Validation of Artificial Intelligence for Bone Age Assessment in Hong Kong Children
ORIGINAL ARTICLE
Hong Kong J Radiol 2024;27:Epub 28 November 2024
Validation of Artificial Intelligence for Bone Age Assessment in Hong Kong Children
C Cheung, JPK Chan, CWK Ng, WT Lai, KKF Fung, EYL Kan
Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr C Cheung, Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China. Email: cc755@ha.org.hk
Submitted: 16 June 2023; Accepted: 4 December 2023. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. CC, JPKC, WTL and KKFF acquired the data. CC, JPKC and KKFF analysed the data. CC and
KKFF drafted the manuscript. CC, JPKC, CWKN and EYLK critically revised the manuscript for important intellectual content. All authors had
full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Hospital Authority Central Institutional Review Board – Paediatrics Panel, Hong Kong (Ref No.: PAED-2023-049). The requirement for patient consent was waived by Board due to the retrospective nature of the research.
Abstract
Introduction
We sought to evaluate the accuracy of an artificial intelligence (AI)–automated bone age analysis software, BoneXpert 3.0, in determining bone age in children in Hong Kong.
Methods
All radiographs of the left hand and the wrist for bone age assessment at a tertiary referral centre in Hong
Kong from January to December 2019 were included. We compared the bone ages from these radiographs assessed
by two experienced paediatric radiologists with analysis by BoneXpert using the Greulich and Pyle method. Gender-based
bone age comparisons were also performed. The assessment involved calculating the Spearman’s correlation
(r), the coefficient of determination (R2), and accuracy (root mean square error). Agreement between manual and
AI-generated assessments was evaluated by Bland-Altman analysis.
Results
A total of 99 bone age radiographs were analysed. The mean chronological age was 9.8 years (standard deviation [SD] = 3.9 years). Manual and AI analyses showed a strong correlation (r = 0.98, R2 = 0.97; p < 0.001). Bland-Altman analysis showed a mean difference of -0.08 year (SD = 0.73 year) and limits of agreement between 1.35 and -1.51 years. The correlation between visual and AI-generated bone age assessment remained strong after stratification by sex (r = 0.98, R2 = 0.97; p < 0.001). Accuracy of the AI bone age analysis was 0.74 year for all studies, 0.79 year for females, and 0.65 year for males.
Conclusion
BoneXpert is reliable and accurate in bone age assessment in the local paediatric population.
Key Words: Algorithms; Artificial intelligence; Bone and bones; Pediatrics; Radiologists
中文摘要
人工智能對香港兒童骨齡評估的驗證
張樂人、陳沛君、吳穎琦、黎永德、馮建勳、簡以靈
引言
我們評估人工智能自動骨齡分析軟體BoneXpert 3.0在確定香港兒童骨齡方面的準確性。
方法
本研究納入2019年1月至12月期間在香港一所三級轉診中心進行骨齡評估的所有左手/手腕 X光片,比較了兩位經驗豐富的兒科放射科醫生評估這些X光片骨齡與BoneXpert使用Greulich和Pyle方法的分析結果,並比較了基於性別的骨齡。我們使用的比較方法包括Spearman相關性(r)、決定系數(R2)和準確性(均方根誤差),並使用Bland-Altman分析評估人工評估和人工智能評估之間的一致性。
結果
本研究總共分析了99張骨齡 X光片,平均實際年齡為9.8歲(標準差 = 3.9 歲)。人工和人工智能分析顯示出強相關(r = 0.98,R2 = 0.97;p < 0.001)。Bland-Altman分析顯示平均差異為-0.08年(標準差 = 0.73年),一致限度為1.35至-1.51年。按性別分層後,人工骨齡評估和人工智能骨齡評估之間的相關性仍然強(r = 0.98,R2 = 0.97;p < 0.001)。所有研究的人工智能骨齡分析準確度為0.74歲,女性為0.79歲,男性為0.65歲。
結論
BoneXpert 對本港兒科族群的骨齡評估可靠且準確。
INTRODUCTION
Bone age assessment is an integral part in the evaluation
of paediatric growth and pubertal disorders. Accurate
determination of bone age is important in assessing
growth potential and timing of therapeutic interventions.
For instance, in children with idiopathic short stature
undergoing growth hormone treatment, continuous
monitoring of bone age is vital to estimate potential
height gain and adjust the treatment dosage.[1]
Conventional bone age assessment most frequently
utilises the Greulich and Pyle (GP) or the Tanner and
Whitehouse (TW) methods, both of which rely on visual
comparison of radiographs of the left hand and the wrist
of the patient against matching reference radiographs
stratified by age and sex. However, these manual grading
methods are subjective and prone to inter- and intra-rater
variability.[1] [2] [3] Longitudinal assessment of multiple
bone age radiographs for the same patient over time can
yield inconsistent results when interpreted by different
radiologists. Moreover, manual bone age assessment is
time-consuming, particularly for inexperienced raters,
with average reported rating time being 1.4 minutes for
the GP method and 7.9 minutes for the TW method.[4]
In addition, calculations of standard deviations of bone
age using data in the atlas may introduce errors in the
reports.
To address these challenges, artificial intelligence
(AI)–based algorithms have been developed to reduce
inconsistencies and eliminate inter-rater and intra-rater
variability in bone age assessment in children. The
evolution of AI-based bone age analysis has closely
followed the advancements in machine learning
through the decades.[5] BoneXpert (Visiana, Hørsholm,
Denmark), launched in 2009, is the first AI-automated
bone age assessment software that is commercially
available and licensed for use in Europe.[6] The program
utilises traditional machine-learning methodology and
determines bone age based on shape, intensity, and
texture scores. The algorithm segments the radius,
ulna, metacarpals, and phalanges and determines an
independent bone age value for each. A self-validation
mechanism exists to reject bones for analysis if their
morphologies lie out of the expected range of the bone-finding
model or if their bone age values deviate by
more than a predefined threshold from the mean bone
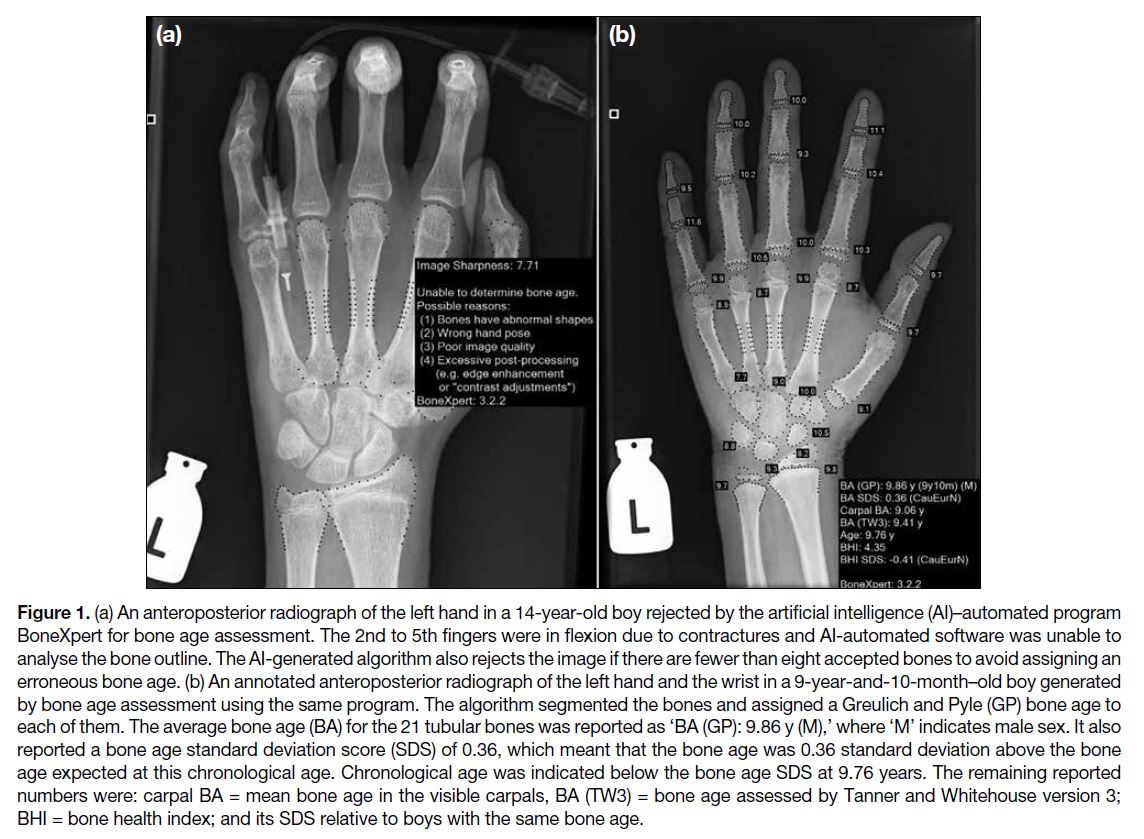
age determined from all the tubular bones (Figure 1a).
The algorithm also rejects the image if there are fewer
than eight accepted bones to prevent erroneous bone age
assessments.[6] [7]
Figure 1. (a) An anteroposterior radiograph of the left hand in a 14-year-old boy rejected by the artificial intelligence (AI)–automated program
BoneXpert for bone age assessment. The 2nd to 5th fingers were in flexion due to contractures and AI-automated software was unable to
analyse the bone outline. The AI-generated algorithm also rejects the image if there are fewer than eight accepted bones to avoid assigning an
erroneous bone age. (b) An annotated anteroposterior radiograph of the left hand and the wrist in a 9-year-and-10-month–old boy generated
by bone age assessment using the same program. The algorithm segmented the bones and assigned a Greulich and Pyle (GP) bone age to
each of them. The average bone age (BA) for the 21 tubular bones was reported as ‘BA (GP): 9.86 y (M),’ where ‘M’ indicates male sex. It also
reported a bone age standard deviation score (SDS) of 0.36, which meant that the bone age was 0.36 standard deviation above the bone
age expected at this chronological age. Chronological age was indicated below the bone age SDS at 9.76 years. The remaining reported
numbers were: carpal BA = mean bone age in the visible carpals, BA (TW3) = bone age assessed by Tanner and Whitehouse version 3;
BHI = bone health index; and its SDS relative to boys with the same bone age.
The final result is computed as the mean age of all the
included bones.[6] The process is almost instantaneous
and produces an annotated Digital Imaging and Communications in Medicine file (Figure 1b) with the
software-calculated bone age data, which can be stored
in a picture archiving and communication system as a
permanent electronic medical record.
While the program has been validated in different
Asian ethnicities,[8] [9] including Chinese[10] and Japanese[11]
children, it is crucial to validate its accuracy before
implementing it in our local paediatric population in
Hong Kong. The objective of our study is to evaluate the
accuracy of this program in determining the bone age of
children in Hong Kong, compared with visual bone age
assessment by experienced paediatric radiologists.
METHODS
This study was performed as part of a quality assurance
initiative. We retrospectively reviewed all bone age
radiographs of the left hand and the wrist, as well
as their radiology reports performed at Hong Kong
Children’s Hospital, a tertiary referral centre in Hong Kong, from January to December 2019. In accordance
with our institutional practice, each radiograph was
evaluated by two of seven experienced paediatric
radiologists (with 6 to 7 years of experience in bone age
assessment) using the GP method. The manual bone
age of the patient was determined by consensus and
recorded in the radiology report. Patient demographics,
including sex, chronological age, diagnosis, and
ethnicity (Chinese, South Asian, and Caucasian), were
retrieved from the electronic patient record. All of the
bone age radiographs were then analysed by BoneXpert
3.0 utilised in this study. The AI-generated bone age
of the patient (the GP method) was determined by
the aforementioned algorithm and was documented
in an annotated image which was stored in a picture
archiving and communication system. The interpreting
radiologists were completely blinded to the AI-generated
analysis results at the time of reporting. The
Guidelines for Reporting Reliability and Agreement
Studies were implemented.[12]
We compared the AI-generated bone age to the manual
bone age for each patient. We also performed the
comparison based on sex. We used the Spearman’s
correlation (r) and the coefficient of determination (R2) when comparing AI-generated and manual bone age.
Bland-Altman analysis was used to assess agreement
between AI-generated and manual ratings. The accuracy
of the AI-generated rating compared to manual rating
using the GP method was defined as the root mean square
error (RMSE) measured in years. Quantitative data are
expressed in means ± standard deviations for comparing
bone age as determined by the manual method versus
the AI-automated method. Agreement was evaluated by
Bland-Altman analysis. A p value < 0.05 was defined
as statistically significant. All statistical analyses were performed with commercial software SPSS (Windows
version 26.0; IBM Corp, Armonk [NY], United States).
RESULTS
Patients and Studies
A total of 99 bone age radiographs from January to
December 2019 were analysed, 38 of which were from
female patients and 61 were from male patients. The
mean chronological age of the cohort was 9.8 ± 3.9
years (range, 1.5-17.8). The majority of patients (n = 94,
94.9%) were Chinese, with the rest being South Asian
(n = 4, 4.0%) and Caucasian (n = 1, 1.0%). Regarding the
indications for bone age assessment, 23 were evaluated
for pubertal disorders, 51 for growth disorders, 21 for
bone marrow transplant workup, two for adrenal disease,
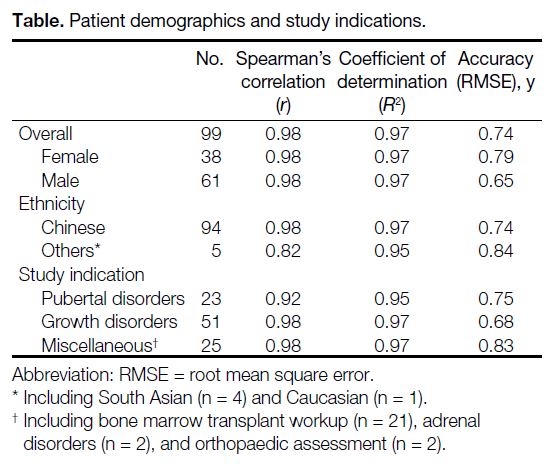
and two for orthopaedic assessment (Table).
Table. Patient demographics and study indications.
For manual bone age, an exact bone age was determined
in 93 radiographs while a bone age-range (e.g., between
3 years and 3 years and 6 months) was provided for six
radiographs. For these six radiographs, the midpoint of
bone age range was calculated as the manually rated
bone age. For AI-generated bone age, the AI software
was able to determine an exact bone age for all 99
radiographs in the sample. None of the radiographs was
rejected by the software.
Comparison Between Artificial Intelligence–Generated and Manual Bone Age Analysis
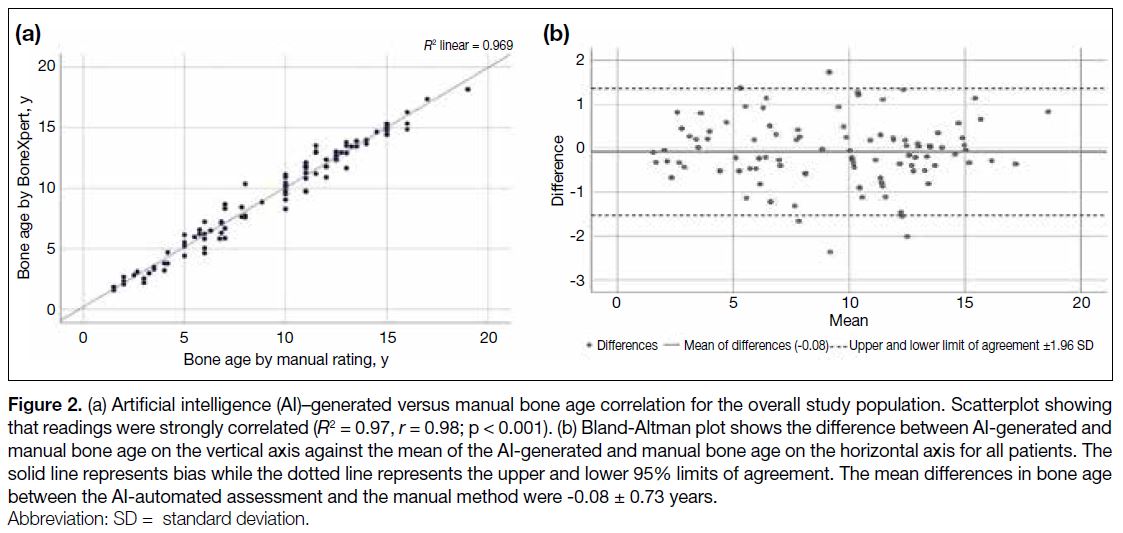
A strong correlation was demonstrated between AIgenerated
and manual bone age, with r of 0.98 and R2 of
0.97 (p < 0.001) [Figure 2a]. The Bland-Altman analysis also showed good agreement between manual rating and
AI-generated bone age. The mean of differences was
-0.08 ± 0.73 year and limits of agreement was between
1.35 and -1.51 years (Figure 2b). When stratified based
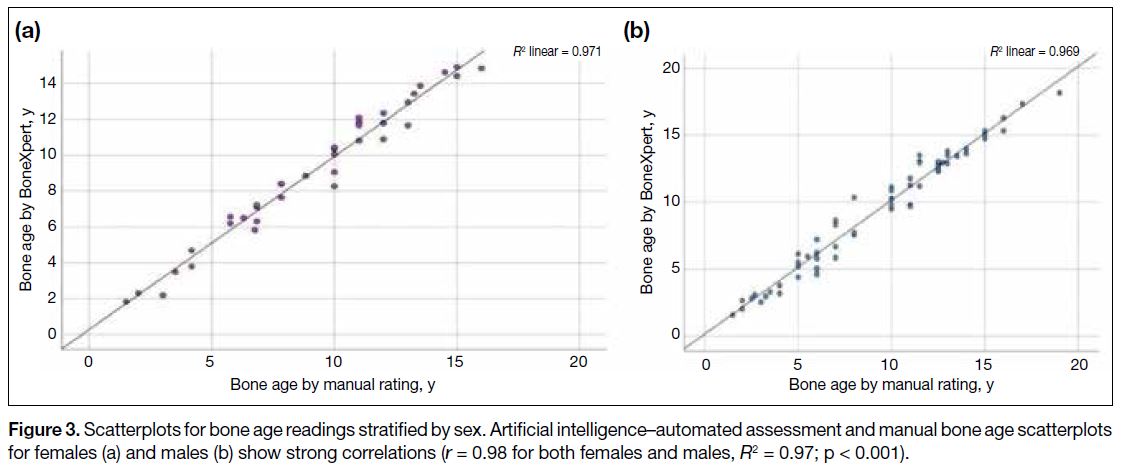
on sex, the correlation between manual and AI-generated
bone age assessment remained strong, with r of both
male and female subgroups being 0.98 and R2 being 0.97
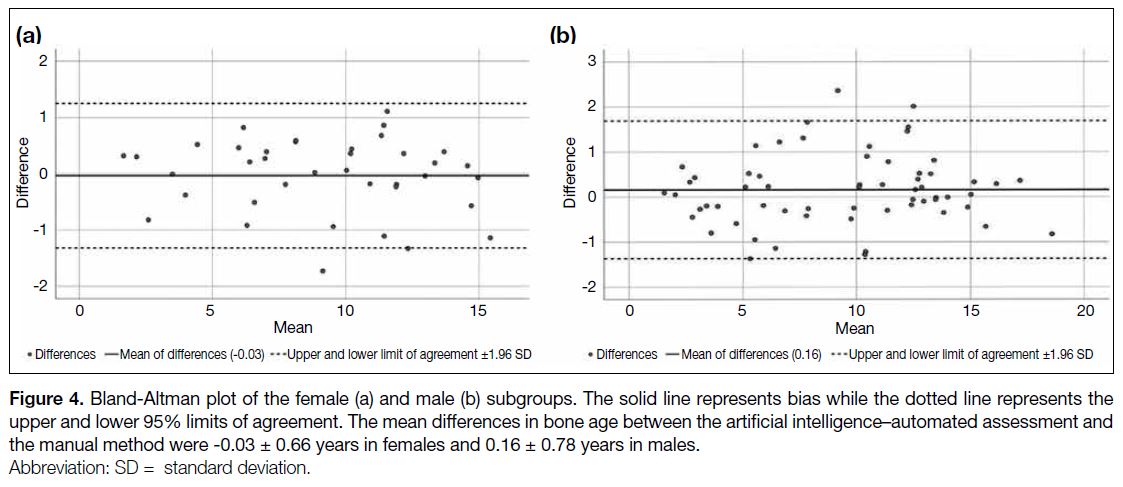
(p < 0.001) [Figure 3]. The Bland-Altman bias was 0.16
± 0.78 years in males and -0.03 ± 0.66 years in females
(Figure 4). RMSE of the AI-generated bone age analysis
was 0.74 year for all studies, 0.79 years for females, and
0.65 years for males.
Figure 2. (a) Artificial intelligence (AI)–generated versus manual bone age correlation for the overall study population. Scatterplot showing
that readings were strongly correlated (R2 = 0.97, r = 0.98; p < 0.001). (b) Bland-Altman plot shows the difference between AI-generated and
manual bone age on the vertical axis against the mean of the AI-generated and manual bone age on the horizontal axis for all patients. The
solid line represents bias while the dotted line represents the upper and lower 95% limits of agreement. The mean differences in bone age
between the AI-automated assessment and the manual method were -0.08 ± 0.73 years.
Figure 3. Scatterplots for bone age readings stratified by sex. Artificial intelligence–automated assessment and manual bone age scatterplots
for females (a) and males (b) show strong correlations (r = 0.98 for both females and males, R2 = 0.97; p < 0.001).
Figure 4. Bland-Altman plot of the female (a) and male (b) subgroups. The solid line represents bias while the dotted line represents the
upper and lower 95% limits of agreement. The mean differences in bone age between the artificial intelligence–automated assessment and
the manual method were -0.03 ± 0.66 years in females and 0.16 ± 0.78 years in males.
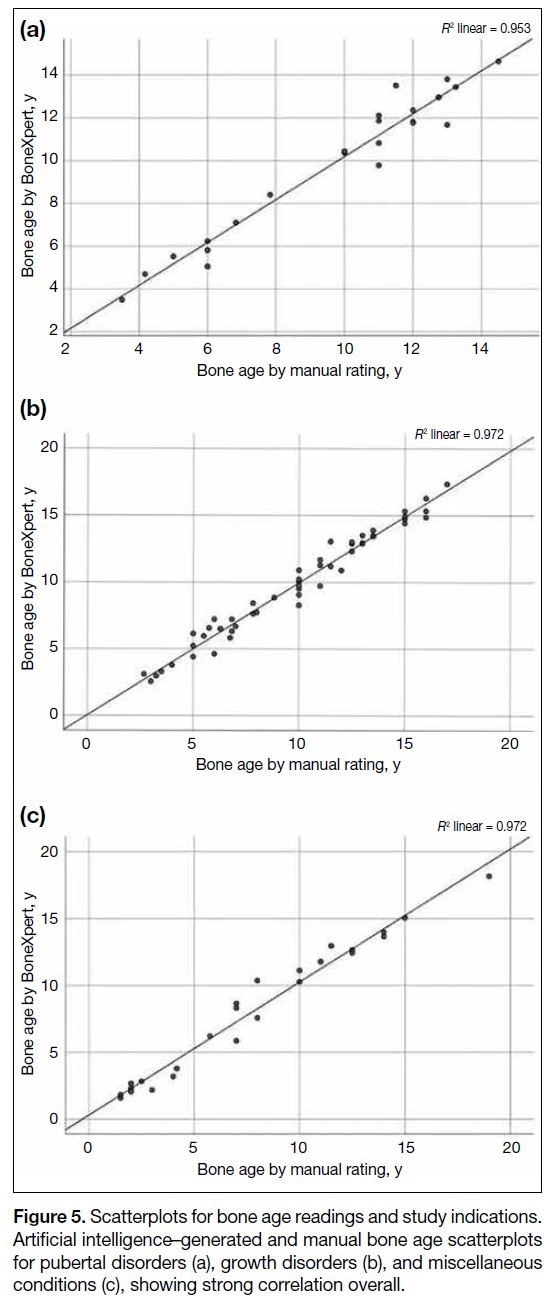
When comparing bone ages for different study
indications, a strong correlation remained between manual and AI-generated bone age. The r for growth
disorders and miscellaneous conditions were 0.98 while
that for pubertal disorders was 0.92. RMSE was best for
growth disorders (0.68 year) and worst for miscellaneous
conditions (0.83 year) [Figure 5].
Figure 5. Scatterplots for bone age readings and study indications.
Artificial intelligence–generated and manual bone age scatterplots
for pubertal disorders (a), growth disorders (b), and miscellaneous
conditions (c), showing strong correlation overall.
DISCUSSION
Good agreement between the manual and AI-generated
bone age rating was demonstrated in our local paediatric
population in this study, with correlation remaining strong
after stratification by sex. Minimal bias was detected
in the Bland-Altman analysis. The small discrepancies
amongst the ratings may be attributed to inclusion of the
carpal bones or the presence of a sesamoid bone during
manual bone age assessment, neither of which is included in the AI-generated assessment. These findings are
similar to previous studies performed in healthy children
of different races from different countries.[3] [8] [9] [13] [14]
One of the strengths of our study is that it demonstrates
the high accuracy of AI-automated assessment when applied in real-life clinical practice. Many of the previous
validation studies for AI-automated assessment included
healthy children as subjects,[3] [9] [10] while the radiographs
included in our study were performed in patients with
pathologies clinically indicated for bone age assessment.
These radiographs reflected actual clinical scenarios and
the rating radiologists assessed the radiographs as part of
their routine clinical practice. AI-automated assessment
maintained high accuracy in our local paediatric
population in Hong Kong (0.75 year) and this level of
accuracy is comparable to validation studies previously
published in healthy children in the Dutch population
(0.71 year),[3] the Northern American population of four
races (Caucasian, African-American, Hispanic and
Asian) [0.74 year],[9] and in healthy Chinese (0.64 year)[10]
and Japanese (0.71 year)[11] children.
The image rejection rate by the AI-automated analysis
was 0% in our study. Other studies have reported an
image rejection rate from 1.3% to 2%.[14] [15] This very low
rejection rate was likely a result of the absence of skeletal
dysplasia cases in our study. As our service expands
with wider clinical indications for bone age assessment,
it is anticipated that there will be an increased number
of rejected cases in clinical application of the software.
Other studies have shown that AI-automated assessment
was able to reject bone age radiographs with abnormal
bone morphology and alert the reporting radiologist that
an underlying metabolic or genetic bone disorder was
possible, indicating the need for manual assessment.[14] [15]
Monitoring the rejection rate and the reasons for
rejection of radiographs as part of a continuous quality
improvement process would be helpful to monitor the
performance of AI-automated assessment.
Limitations
There are a few limitations to our study. Firstly, the
longitudinal bone age assessment of the same patient
was not evaluated due to the relatively short study period.
With the inherent nature of AI-automated assessment,
the risk of intra- and inter-rater variability is eliminated
and previous studies have proven that repeated bone
age assessment by AI-automated assessment has
good agreement with manual rating in terms of bone
age maturation.[14] The time required for manual bone
age assessment was not formally documented in our
study, limiting our ability to assess how AI-automated
assessment can shorten reporting time. From our
experience, manual bone age determination using the
GP method commonly requires around 5 to 10 minutes
to determine bone age from a radiograph of the left hand and the wrist. Compared with the almost instantaneous
process of bone age determination by AI-automated
assessment, this significantly shortens reporting time
and improves the efficiency of radiologists. Another
limitation is that our study did not compare the agreement
and accuracy of AI-automated assessment against
manual rating using the TW method, which is utilised in
a small number of centres in our locality.
CONCLUSION
BoneXpert, an AI-automated bone age analysis
algorithm, was reliable and accurate in a real-life clinical
setting in our local paediatric population in Hong Kong.
REFERENCES
1. Lepe GP, Villacrés F, Silva Fuente-Alba C, Guiloff S. Correlation
in radiological bone age determination using the Greulich and Pyle
method versus automated evaluation using BoneXpert software [in
Spanish]. Rev Chil Pediatr. 2018;89:606-11. Crossref
2. Tajmir SH, Lee H, Shailam R, Gale HI, Nguyen JC, Westra SJ,
et al. Artificial intelligence–assisted interpretation of bone age
radiographs improves accuracy and decreases variability. Skeletal
Radiol. 2018;48:275-83. Crossref
3. van Rijn RR, Lequin MH, Thodberg HH. Automatic determination
of Greulich and Pyle Bone age in healthy Dutch children. Pediatric
Radiol. 2009;39:591-7. Crossref
4. Satoh M. Bone age: assessment methods and clinical applications.
Clin Pediatr Endocrinol. 2015;24:143-52. Crossref
5. Dallora AL, Anderberg P, Kvist O, Mendes E, Diaz Ruiz S,
Sanmartin Berglund J. Bone age assessment with various machine
learning techniques: a systematic literature review and metaanalysis.
PLoS One. 2019;14:e0220242. Crossref
6. Thodberg HH, Thodberg B, Ahlkvist J, Offiah AC. Autonomous
artificial intelligence in pediatric radiology: the use and
perception of BoneXpert for bone age assessment. Pediatr Radiol.
2022;52:1338-46. Crossref
7. Martin DD, Calder AD, Ranke MB, Binder G, Thodberg HH.
Accuracy and self-validation of automated bone age determination.
Sci Rep. 2022;12:6388. Crossref
8. Prokop-Piotrkowska M, Marszałek-Dziuba K, Moszczyńska E,
Szalecki M, Jurkiewicz E. Traditional and new methods of bone
age assessment–an overview. J Clin Res Pediatr Endocrinol.
2021;13:251-62. Crossref
9. Thodberg HH, Sävendahl L. Validation and reference values of
automated bone age determination for four ethnicities. Acad Radiol.
2010;17:1425-32. Crossref
10. Zhang SY, Liu G, Ma CG, Han YS, Shen XZ, Xu RL, et al.
Automated determination of bone age in a modern Chinese
population. ISRN Radiol. 2013;874570. Crossref
11. Martin DD, Sato K, Sato M, Thodberg HH, Tanaka T. Validation of
a new method for automated determination of bone age in Japanese
children. Horm Res Paediatr. 2010;73:398-404. Crossref
12. Kottner J, Audigé L, Brorson S, Donner A, Gajewski BJ,
Hróbjartsson A, et al. Guidelines for Reporting Reliability and
Agreement Studies (GRRAS) were proposed. J Clin Epidemiol.
2011;64:96-106. Crossref
13. Artioli TO, Alvares MA, Carvalho Macedo VS, Silva TS,
Avritchir R, Kochi C, et al. Bone age determination in eutrophic,
overweight and obese Brazilian children and adolescents: a
comparison between computerized BoneXpert and Greulich-Pyle
methods. Pediatr Radiol. 2019;49:1185-91. Crossref
14. Bowden JJ, Bowden SA, Ruess L, Adler BH, Hu H,
Krishnamurthy R, et al. Validation of automated bone age analysis
from hand radiographs in a North American pediatric population.
Pediatr Radiol. 2022;52:1347-55. Crossref
15. Offiah AC. Current and emerging artificial intelligence applications
for pediatric musculoskeletal radiology. Pediatric Radiol.
2022;52:2149-58. Crossref