year-old female patient with left renal transcatheter arterial embolisation complicated by renal artery dissection. (a) Post-embolisation
angiogram showing a small dissection (1.5 cm long and 0.2 cm wide) in the left main renal artery (arrow). Conservative
management was decided upon. Sagittal reformat of follow-up computed tomography the next day (b) and after 15 months (c) showed that
the dissection remained unchanged (arrow in [c]).
The mean hospitalisation days were 1.7 (SD = 1.6) after embolisation. Four (12.5%) patients had hospitalisations
>2 days, including the day of admission to the ward
before doing the procedure. Among these four patients,
one case was complicated by renal artery dissection
as discussed above, and three cases were complicated
by post-embolisation syndrome, which responded to
analgesics.
All 32 patients had follow-up CT or US with a mean
imaging follow-up duration of 23.8 months (SD = 18.7).
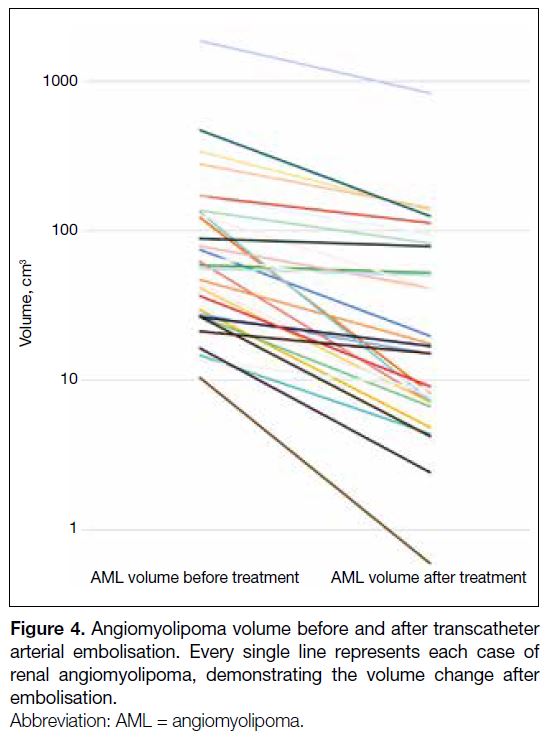
All AMLs showed reduction in volume with a mean of 59.8% (SD = 25%) observed (Figure 4). A paired t test indicated that there was a statistically significant
difference between AML size before and after TAE
(mean ± SD = 148.45 ± 332.96 cm3 vs. 62.63 ± 147.43
cm3; p = 0.015).
Figure 4. Angiomyolipoma volume before and after transcatheter arterial embolisation. Every single line represents each case of renal angiomyolipoma, demonstrating the volume change after embolisation.
The clinical and lesion characteristics were analysed to
explore the potential association with treatment response
(i.e., percentage of AML volume reduction). Table 1 summarises the clinical and lesion characteristics
with good versus poor response to TAE. Statistical
analysis revealed that a predominant angiomyogenic
component of AML was significantly associated with
good response to TAE (p = 0.002). Although the mean
of pre-TAE AML volume of the good response group
was greater than that of the poor response group, it
did not reach statistical significance (186.89 cm3 vs.
92.27 cm3; p = 0.358). Similarly, the mean volume
of ethanol-Lipiodol mixture injected was higher
in the good response group; however, this did not
reach statistical significance (4.54 mL vs. 2.25 mL;
p = 0.130). There were no significant differences in
patient age (p = 0.533), sex (p = 0.403), or TSC incidence
(p = 1.000) [Table 1].
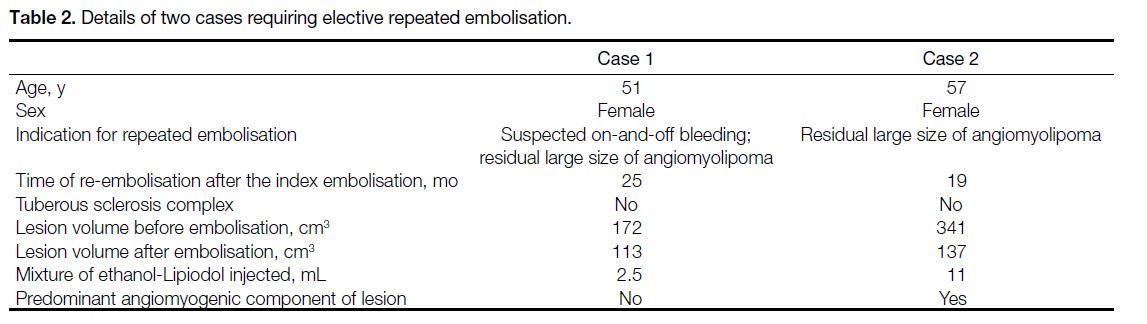
None of the cases required further urgent TAE for the same AML. Two (6.3%) patients had repeated elective
TAE for the same AML after multidisciplinary meeting
discussion. One was due to residual large size of the
lesion, while the other one was due to residual large size
as well as occasional flank pain suspected to be caused
by intermittent bleeding despite no active contrast
extravasation on serial CT. Details of the two cases are
summarised in Table 2.
Table 2. Details of two cases requiring elective repeated embolisation.
DISCUSSION
The study shows that TAE with an ethanol-Lipiodol
mixture is effective and safe in the prophylactic treatment of renal AML, with a high technical success rate in our
centre (100%) and a low major complication rate (3.1%).
Oesterling et al[9] reported the correlation of symptoms and AML size, showing that 82% of AML with
diameter >4 cm were symptomatic. Further evidence
from Yamakado et al[17] supported these findings, with
a lesion size >4 cm predicting the risk of rupture with
a sensitivity of 100% and specificity of 38%, and an
aneurysm size of 5 mm predicting the risk of rupture
with a sensitivity of 100% and specificity of 86%. In our
institution, we adopted these criteria (AML dimension
>4 cm or pseudo-aneurysm size ≥5 mm) for prophylactic
AML embolisation, and emergency embolisation for
those presenting with acute haemorrhage.
Various embolic agents have been used for embolisation
of AMLs, including pure ethanol, ethanol-Lipiodol
mixture, polyvinyl alcohol particles, absorbable gelatin
powder (Gelfoam; Pfizer Inc, New York [NY], United
States), N-butyl cyanoacrylate, and coils.[18] [19] [20] In our
centre, the ethanol-Lipiodol mixture is the preferred
agent for embolisation. Ethanol is a potent liquid embolic
agent that can cause permanent arterial thrombosis
and endothelial damage resultant in necrosis of viable
tissue,[21] whereas Lipiodol is iodised poppyseed oil,
being radiopaque under fluoroscopy, providing better
control of injection and preventing reflux.[22] The optimal
ratio of the ethanol-Lipiodol mixture has been discussed
in different literature, ranging from 2:1 to 4:1.[22] [23] [24] [25] [26] In an
animal study by Gao et al27, the effect and safety of TAE with various volume ratios of ethanol and Lipiodol in
a rabbit VX2 tumour model were investigated, showing
that the volume ratios of ethanol to Lipiodol from 1:2 to
4:1 were equally effective. The information gleaned from
these results could provide insight to future research on
the optimal ratio of ethanol-Lipiodol mixture in TAE of
human renal AML. In our centre, we adopted a ratio of
2:1 ethanol to Lipiodol after balancing and optimising the
therapeutic embolic effect and fluoroscopic visualisation.
The drawback of using ethanol is the potential risk of non-targeted embolisation and alcohol toxicity, which
include central nervous system depression, haemolysis,
and cardiac arrest. Monitoring for systemic toxicity
is crucial when the dose of ethanol >1 mL/kg.[24] In our
centre, we do not inject ethanol >1 mL/kg (i.e., 60 mL
of ethanol for a 60 kg adult). In our study, we injected
1 mL to 26.0 mL (mean = 3.61) of 2:1 ethanol-Lipiodol
mixture, which is below the limit.
The study also identified AML volume reduction
percentage associated with the dominant angiomyogenic
component, which was compatible with published
literature.[28] [29] This also correlates with the theory that
ethanol causes permanent arterial damage, and hence,
tumoural necrosis in the angiomyogenic component
of AML, and therefore the larger the angiogenic
component of the lesion, the better treatment response.
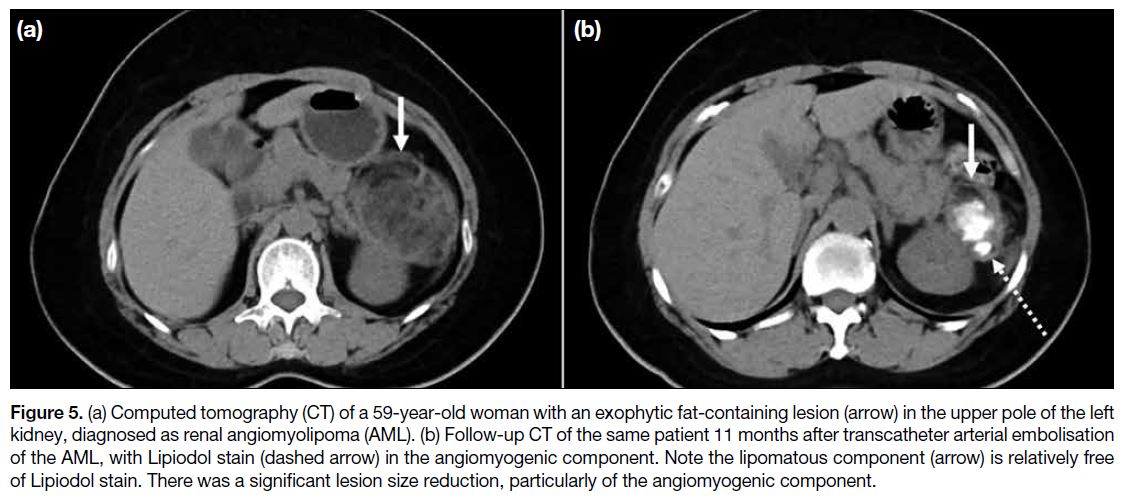
This is demonstrated in Figure 5 showing pre- and post-embolisation
CT, with Lipiodol predominantly staining
the angiomyogenic component of the AML.
Figure 5. (a) Computed tomography (CT) of a 59-year-old woman with an exophytic fat-containing lesion (arrow) in the upper pole of the left
kidney, diagnosed as renal angiomyolipoma (AML). (b) Follow-up CT of the same patient 11 months after transcatheter arterial embolisation
of the AML, with Lipiodol stain (dashed arrow) in the angiomyogenic component. Note the lipomatous component (arrow) is relatively free
of Lipiodol stain. There was a significant lesion size reduction, particularly of the angiomyogenic component.
Previous literature also reported initial AML volume correlates with post-embolisation volume reduction.[28] In
our study, we identified that the pre-embolisation AML
volume of the good response group was greater than that
of the poor response group; however, it did not reach
statistical significance (Table 1).
TSC-associated AML is known to develop at a younger
age and tends to exhibit a much faster growth rate
over time than sporadic AML. Multiple studies have
demonstrated that, in contrast to sporadic AMLs, TSC-associated
AMLs tend to regrow after TAE, with a
recurrence rate up to 60%.[30] [31] [32] Furthermore, TSC-related
AMLs tend to develop in both kidneys; therefore, medical
therapy would be required. In cases of asymptomatic
TSC-associated AMLs >3 cm in size, mammalian target
of rapamycin inhibitors are considered as the first-line
treatment.[33] However, in our study, we observed only
two unilateral cases of TSC, and none of the TSC-associated
AMLs required re-embolisation or showed
an increase in size during the follow-up imaging at 7 months and 49 months post-embolisation, respectively.
Nevertheless, further extended follow-up is necessary to
conclusively ascertain the absence of AML recurrence
or size escalation.
Limitations
We acknowledge this study’s limitations, such as its retrospective nature and the small and heterogeneous
population. The imaging follow-up periods were variable,
ranging from 3 to 77 months (mean = 23.8). Also, the
post-embolisation AML volume assessment was based
on CT or US, which could result in measurement
differences between the two imaging modalities.
CONCLUSION
TAE of renal AML using a mixture of ethanol and Lipiodol in 2:1 ratio is an effective and safe treatment option that
significantly reduces the AML volume. A predominant
angiomyogenic component of AML predicts significant
AML volume reduction after embolisation.
REFERENCES
1. Vos N, Oyen R. Renal angiomyolipoma: the good, the bad, and the ugly. J Belg Soc Radiol. 2018;102:41. Crossref
2. Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of renal angiomyolipoma. J Urol. 2016;195:834-46. Crossref
3. Hatano T, Egawa S. Renal angiomyolipoma with tuberous sclerosis
complex: how it differs from sporadic angiomyolipoma in both
management and care. Asian J Surg. 2020;43:967-72. Crossref
4. Gray RE, Harris GT. Renal cell carcinoma: diagnosis and management. Am Fam Physician. 2019;99:179-84.
5. Yang R, Wu J, Sun L, Lai S, Xu Y, Liu X, et al. Radiomics of small
renal masses on multiphasic CT: accuracy of machine learning-based
classification models for the differentiation of renal cell
carcinoma and angiomyolipoma without visible fat. Eur Radiol.
2020;30:1254-63. Crossref
6. Yousaf A, Nabi U, Hussein ML, Twair A, Gashir MB. Fat-containing
renal cell carcinoma mimicking angiomyolipoma: a
radiological and histopathological diagnostic challenge. Cureus.
2020;12:e6721. Crossref
7. Park BK. Renal angiomyolipoma based on new classification: how
to differentiate it from renal cell carcinoma. AJR Am J Roentgenol.
2019;212:582-8. Crossref
8. Parmar N, Langdon J, Kaliannan K, Mathur M, Guo Y, Mahalingam S.
Wunderlich syndrome: wonder what it is. Curr Probl Diagn Radiol.
2022;51:270-81. Crossref
9. Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The
management of renal angiomyolipoma. J Urol. 1986;135:1121-4. Crossref
10. Wang C, Li X, Peng L, Gou X, Fan J. An update on recent
developments in rupture of renal angiomyolipoma. Medicine
(Baltimore). 2018;97:e0497. Crossref
11. Muller A, Rouvière O. Renal artery embolization-indications, technical approaches and outcomes. Nat Rev Nephrol. 2015;11:288-301. Crossref
12. Nozadze G, Larsen SB, Heerwagen S, Juhl Jensen R, Lönn L,
Røder MA. Selective arterial embolization of renal angiomyolipomas:
a 10-year experience. BJUI Compass. 2021;3:86-92. Crossref
13. Murray TE, Lee MJ. Are we overtreating renal angiomyolipoma:
a review of the literature and assessment of contemporary
management and follow-up strategies. Cardiovasc Intervent Radiol.
2018;41:525-36. Crossref
14. Lee S, Park HS, Hyun D, Cho SK, Park KB, Shin SW, et al.
Radiologic and clinical results of transarterial ethanol embolization
for renal angiomyolipoma. Eur Radiol. 2021;31:6568-77. Crossref
15. Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC,
et al. Quality improvement guidelines for percutaneous transcatheter
embolization: Society of Interventional Radiology Standards of
Practice Committee. J Vasc Interv Radiol. 2010;21:1479-86. Crossref
16. Tsuchiya S, Saiga A, Yokota H, Kubota Y, Wada T, Akutsu A,
et al. Prophylactic steroids for preventing postembolization syndrome
after transcatheter arterial embolization of renal angiomyolipoma: a
comparative study. Interv Radiol (Higashimatsuyama). 2023;8:1-6. Crossref
17. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225:78-82. Crossref
18. Chapman D, Tyson M, Buckley B. Single-institution, retrospective
review of elective and emergency embolization of renal
angiomyolipoma. Can Urol Assoc J. 2021;15:E598-602. Crossref
19. Ahmadov J, Çay F, Eldem G, Akdoğan B, Bilen CY, Aki FT,
et al. Endovascular management of renal angiomyolipomas: do
coils have a benefit in terms of clinical success rates? Diagn Interv
Radiol. 2022;28:597-602. Crossref
20. Kocakgol DO, Cayli E, Oguz S, Dinc H. Selective arterial
embolization of giant renal angiomyolipoma associated with
tuberous sclerosis complex using particular and liquid embolic
agents. Eurasian J Med. 2018;50:130-3. Crossref
21. Le Daré B, Gicquel T. Therapeutic applications of ethanol: a review.
J Pharm Pharm Sci. 2019;22:525-35. Crossref
22. Tomita K, Matsumoto T, Kamei S, Yamamoto S, Suda S, Zakoji H,
et al. Transcatheter arterial embolization for unruptured renal
angiomyolipoma using a 1.8-Fr tip microballoon catheter with a
mixture of ethanol and Lipiodol. CVIR Endovasc. 2020;3:3. Crossref
23. Bishay VL, Crino PB, Wein AJ, Malkowicz SB, Trerotola SO,
Soulen MC, et al. Embolization of giant renal angiomyolipomas:
technique and results. J Vasc Interv Radiol. 2010;21:67-72. Crossref
24. Guimaraes M, Lencioni R, Siskin GP. Embolization therapy:
principles and clinical applications. Lippincott Williams & Wilkins; 2014.
25. Idil Soylu A, Uzunkaya F, Belet Ü, Akan H. Selective transarterial
embolization of acute renal hemorrhage: a retrospective study.
Minim Invasive Ther Allied Technol. 2020;29:326-33. Crossref
26. Sanampudi S, Raissi D. Optimal ethanol-ethiodol emulsion ratio
in renal angiomyolipoma embolization: a question that remains
unanswered. J Clin Imaging Sci. 2019;9:16. Crossref
27. Gao F, Qian T, Chen MZ, Yin HB, Xu YL. Therapeutic effects of
transarterial infusion of lipiodol and ethanol in various ratios in a
rabbit VX2 tumor model. Diagn Interv Radiol. 2015;21:241-6. Crossref
28. Hocquelet A, Cornelis F, Le Bras Y, Meyer M, Tricaud E,
Lasserre AS, et al. Long-term results of preventive embolization of
renal angiomyolipomas: evaluation of predictive factors of volume
decrease. Eur Radiol. 2014;24:1785-93. Crossref
29. Kato H, Kuwatsuru R, Inoue T, Okada S, Aida M, Yamashiro Y.
Superselective transcatheter arterial embolization for large
unruptured renal angiomyolipoma in lymphangioleiomyomatosis.
J Vasc Interv Radiol. 2018;29:958-65. Crossref
30. Mbengue M, Bigirimana B, Diagne S, Niang A. Renal
angiomyolipoma in tuberous sclerosis complex: case series and
literature review. Clin Nephrol Case Stud. 2023;11:29-34. Crossref
31. Ewalt DH, Diamond N, Rees C, Sparagana SP, Delgado M,
Batchelor L, et al. Long-term outcome of transcatheter embolization
of renal angiomyolipomas due to tuberous sclerosis complex. J
Urol. 2005;174:1764-6. Crossref
32. Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD,
Crino PB, et al. Renal angiomyolipoma: long-term results after
arterial embolization. J Vasc Interv Radiol. 2005;16:45-50. Crossref
33. Krueger DA, Northrup H; International Tuberous Sclerosis
Complex Consensus Group. Tuberous sclerosis complex
surveillance and management: recommendations of the 2012
International Tuberous Sclerosis Complex Consensus Conference.
Pediatr Neurol. 2013;49:255-65. Crossref