Journal
Vol. 28 No. 4, 2025
Table of Contents
EDITORIAL
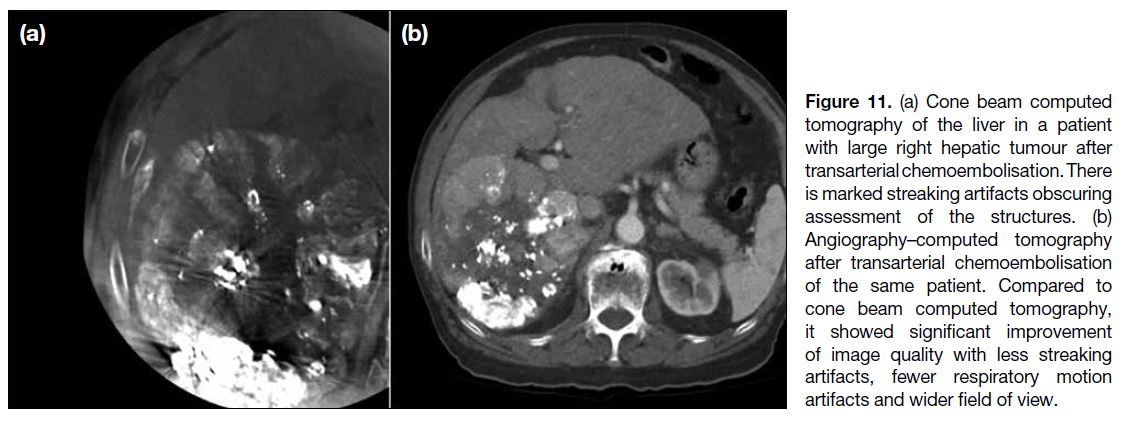
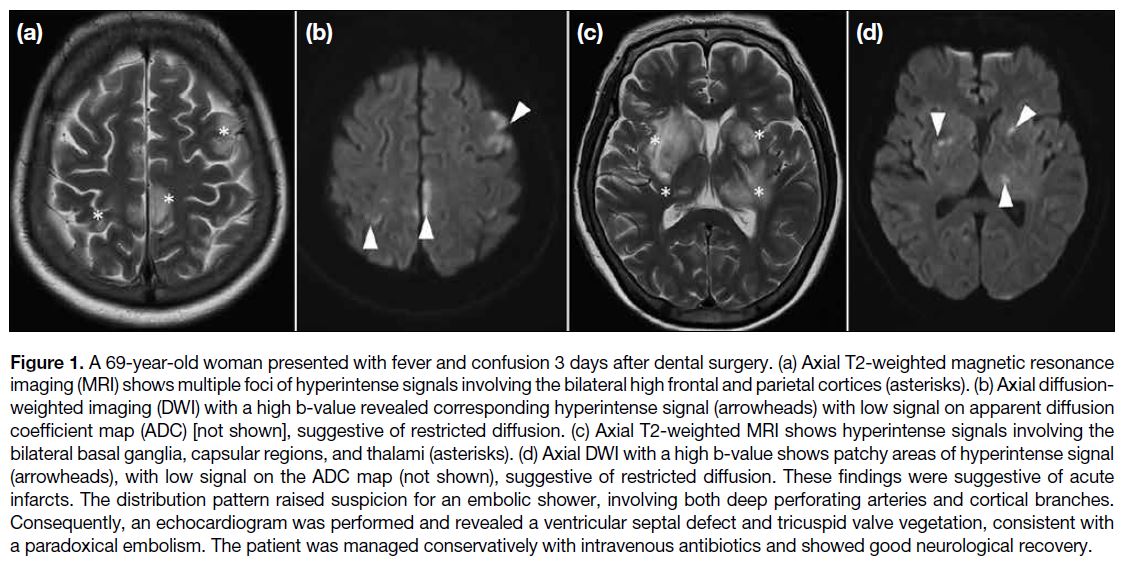
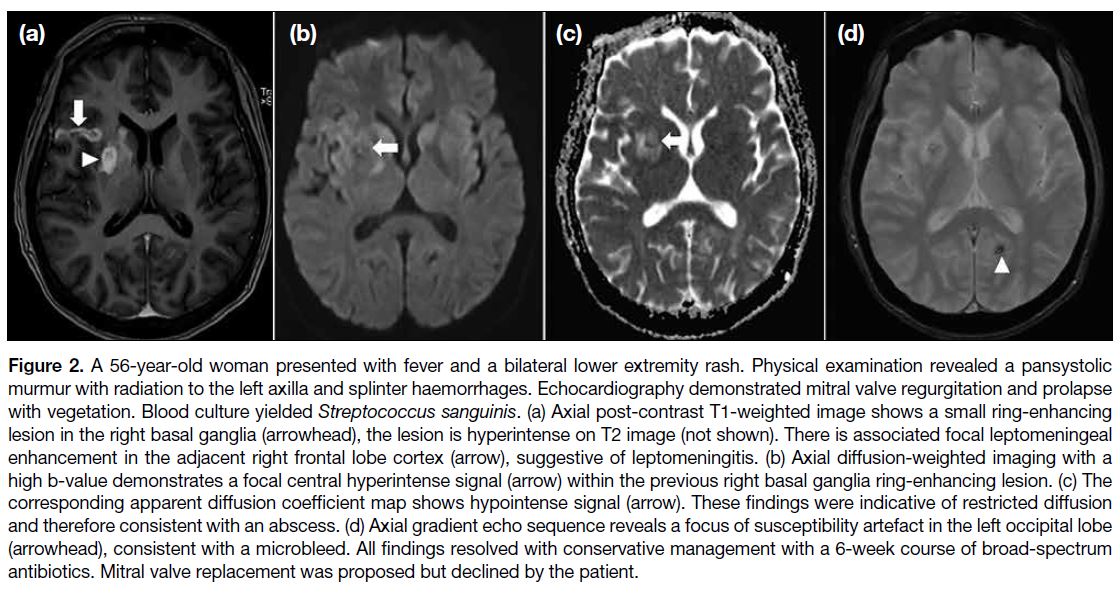
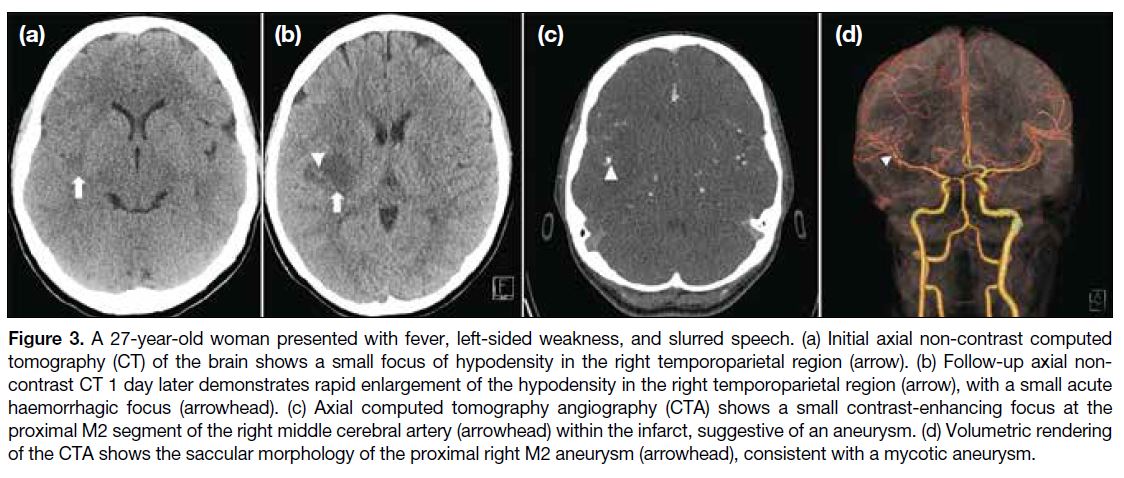
More than the Sum of Its Parts: The Synergy of Hybrid Angiography–Computed Tomography Systems in Interventional Radiology
EDITORIAL
More than the Sum of Its Parts: The Synergy of Hybrid
Angiography–Computed Tomography Systems in Interventional Radiology
KKF Fung
Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr KKF Fung, Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China. Email: k.fung@ha.org.hk
Contributors: The author solely contributed to the editorial, approved the final version for publication, and takes responsibility for its accuracy and integrity.
Conflicts of Interest: The author of this editorial is also a co-author of the article by Wong et al (Reference 4), published in the same issue. This
editorial represents the author’s objective interpretation of the topic and was not subject to internal peer review.
The introduction of cross-sectional imaging during
procedures has greatly improved treatment precision and
allowed real-time assessment of therapeutic outcomes,
particularly in the realm of interventional oncology. The
first hybrid angiography–computed tomography (angio-CT) system was developed in the early 1990s by Professor
Yasuaki Arai at Aichi Medical Centre in Japan.[1] In its
early stages, integration between the two modalities
was minimal, with each operating largely independently
and requiring the operator to manually combine the
imaging data.[2] The development of flat-panel detectors
in the late 1990s paved the way for the rapid adoption of
cone beam CT (CBCT). Compared to angio-CT, CBCT
has since become the more widely utilised modality,[3]
largely attributable to the higher cost and infrastructural
demands of angio-CT systems, particularly the need to
accommodate a sliding gantry. However, recent advances
in workflow efficiency, decreasing relative costs, and
the multipurpose capabilities of angio-CT systems have
led to renewed interest. Increasingly, institutions are
accepting the higher upfront investment, recognising the
potential long-term benefits in terms of improved patient
outcomes and enhanced procedural room utilisation.
In this issue of the Hong Kong Journal of Radiology,
Wong et al[4] presented a case-based review that explores
the advantages of angio-CT technology through a series
of illustrative examples. While most of the interventional
radiology (IR) literature has focused primarily on
oncologic applications, this review highlights its broader utility in various vascular interventions, including
embolotherapy for acute haemorrhage, management
of pulmonary arteriovenous fistulae, and adrenal
venous sampling. Additional potential non-oncological applications—though not addressed in this review—
include complex drain placements, acute trauma
management, prostate artery embolisation, geniculate
artery embolisation, complex venous reconstruction, and
lymphatic interventions.[5] [6] These examples underscore
the versatility of angio-CT systems across a wide
spectrum of IR procedures.
The most significant advantage of angio-CT lies in
its ability to integrate high-resolution CT imaging
with selective angiography and fluoroscopy, thereby
eliminating the need to transfer patients to a separate
CT scanner during critical procedural steps. Motion
and beam-hardening artefacts commonly encountered
with CBCT are minimised with angio-CT due to its
superior temporal resolution. The capacity to accurately
delineate perfused tissue volume is particularly
valuable in procedures where extra-target embolisation
may have serious consequences, such as geniculate
artery embolisation and prostate artery embolisation.
Additionally, the ability to provide three-dimensional
needle guidance is invaluable for complex interventions
such as sharp recanalisation in venous reconstructions
and antegrade percutaneous puncture of the cisterna
chyli. In the setting of acute polytrauma and stroke,
angio-CT enables both diagnostic CT and subsequent therapeutic embolisation to be performed within the
same gantry, thereby saving precious time and reducing
the risks associated with transferring critical, often
haemodynamically unstable, patients from one venue to
another.
Converting a conventional IR suite into a hybrid angio-CT
suite has been shown to enhance operational efficiency
across both IR and diagnostic radiology services. This
conversion allows CT scanners, which previously had
to be shared between diagnostic and interventional
services, to be dedicated solely to diagnostic imaging,
while enabling CT-guided procedures to be performed
directly within the hybrid IR suite. For instance, one
institution reported a 19.1% relative increase in the
utilisation of formerly shared CT scanners for diagnostic
imaging, along with a 287.1% relative increase in the use
of the hybrid IR suite, compared with the overall growth
rates of both diagnostic radiology and IR departments.[7] [8]
In addition, the potential use of the angio-CT scanner as
a diagnostic scanner affords scheduling flexibility for
both patients and physicians and contributes to workflow
efficiency and optimisation.
A major limitation of angio-CT systems is the
substantially higher cost of installation compared with
single-modality fluoroscopy suites. In addition, these
systems require a larger physical footprint, typically at
least 50 m2, to accommodate both CT and fluoroscopy
units. To date, robust quantitative evidence demonstrating
improved patient outcomes or cost-effectiveness is
lacking. Comparison of radiation dose between CBCT
and angio-CT is also challenging due to variability in
imaging protocols and technical parameters, which
introduces uncertainties in direct comparisons. Notably,
a CT acquisition during a procedure may reduce the
need for multiple digital subtraction angiography runs,
thereby lowering and more evenly distributing overall
radiation exposure to the patient. While one study found
no significant difference in effective dose per CT scan
between CBCT and angio-CT,[9] limited available data
suggest that angio-CT systems may reduce the overall
effective dose from both CT and angiographic imaging
compared with CBCT systems.[10]
Hybrid angio-CT systems offer institutions a valuable opportunity to expand treatment capabilities and
optimise workflow. The integration of diagnostic-quality
intraprocedural CT with conventional angiography
and fluoroscopy has substantially broadened the scope
of interventional procedures while improving room
utilisation. While the synergistic benefits of angio-CT
are clear, the decision to invest in such a system should
be informed by a comprehensive needs assessment
and institutional considerations, including case mix,
procedural complexity, patient volume, and the potential
to enhance workflow efficiency and translate into
improved patient outcomes.
REFERENCES
1. Tanaka T, Arai Y, Inaba Y, Inoue M, Nishiofuku H, Anai H, et al.
Current role of hybrid CT/angiography system compared with
C-arm cone beam CT for interventional oncology. Br J Radiol.
2014;87:20140126. Crossref
2. Taiji R, Lin EY, Lin YM, Yevich S, Avritscher R, Sheth RA, et al.
Combined angio-CT systems: a roadmap tool for precision
therapy in interventional oncology. Radiol Imaging Cancer.
2021;3:e210039. Crossref
3. Floridi C, Radaelli A, Abi-Jaoudeh N, Grass M, De Lin M,
Chiaradia M, et al. C-arm cone-beam computed tomography in
interventional oncology: technical aspects and clinical applications.
Radiol Med. 2014;119:521-32. Crossref
4. Wong CL, Fung KK, Lo HY, Yeung LH, Ng JC, Lee KH, et al.
Exploring the power of hybrid intervention: utility of angiography–
computed tomography system in interventional radiology. Hong
Kong J Radiol. 2025;28:e257-67. Crossref
5. Wong D, Fung KF, Chen HS, Lun KS, Kan YL. Intranodal conebeam
computed tomographic lymphangiography with water-soluble
iodinated contrast agent for evaluating chylothorax in infants—preliminary experience at a single institution. Pediatr Radiol.
2023;53:179-83. Crossref
6. Wong D, Fung KF, Chen HS, Lun KS, Kan YL. Re: Pediatric
intranodal CT lymphangiography with water-soluble contrast
media. J Vasc Interv Radiol. 2023;34:1451. Crossref
7. Feinberg N, Funaki B, Hieromnimon M, Guajardo S, Navuluri R,
Zangan S, et al. Improved utilization following conversion of a
fluoroscopy suite to hybrid CT/angiography system. J Vasc Interv
Radiol. 2020;31:1857-63. Crossref
8. Kwak DH, Ahmed O, Habib H, Nijhawan K, Kumari D, Patel M.
Hybrid CT-angiography (angio-CT) for combined CT and
fluoroscopic procedures in interventional radiology enhances
utilization. Abdom Radiol (NY). 2022;47:2704-11. Crossref
9. Marshall EL, Guajardo S, Sellers E, Gayed M, Lu ZF, Owen J, et al.
Radiation dose during transarterial radioembolization: a dosimetric
comparison of cone-beam CT and angio-CT technologies. J Vasc
Interv Radiol. 2021;32:429-38. Crossref
10. Piron L, Le Roy J, Cassinotto C, Delicque J, Belgour A, Allimant C,
et al. Radiation exposure during transarterial chemoembolization:
angio-CT versus cone-beam CT. Cardiovasc Intervent Radiol.
2019;42:1609-18. Crossref
ORIGINAL ARTICLES
Efficacy of Prophylactic Embolisation of Renal Angiomyolipomas Using Semi-automatic Segmentation for Volume Measurement
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2025 Dec;28(4):e234-42 | Epub 17 November 2025
Efficacy of Prophylactic Embolisation of Renal Angiomyolipomas Using Semi-automatic Segmentation for Volume Measurement
PL Lam1, JC Ng1, KH Lee1, KKF Fung2, DHY Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr PL Lam, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR,
China. Email: lpl404@ha.org.hk
Submitted: 28 August 2024; Accepted: 29 October 2024.
Contributors: All authors designed the study. PLL acquired the data. All authors analysed the data. PLL drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, KKFF was not involved in the peer review process. Other authors have disclosed no conflicts of
interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-022-4). The requirement for informed patient consent was waived by the Board due to the retrospective nature of the research.
Abstract
Introduction
We aimed to assess the efficacy of prophylactic embolisation of renal angiomyolipomas (AMLs) by
determining post-embolisation rupture risk, as well as changes in total volume and in angiomyogenic and fatty
components using semi-automatic segmentation.
Methods
This was a retrospective study of 22 adult patients with prophylactic embolisation of AML performed
between January 2009 and January 2024. Patients were followed up for any post-embolisation rupture. Pre- and
post-embolisation computed tomography (CT) data were assessed using the open-source software 3D Slicer for
semi-automatic segmentation. Volumetric changes of AMLs were compared using the Wilcoxon signed-rank test
for paired data and Mann-Whitney U test for unpaired data. Spearman’s rank correlation coefficient was used to
identify any associations between variables.
Results
There were 25 prophylactic embolisations performed on the 22 adult patients with AML (18 females
[81.8%]), with a median age of 60.0 years (interquartile range [IQR], 15.0). No procedure-related complications
were encountered. The median follow-up was 49.0 months (IQR, 56.0) with no post-embolisation rupture. Pre- and
post-treatment median tumour volumes were 67.5 cm3 (IQR, 116.1) and 35.7 cm3 (IQR, 82.1), respectively. There
was a significant reduction in total tumour volume (41.4%), including angiomyogenic (73.6%) and fatty components
(14.0%) [all p < 0.001]. Factors associated with greater tumour volume reduction included a higher proportion of
angiomyogenic and a lower proportion of fatty components (both p < 0.001).
Conclusion
Prophylactic embolisation of AML effectively reduced tumour volume, with more significant changes
in its angiomyogenic than fatty components. No post-embolisation rupture was documented with a median follow-up of over 4 years.
Key Words: Angiomyolipoma; Embolization, therapeutic; Hemorrhage; Kidney neoplasms; Tumor burden
中文摘要
半自動分割體積測量法評估預防性栓塞治療腎血管平滑肌脂肪瘤的療效
林栢麟、吳昆倫、李家灝、馮建勳、曹慶恩
引言
本研究旨在透過半自動分割技術評估腎血管平滑肌脂肪瘤(AML)預防性栓塞的療效,具體方法包括確定栓塞術後破裂風險以及腫瘤總體積、血管肌源性成分和脂肪成分的變化。
方法
本研究為回顧性研究,納入2009年1月至2024年1月期間接受AML預防性栓塞的22位成年患者。所有患者均接受隨訪,觀察栓塞術後是否發生破裂。我們採用開源軟件3D Slicer對栓塞術前及術後的電腦斷層掃描圖像進行半自動分割,並採用Wilcoxon 符號排序檢定(配對資料)和Mann-Whitney U 檢定(非配對資料)比較AML的體積變化,以及採用Spearman秩相關系數分析各變數間的相關性。
結果
22位成年AML患者(18位女性[81.8%])接受了25次預防性栓塞治療,中位年齡為60.0歲(四分位數間距[IQR]為15.0)。沒有發生手術相關併發症。中位隨訪時間為49.0個月(IQR為56.0),沒有發生栓塞後破裂。治療前後腫瘤體積中位數分別為67.5 cm3(IQR為116.1)及35.7 cm3(IQR為82.1)。腫瘤總體積顯著縮小(41.4%),其中血管肌源性成分縮小73.6%,脂肪成分縮小14.0% [所有p < 0.001]。腫瘤體積顯著縮小的相關因素包括血管肌源性成分比例較高和脂肪成分比例較低(兩者 p < 0.001)。
結論
預防性栓塞治療AML可有效縮小腫瘤體積,且血管肌源性成分的變化比脂肪組成的變化更為顯著。中位隨訪時間超過4年,未記錄到栓塞後破裂病例。
INTRODUCTION
Renal angiomyolipoma (AML) is the most common
benign solid renal tumour.[1] The majority (approximately
80%) occur sporadically, while the rest (approximately
20%) are associated with phakomatoses, most
commonly tuberous sclerosis.[2] AML belongs to the
family of tumours with perivascular epithelioid cellular
differentiation.[3] It typically contains both angiomyogenic
and fatty components, with the latter readily identifiable
in computed tomography (CT) due to its hypoattenuating
nature (< -10 Hounsfield unit [HU]) [Figure 1].[4] [5]
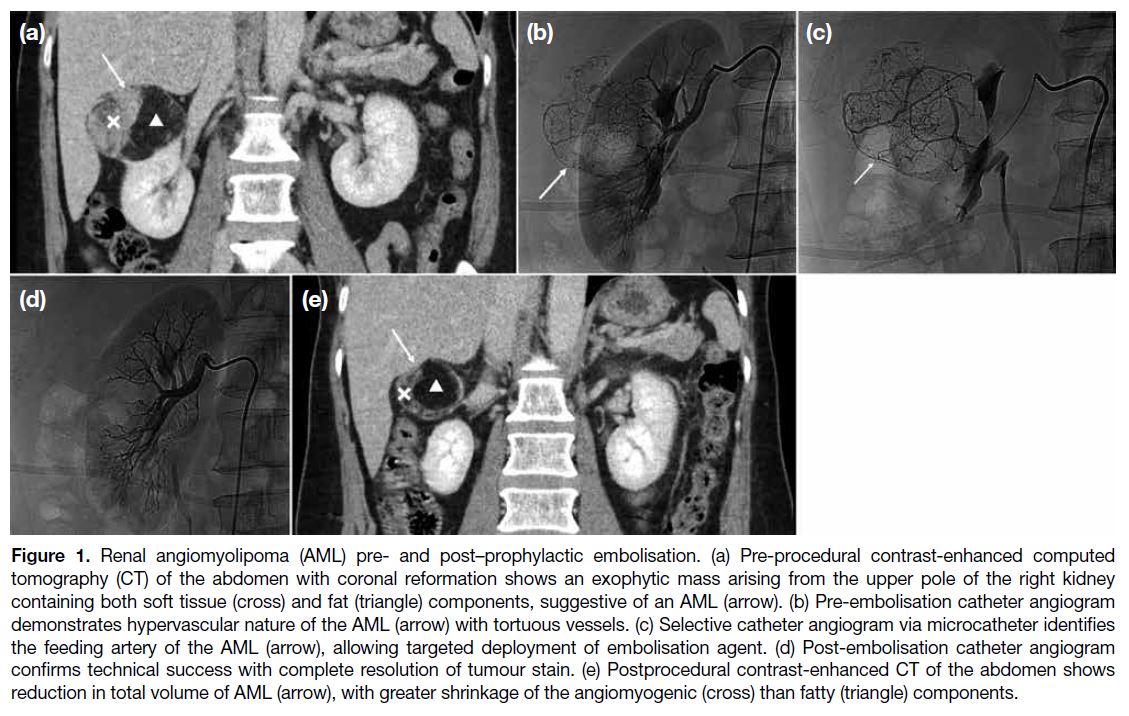
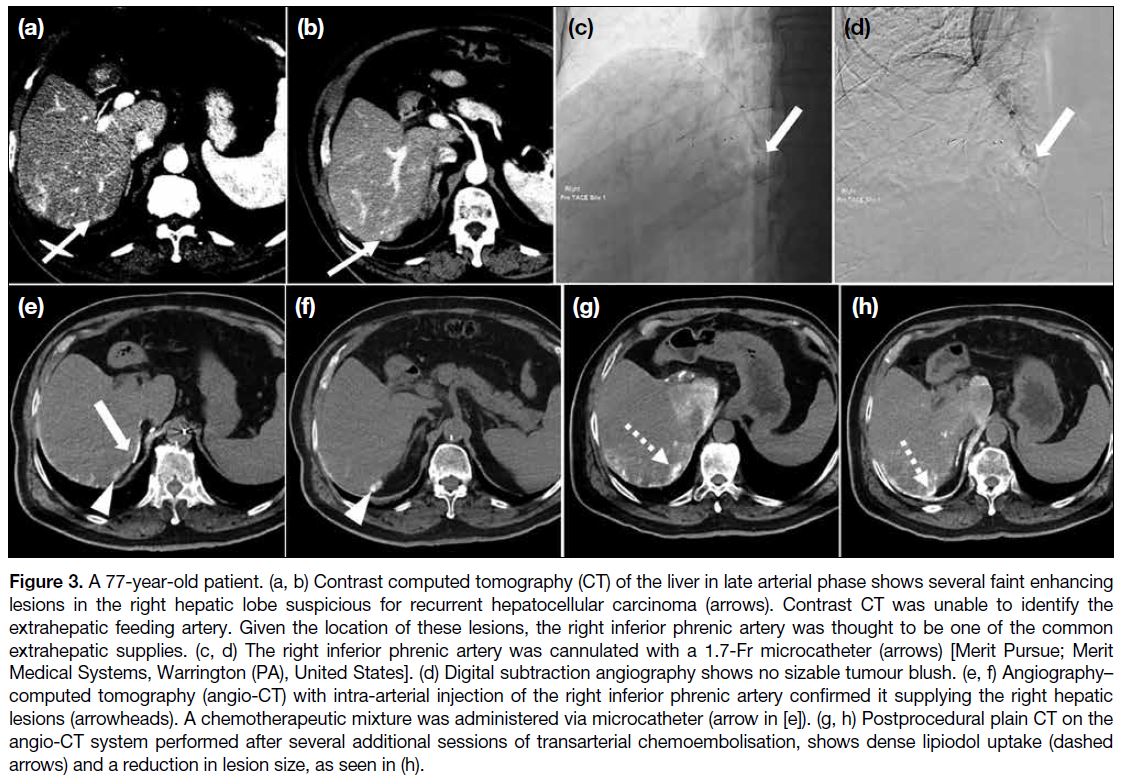
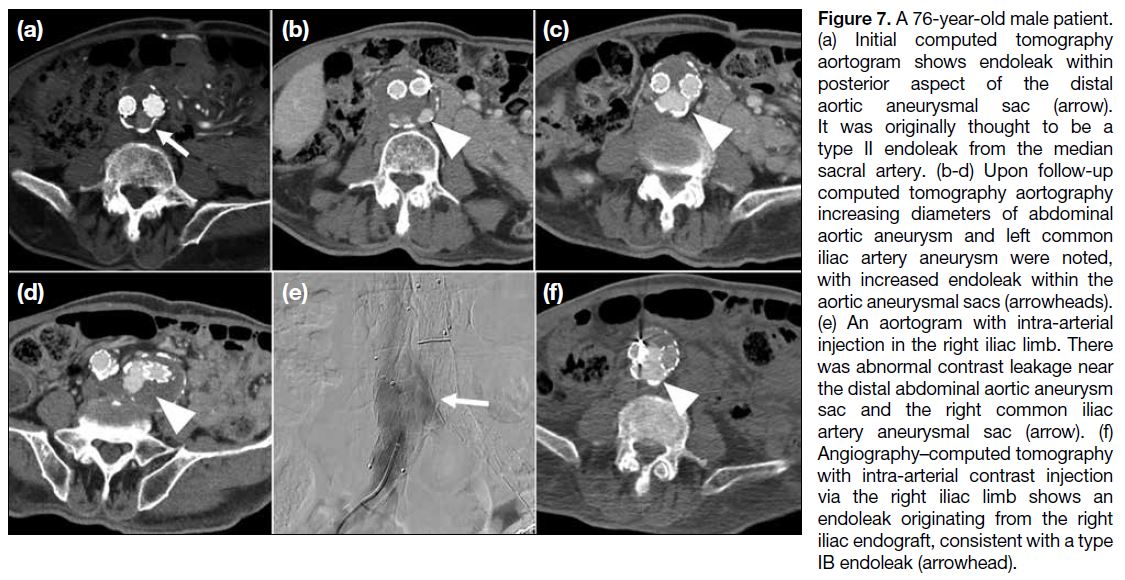
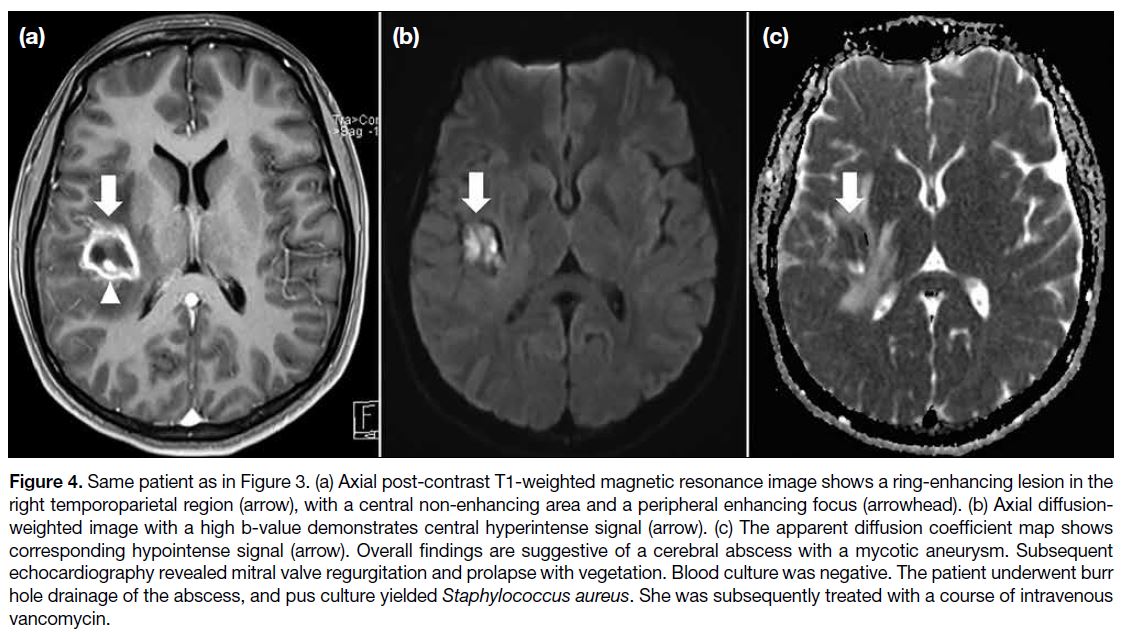
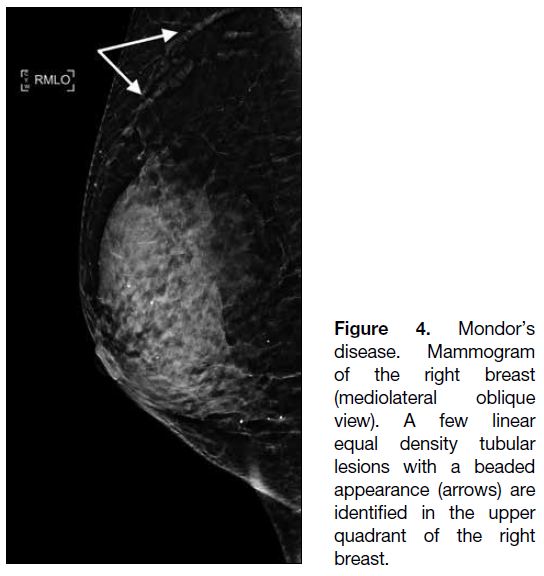
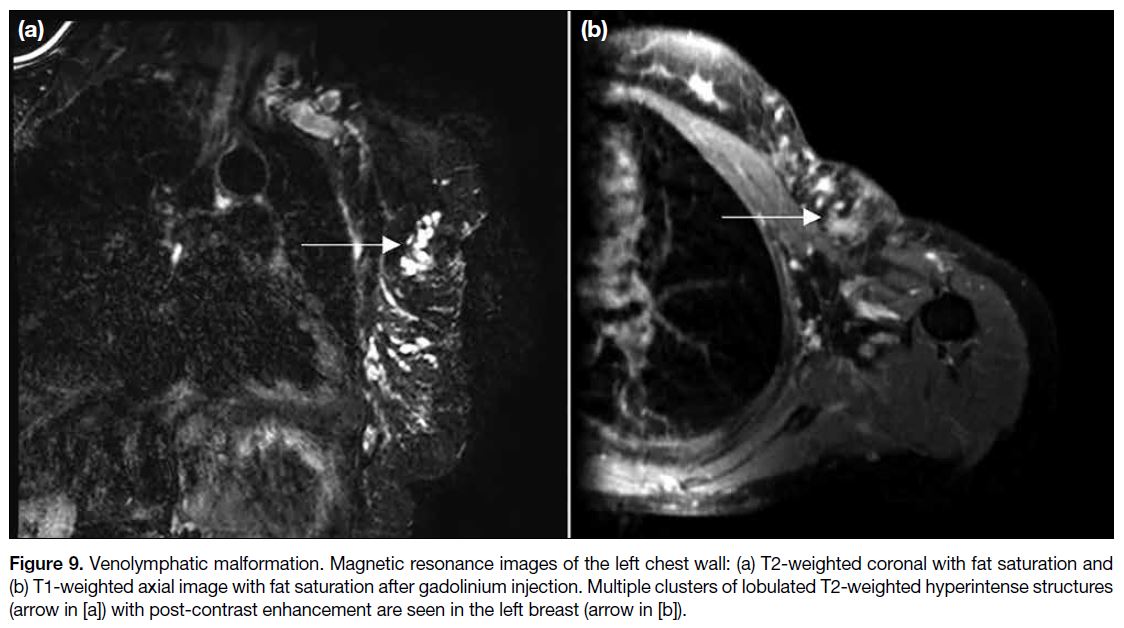
Figure 1. Renal angiomyolipoma (AML) pre- and post–prophylactic embolisation. (a) Preprocedural contrast-enhanced computed
tomography (CT) of the abdomen with coronal reformation shows an exophytic mass arising from the upper pole of the right kidney
containing both soft tissue (cross) and fat (triangle) components, suggestive of an AML (arrow). (b) Pre-embolisation catheter angiogram
demonstrates hypervascular nature of the AML (arrow) with tortuous vessels. (c) Selective catheter angiogram via microcatheter identifies
the feeding artery of the AML (arrow), allowing targeted deployment of embolisation agent. (d) Post-embolisation catheter angiogram
confirms technical success with complete resolution of tumour stain. (e) Postprocedural contrast-enhanced CT of the abdomen shows
reduction in total volume of AML (arrow), with greater shrinkage of the angiomyogenic (cross) than fatty (triangle) components.
It is well recognised that AML carries a risk of rupture
with bleeding, especially for larger tumours, which can
lead to fatal consequences.[6] [7] Treatment options include
transcatheter arterial embolisation and radiofrequency
ablation, as well as partial or radical nephrectomy.[8]
Selective arterial embolisation can be performed in an
emergency setting for AML with active bleeding. It can
also be a prophylactic treatment to reduce tumour size
and its risk of haemorrhage (Figure 1).[9] [10] It has been
suggested that for AML of 4 cm or above in diameter, or
those with microaneurysms 0.5 cm or above in the feeding
artery, prophylactic selective arterial embolisation is indicated.[11] Different embolisation agents have been
reported in the literature, including microparticles, such
as microspheres, and liquid agents, such as ethanol. Past
studies have shown that prophylactic embolisation could
reduce the size of AML, thus reducing its haemorrhagic
risk.[9] [10] [12] In addition, the risk of haemorrhage is mainly
attributed to the angiomyogenic component of AML.[13] [14] [15]
Yet, there are limited studies accurately assessing how
tumour composition changes after treatment.
For AML, tumour size has been shown to be associated
with risk of spontaneous rupture, with larger ones
more likely to bleed.[6] [7] [11] This study therefore aimed
to assess the efficacy of prophylactic selective arterial
embolisation in determining the rupture risk post-embolisation
and reducing the volume of AML using
semi-automatic segmentation as a measurement tool.
Changes in its angiomyogenic and fatty components
were also evaluated.
METHODS
Patient Selection
This was a single-centre, single-arm retrospective
study. Consecutive adult patients (≥18 years old) who underwent prophylactic embolisation of AML (Figure 2) in a public acute general hospital between January
2009 and January 2024 were included. Exclusion criteria
included paediatric patients (<18 years old), patients
who underwent emergency embolisation of ruptured
AML, and patients without pre- or postprocedural CT.
Figure 2. Protocol for prophylactic embolisation of renal angiomyolipoma.
Data Collection
Clinical data of the included patients were retrieved from
the radiology information system of the hospital network.
They included demographics and medical history, such
as tuberous sclerosis status. Presenting symptoms and
postprocedural complaints were recorded. Pre- and post-intervention blood tests, such as haemoglobin level and
renal function tests, were documented.
Details of prophylactic embolisation of AML were
logged. They encompassed the type and amount of
embolisation agents deployed, as well as catheters
and guidewires used, which were chosen based on the
operators’ preference. Data on technical success, defined
as complete angiographic resolution of tumour stain
and microaneurysms, as well as contrast stasis of the
feeding artery, were documented. Intraoperative and
immediate postprocedural complications were recorded.
Subsequent clinical follow-up was reviewed for post-embolisation
tumour rupture.
Radiological Assessment
Pre- and post-embolisation plain and contrast-enhanced
CTs of the abdomen in DICOM (Digital Imaging and
Communications in Medicine) format were obtained
from the picture archiving and communication system
of the hospital network. The time interval between the
day of CT examination and interventional procedure
was logged. DICOM images were assessed using 3D
Slicer (macOS version 5.6.2; The Slicer Community),
an open-source image computing platform.[16] Semi-automatic
segmentation of AMLs was performed in
the following sequence (Figure 3): (1) reformation of
contrast-enhanced CT images in axial, coronal and
sagittal planes; (2) manual contouring of tumour and
non-tumour regions on limited CT slices (<5); (3)
automatic segmentation of tumour and non-tumour
regions using the ‘grow from seeds’ algorithm; (4)
manual refinement of segmented regions using ‘paint’
and ‘erase’ algorithms; (5) automatic differentiation
between angiomyogenic and fatty components of AML
using a ‘threshold’ algorithm, with the threshold set
at ≥ -10 HU for the angiomyogenic component and
< -10 HU for the fatty component; (6) automatic volume
rendering of tumour and non-tumour regions; and (7)
automatic volumetric computation of the entire AML,
as well as its angiomyogenic and fatty components. In
addition, laterality and polarity of AML, as well as the
presence or absence of aneurysms, were documented.
In postprocedural CT, any complications, including
renal parenchymal infarction, haematoma, abscess,
pyelonephritis, or hydronephrosis, were recorded.
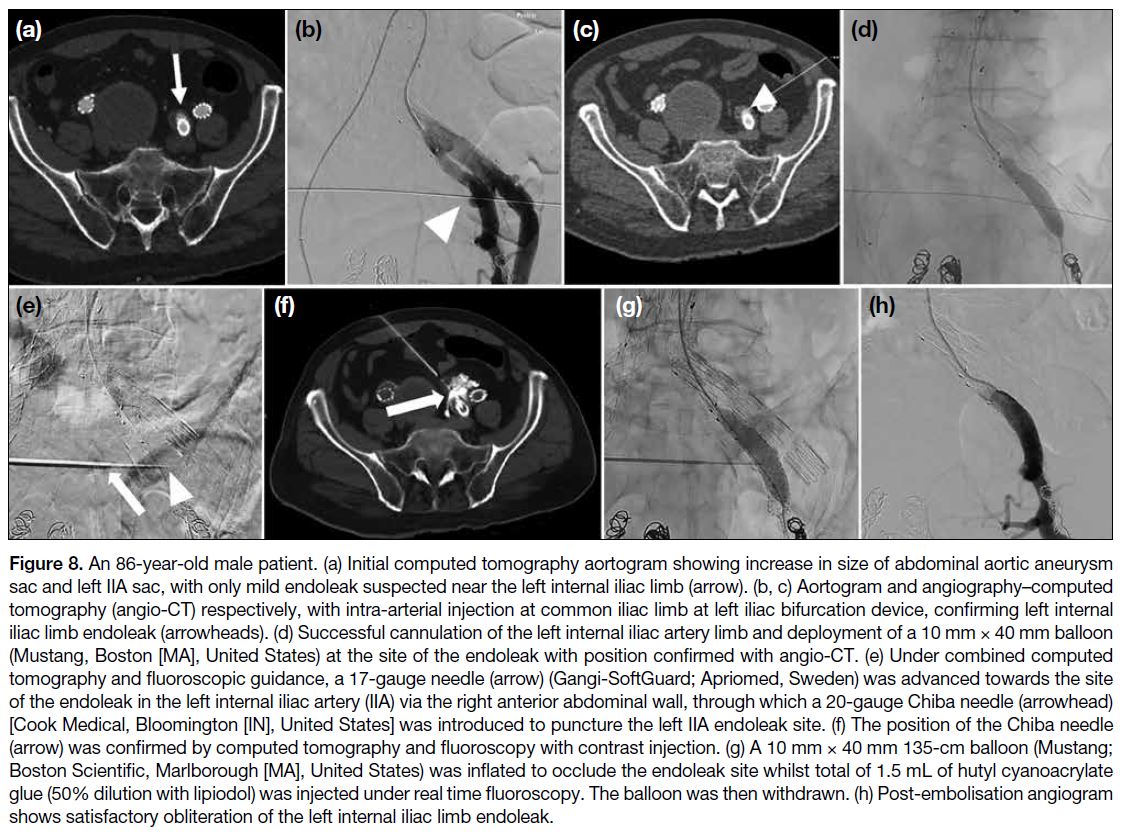
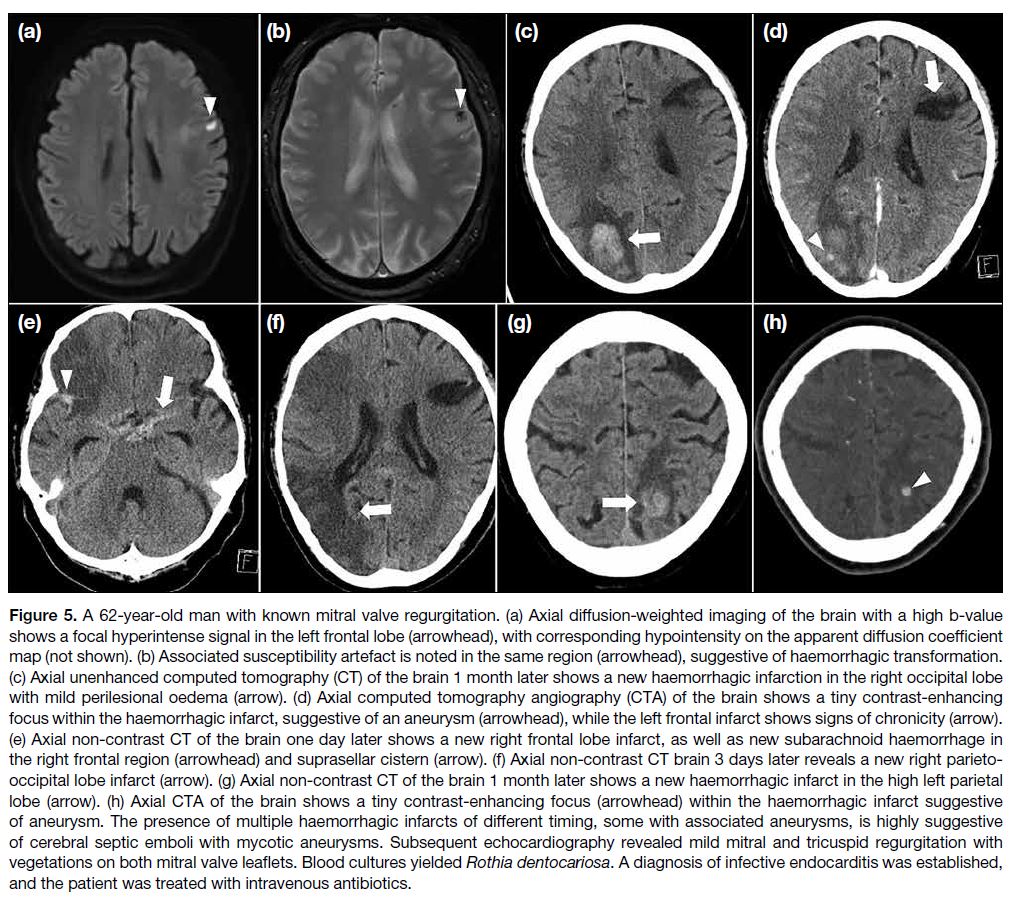
Figure 3. Semi-automatic segmentation of renal
angiomyolipoma (AML) using 3D Slicer. (a) Digital Imaging
and Communications in Medicine images of contrastenhanced
computed tomography (CT) of the abdomen
are reformatted in the axial (upper left), coronal (lower
left), and sagittal planes (lower right). An AML (arrows)
arises from the upper pole of the left kidney. (b) Contrast-enhanced
CT of the abdomen reformatted in sagittal
planes. Contours of AML (arrows) and other non-tumour
regions are drawn manually on several (<5) CT slices. (c)
Automatic segmentation of AML (arrows) is performed
in the axial (upper left), coronal (lower left), and sagittal
planes (lower right) using the ‘grow from seeds’ algorithm.
Further refinement of the segmented regions is possible
using the ‘paint’ and ‘erase’ algorithms. Automatic three-dimensional
rendering of (d) non-tumour regions and (e)
the AML are shown. Further volumetric analysis, such as
determining the angiomyogenic and fatty components of
the AML using -10 Hounsfield unit as the threshold, can
be performed.
Statistical Analysis
Statistical analysis was performed using SPSS (macOS
version 29.0; IBM Corp, Armonk [NY], United States).
The distribution of all numerical data was first tested for normality using the Shapiro-Wilk test. The Wilcoxon
signed-rank test was used to compare paired data,
such as pre- and postprocedural volumetric changes
in each AML. The Mann-Whitney U test was used for
comparison between unpaired data. Spearman’s rank
correlation coefficient was used to identify association
between variables. A p value of < 0.05 was considered
statistically significant.
This manuscript was prepared in accordance with the
STROBE (Strengthening the Reporting of Observational
Studies in Epidemiology) guidelines.
RESULTS
Patient Demographics and Clinical
Information
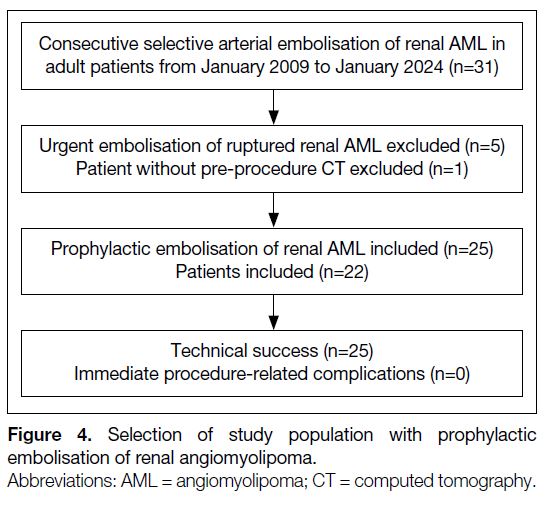
There were 31 prophylactic embolisations of AMLs
in adult patients performed from January 2009 to
January 2024. Five patients underwent emergent
embolisation of ruptured AMLs. One patient did not
have preoperative CT available for assessment. These
six patients were therefore excluded. A total of 25
prophylactic embolisations of AML in 22 patients were
finally included in the study, of which three patients had
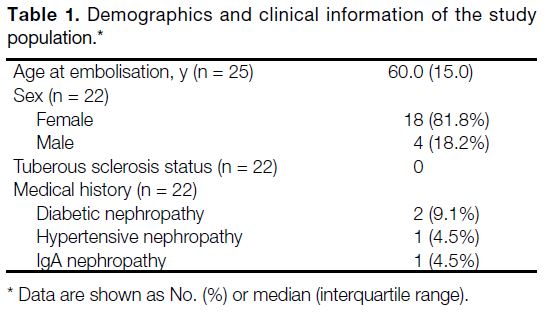
repeated embolisation (n = 3, 13.6%) [Figure 4]. The median age of the patients on the day of embolisation
was 60.0 years (interquartile range, 15.0). Most patients
were female (n = 18, 81.8%). There was no patient with
tuberous sclerosis. Four patients (18.2%) had known
chronic kidney disease due to diabetic nephropathy
(n = 2, 9.1%), hypertensive nephropathy (n = 1, 4.5%)
and IgA nephropathy (n = 1, 4.5%) [Table 1].
Figure 4. Selection of study population with prophylactic embolisation of renal angiomyolipoma.
Table 1. Demographics and clinical information of the study population.
Prophylactic Embolisation of Renal
Angiomyolipoma
All prophylactic embolisations of AMLs were performed
due to large tumour size (≥4 cm in diameter). In one
case (4.0%), there was a 0.5-cm aneurysm identified
in preprocedural assessment, which was successfully
embolised. Microspheres (Embosphere Microsphere;
Merit Medical Systems, South Jordan [UT], United
States) were employed in two-thirds of all interventions
(n = 17, 68.0%). The sizes of the microparticles ranged
between 100-300 μm (n = 2, 11.8%), 300-500 μm
(n = 6, 35.3%) and 500-700 μm (n = 9, 52.9%). Ethanol
was used in the remaining one-third of the cases (n = 8,
32.0%). Ethanol was radio-opacified with ethiodised
oil in a ratio of 7:3 for embolisation of the other cases.
Selective arterial embolisation with microcatheters
and microguidewires was performed in every case.
All prophylactic embolisation achieved technical success. There were no intra-procedural or immediate
postprocedural complications encountered. The median
time intervals between pre- and postprocedural CT with
prophylactic embolisation were 90.0 days and 107.0 days,
respectively. Postprocedural CT showed a small (2.0 cm
in diameter) subsegmental renal infarction in one case
(4.0%). No other complications were seen. There were
no significant changes in haemoglobin level or renal
function tests before and after prophylactic embolisation.
Median clinical follow-up duration was over 4 years
(49.0 months), with a minimum of 6 months. There were
no post-embolisation rupture of AML (Table 2).
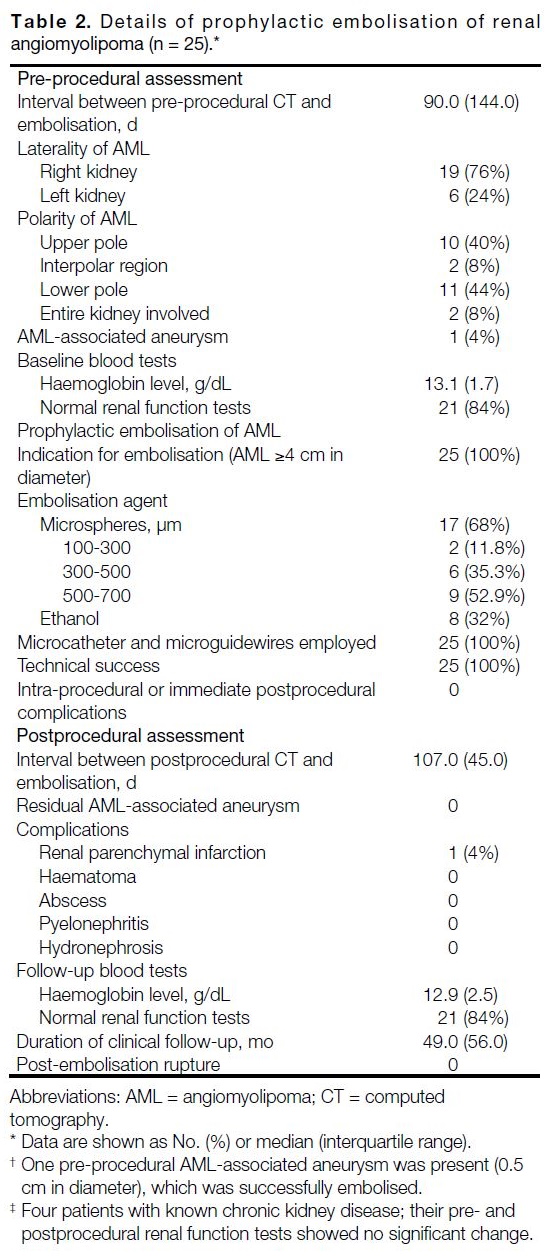
Table 2. Details of prophylactic embolisation of renal angiomyolipoma (n = 25).
Volumetric Analysis of Renal Angiomyolipoma
The median total volume of AMLs in pre- and
postprocedural CTs were 67.5 cm3 and 35.7 cm3,
respectively, showing significant interval shrinkage, with 41.4% total tumour volume reduction. Both
angiomyogenic and fatty components showed
significant interval reduction in size, attaining 73.6% and
14.0% volume loss, respectively. The angiomyogenic
component of the AMLs showed significantly greater
reduction in size compared to the fatty component
(Table 3).
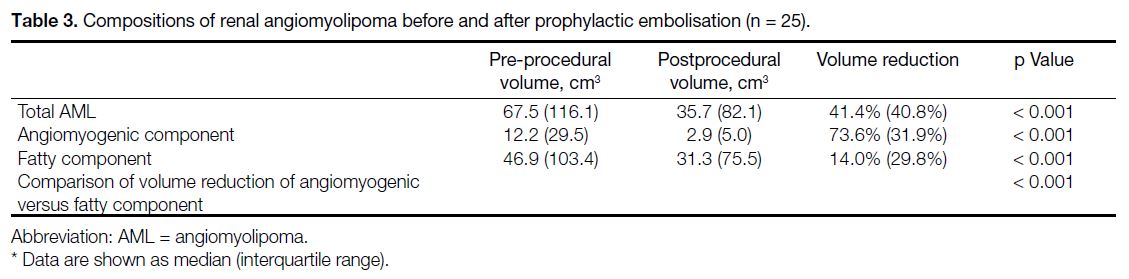
Table 3. Compositions of renal angiomyolipoma before and after prophylactic embolisation (n = 25).
Correlation analysis revealed AMLs with a greater
proportion of angiomyogenic component and smaller
proportion of fatty component in preprocedural CT
were associated with greater tumour volume reduction
after prophylactic embolisation. No other clinical or
procedural factors associated with total tumour shrinkage
were identified (Table 4).
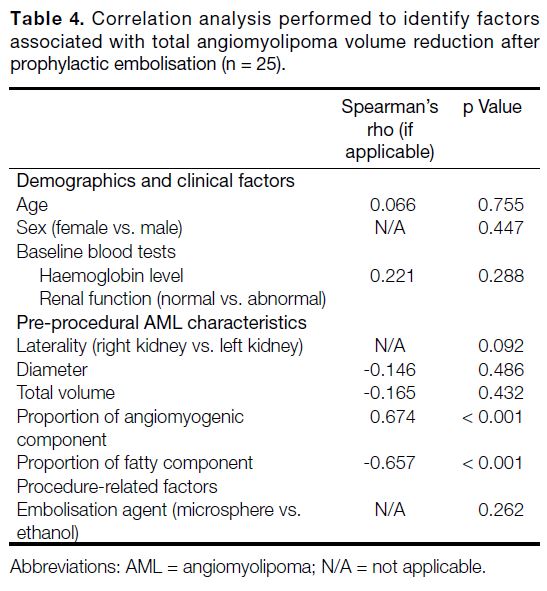
Table 4. Correlation analysis performed to identify factors associated with total angiomyolipoma volume reduction after prophylactic embolisation (n = 25).
Prophylactic embolisation of AML with either
microspheres or ethanol achieved significant reduction
in total tumour size, with 26.2% (p < 0.001) and 42.7%
(p = 0.008) volume loss, respectively. Using either
embolisation agent, there were significant reduction
in volume of both angiomyogenic (microspheres:
71.6%, p < 0.001; ethanol: 81.0%, p = 0.008) and fatty
components (microspheres: 12.7%, p < 0.001; ethanol:
29.7%, p = 0.008), with the angiomyogenic component showing significantly greater volume loss than the fatty
component using either embolisation agent (both p < 0.001). Comparing microspheres and ethanol, there were
no statistically significant differences in their efficacy of
reduction of the total tumour volume, angiomyogenic or
fatty components of AML (Table 5).
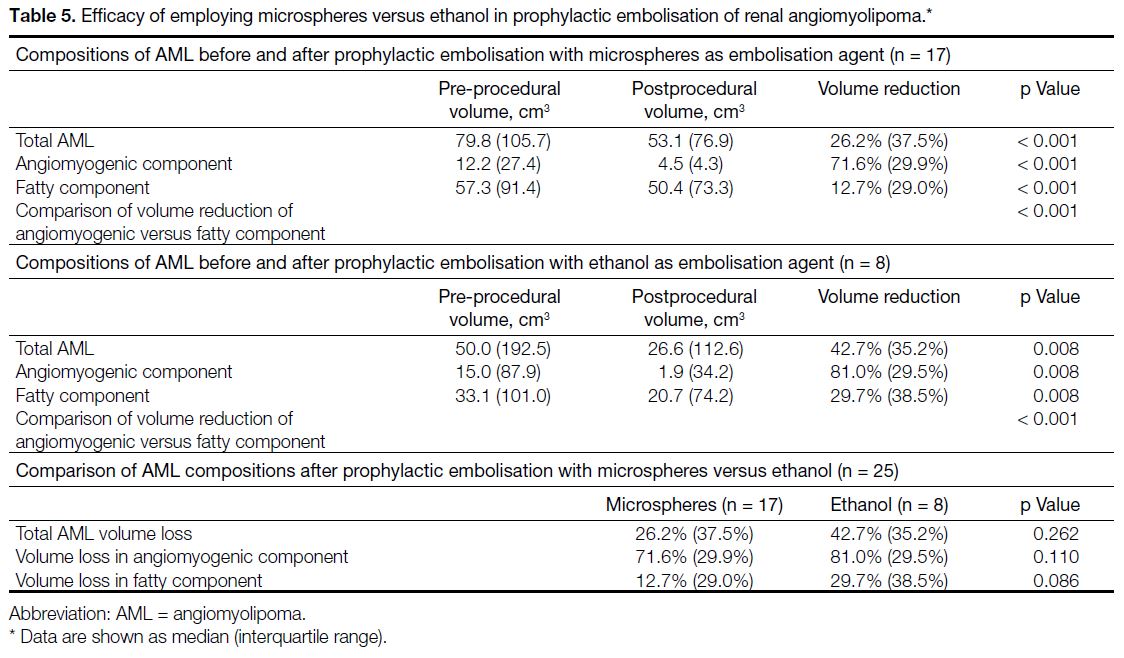
Table 5. Efficacy of employing microspheres versus ethanol in prophylactic embolisation of renal angiomyolipoma.
DISCUSSION
Transcatheter embolisation of AML is recognised as
a safe intervention.[9] [10] Compared to more invasive
treatment options such as partial or radical nephrectomy,
transcatheter selective arterial embolisation typically
only requires local anaesthesia, has lower risks of
bleeding and infection, and allows shorter admission
times. Some authors therefore suggest transcatheter
embolisation as the first-line treatment option.[8] In our
study, no intra-procedural or immediate complications
were encountered. However, in one patient, a small
subsegmental renal infarction was seen in postprocedural
CT. This highlights the importance of follow-up imaging,
which encompasses assessment of treatment efficacy, as
well as identification of complications.
There was significant change in tumour volume after
prophylactic embolisation of AML, achieving over 40%
reduction in median total volume amongst our study
population. With decreased tumour volume, the risk of
spontaneous haemorrhage would be lowered.[6] [11] It was
reassuring that none of the included patients encountered
post-embolisation tumour rupture in clinical follow-up
with a median duration of over 4 years. These findings
demonstrate that prophylactic embolisation of AML is
a safe and effective means to reduce haemorrhagic risk,
concurrent with previous studies.[9] [10]
Various materials for prophylactic embolisation of
the kidney have been suggested in the literature,
with microspheres and ethanol being two of the
most commonly adopted agents. In this study, both microspheres and ethanol effectively reduced the size
of AMLs by over 25%, without a statistically significant
difference between the two agents. To the best of our
knowledge, there is no large-scale study establishing
whether microspheres or ethanol is the superior
prophylactic embolisation agent for AML.[9] [10] [12] In our centre, this choice depended on the operators’ preference.
It has been proposed that the effectiveness of
prophylactic embolisation in achieving volume reduction
depends on the composition of the AML, which has
variable angiomyogenic and fatty components. The
angiomyogenic component usually demonstrates
greater response to embolisation due to its vascular
nature, whereas the fatty component is hypovascular
and more treatment-resistant.[9] [17] A study by Han et al[17]
showed near-complete resolution of the angiomyogenic
component after prophylactic embolisation, but the
fatty component only partially shrank. In their study,
the proportion of angiomyogenic and fatty components
were evaluated on a transverse image at the middle
of the tumour. However, this might not reflect the
actual composition of the entire AML. In our study,
semi-automatic segmentation was performed, and the
angiomyogenic and fatty components were differentiated
using -10 HU as the threshold. This allowed a more accurate volumetric assessment of AML. Similar to
prior studies, there was significantly greater reduction in
the angiomyogenic component than the fatty component
after embolisation.
AMLs with a greater proportion of angiomyogenic
component and smaller proportion of fatty component
are associated with greater total volume reduction
after embolisation. For AML with high fatty content,
patients and clinicians may be concerned that about the
smaller postprocedural volume reduction. However,
the angiomyogenic component of AML, which is the
main culprit in haemorrhage, has shown good response
to embolisation.[9] [17] In our study, the angiomyogenic
component achieved over 70% volume reduction, which
could be reassuring to both patients and clinicians.
Limitations
First, none of the patients in our study had tuberous
sclerosis. Treatment efficacy for sporadic and tuberous
sclerosis–associated AML may differ and have not
been explored. Second, there was a lack of a control
group to compare rupture risk in patients who received
prophylactic embolisation versus those who did not. A
double-arm study could better assess treatment effect.
Third, the sample size was limited. This may be partly attributed to the relatively low prevalence of AML, which
is below 0.5% in the population.[18] A multi-centre study
with larger sample sizes is a potential future direction.
CONCLUSION
Prophylactic embolisation of AML effectively reduced
tumour volume, with more significant changes in the
angiomyogenic component compared to the fatty
component. No rupture or haemorrhage was documented
post-embolisation with a median follow-up of over 4
years.
REFERENCES
1. Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN.
Benign renal neoplasms in adults: cross-sectional imaging findings.
AJR Am J Roentgenol. 2008;190:158-64. Crossref
2. Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150:1782-6. Crossref
3. Chan TY. World Health Organization classification of tumours: pathology & genetics of tumours of the urinary system and male
genital organs. Urology. 2005;65:214-5. Crossref
4. Halpenny D, Snow A, McNeill G, Torreggiani WC. The radiological diagnosis and treatment of renal angiomyolipoma—current status.
Clin Radiol. 2010;65:99-108. Crossref
5. Park BK. Renal angiomyolipoma: radiologic classification and
imaging features according to the amount of fat. AJR Am J
Roentgenol. 2017;209:826-35. Crossref
6. Ruud Bosch JL, Vekeman F, Duh MS, Neary M, Magestro M, Fortier J, et al. Factors associated with the number and size of renal
angiomyolipomas in sporadic angiomyolipoma (sAML): a study
of adult patients with sAML managed in a Dutch tertiary referral
center. Int Urol Nephrol. 2018;50:459-67. Crossref
7. Wang C, Li X, Peng L, Gou X, Fan J. An update on recent developments in rupture of renal angiomyolipoma. Medicine
(Baltimore). 2018;97:e0497. Crossref
8. Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of
renal angiomyolipoma. J Urol. 2016;195:834-46. Crossref
9. Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Crino PB, et al. Renal angiomyolipoma: long-term results after
arterial embolization. J Vasc Interv Radiol. 2005;16:45-50. Crossref
10. Lenton J, Kessel D, Watkinson AF. Embolization of renal angiomyolipoma: immediate complications and long-term
outcomes. Clin Radiol. 2008;63:864-70. Crossref
11. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M,
Takeda K. Renal angiomyolipoma: relationships between tumor
size, aneurysm formation, and rupture. Radiology. 2002;225:78-82. Crossref
12. Chatziioannou A, Gargas D, Malagari K, Kornezos I, Ioannidis I,
Primetis E, et al. Transcatheter arterial embolization as therapy of
renal angiomyolipomas: the evolution in 15 years of experience.
Eur J Radiol. 2012;81:2308-12. Crossref
13. Combes A, McQueen S, Palma CA, Benz D, Leslie S, Sved P, et al.
Is size all that matters? New predictors of complications and
bleeding in renal angiomyolipoma. Res Rep Urol. 2023;15:113-21. Crossref
14. Xu XF, Hu XH, Zuo QM, Zhang J, Xu HY, Zhang Y. A scoring
system based on clinical features for the prediction of sporadic renal
angiomyolipoma rupture and hemorrhage. Medicine (Baltimore).
2020;99:e20167. Crossref
15. Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, et al. Large renal angiomyolipomas: digital subtraction
angiographic grading and presentation with bleeding. Clin Radiol.
2006;61:520-6. Crossref
16. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC,
Pujol S, et al. 3D Slicer as an image computing platform for
the Quantitative Imaging Network. Magn Reson Imaging.
2012;30:1323-41. Crossref
17. Han YM, Kim JK, Roh BS, Song HY, Lee JM, Lee YH, et al. Renal
angiomyolipoma: selective arterial embolization—effectiveness
and changes in angiomyogenic components in long-term follow-up.
Radiology. 1997;204:65-70. Crossref
18. Fittschen A, Wendlik I, Oeztuerk S, Kratzer W, Akinli AS, Haenle MM, et al. Prevalence of sporadic renal angiomyolipoma:
a retrospective analysis of 61,389 in- and out-patients. Abdom
Imaging. 2014;39:1009-13. Crossref
Clinical and Imaging Outcomes of Radiosynoviorthesis in Haemophilic Arthropathy
ORIGINAL ARTICLE
Hong Kong J Radiol 2025 Dec;28(4):e243-9 | Epub 9 December 2025
Clinical and Imaging Outcomes of Radiosynoviorthesis in
Haemophilic Arthropathy
KH Chu1, FY Wan1, L Xu1, TWY Chin1, IWC Wong2, CP Lam3, JSM Lau3, MK Chan1, KC Lai1
1 Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Nuclear Medicine Unit, Queen Elizabeth Hospital, Hong Kong SAR, China
3 Department of Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr KH Chu, Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR,
China. Email: ckh975@ha.org.hk
Submitted: 12 December 2024; Accepted: 9 May 2025.
Contributors: KHC and KCL designed the study. All authors acquired and analysed the data. KHC, FYW and LX drafted the manuscript. FYW,
LX, TWYC, IWCW, CPL, JSML, MKC and KCL critically revised the manuscript for important intellectual content. All authors had full access
to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-113-1). A waiver of informed consent was obtained from the Board due to the retrospective nature of the study, with strict protections in
place to ensure patient privacy and the anonymity of personal data.
Abstract
Introduction
Radiosynoviorthesis, the intra-articular injection of radionuclides, is an established treatment for
haemophilic arthropathy. This study aimed to examine the clinical and imaging outcomes of radiosynoviorthesis
in Hong Kong.
Methods
A retrospective review of the radiosynoviorthesis cases performed from 2014 to 2023 in our tertiary
referral centre was conducted. Patients’ demographics, involved joints, injected radionuclides, technical success,
complications, and clinical outcomes (symptoms and frequency of bleeding) were assessed.
Results
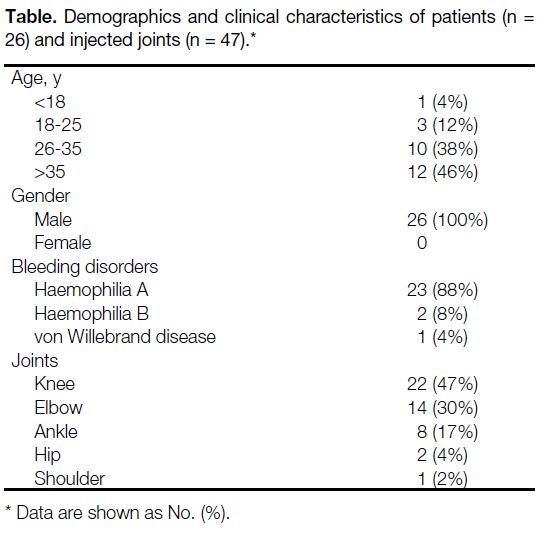
Radiosynoviorthesis was performed on 47 joints (22 knees, 14 elbows, 8 ankles, 2 hips, and 1 shoulder) in
26 patients. Joint injections were performed under fluoroscopic or ultrasound guidance, with a technical success
rate of 98%. Six (13%) joints showed mild systemic absorption, and two (4%) joints developed transient radiation
synovitis. No major complications were encountered. Excellent clinical outcomes were observed, with 83% of cases
demonstrating symptomatic improvement and 91% showing a reduction in bleeding frequency. The mean monthly
bleeding frequency decreased from 2.2 episodes before the procedure to 0.6 episode afterwards (p = 0.005). The
total number of hospitalisations or outpatient clinic visits due to haemarthrosis decreased from 60 to 31 in the year
following the procedure (p = 0.01).
Conclusion
Our case series suggests that radiosynoviorthesis is a safe and effective procedure that can improve
clinical symptoms and reduce bleeding frequency in haemophilic arthropathy. It should be considered as part of a
multidisciplinary management approach.
Key Words: Hemarthrosis; Hemophilia A; Injections; Knee; Radiotherapy
中文摘要
血友病性關節病放射性滑膜切除術的臨床和影像學結果
朱僑栩、尹芳盈、徐璐、錢永恩、黃偉宗、林靖邦、劉詩敏、陳文光、黎國忠
引言
放射性滑膜切除術,即關節內注射放射性核素,是治療血友病性關節病的成熟方法。本研究旨在探討放射性滑膜切除術在香港的臨床與影像學成效。
方法
本研究回顧分析了本中心於2014至2023年間進行的放射性滑膜切除術病例。評估內容包括患者的人口學特徵、受累關節、注射的放射性核素種類、技術成功率、併發症,以及臨床療效(症狀及出血頻率)。
結果
共為26位患者的47個關節(包括22個膝關節、14個肘關節、8個踝關節、2個髖關節及1個肩關節)進行放射性滑膜切除術。關節注射在透視或超聲影像引導下進行,技術成功率達98%。其中6個關節(13%)出現輕微的全身性放射性物質吸收,兩個關節(4%)出現短暫性放射性滑膜炎,未見重大併發症。臨床效果理想,83%病例症狀有所改善,91%病例出血頻率下降。平均每月出血次數由術前的2.2次顯著下降至術後的0.6次(p = 0.005)。術後一年內因關節積血而導致的住院及門診就診總次數由60次減少至31次(p = 0.01)。
結論
本病例系列顯示,放射性滑膜切除術是安全且有效的治療方式,能改善血友病性關節病患者的臨床症狀並降低出血頻率,應納入多學科綜合治療方案。
INTRODUCTION
Patients with haemophilia and von Willebrand disease
show an increased tendency to bleed. These patients can
present with recurrent haemarthrosis.[1] The synovium
becomes hypertrophied due to the inflammatory
response to iron deposition within the joint.[2] Increased
vascularity in the inflamed synovium renders it more
prone to bleeding. This creates a vicious cycle, leading to
cartilage and bone damage and resulting in arthropathy.
Prevention and treatment of musculoskeletal damage are
paramount in the care of patients with bleeding disorders.
Prophylactic measures include coagulation factor
replacement therapy and subcutaneous emicizumab
injections to reduce bleeding and prevent subsequent
haemarthropathy.[3] These measures have provided
greater protection for patients and significantly improved
patients’ quality of life. However, recurrent haemarthrosis
remains an issue for some patients despite advances in
medical treatments.[3] In the past, surgical synovectomy
was employed in patients who failed to respond to
medical treatment.[4] Over time, more studies have
reported favourable clinical outcomes with non-surgical
synovectomy, which includes intra-articular injection of
radioisotopes (radiosynoviorthesis) or antibiotics such as rifampicin.[1] [5] [6] [7] These minimally invasive interventions
have gained popularity and are now considered viable
alternatives to surgery.[8] [9] Surgery is reserved for cases
when intra-articular injections are unsuccessful.
Radiosynoviorthesis, also referred to radiation
synovectomy, involves the injection of radionuclides
into affected joints, leading to fibrosis of the inflamed
and hypertrophied synovium.[10] The primary objectives
of this treatment are to reduce bleeding frequency and
alleviate clinical symptoms such as pain and swelling.
Once absorbed by the synovium, the radionuclides emit
high-energy beta particles that induce cell death and
obliterate the capillary blood supply.[11] This results in
fibrosis and sclerosis of the synovial membrane, as well
as a significant decrease in inflammatory activity and
angiogenesis, ultimately reducing the bleeding tendency.
Although international guidelines and studies are
available for Western populations, there remains a
limited focus on Asian haemophilic patients and our
local population.[12] [13] In this retrospective study, we aimed
to evaluate the technical success, efficacy, and safety
of radiosynoviorthesis in our tertiary referral centre in
Hong Kong.
METHODS
All cases of radiosynoviorthesis performed on patients
with haemophilic arthropathy in Queen Elizabeth
Hospital between 2014 and 2023 were retrospectively
reviewed. Data were collected on patient demographics,
type of bleeding disorder, joints treated, radionuclides
administered, technical success, clinical outcomes, and
complications.
Technical success was defined as successful joint
puncture and intra-articular injection of radionuclides,
confirmed by postprocedural scintigraphy. Clinical
outcomes were assessed by evaluating patient records
for changes in joint pain, swelling, and bleeding
frequency. As transient synovitis could cause temporary
symptoms following the procedure, patients’ symptoms
were evaluated at least 3 months afterwards. Clinical
assessments were performed during follow-up visits 6
to 12 months post procedure. Bleeding frequency was
compared by analysing the monthly bleeding episodes
before the procedure and 12 months after the procedure.
The number of hospitalisations or outpatient clinic
appointments due to haemarthrosis during the same
period was also recorded. Comparisons were analysed
using the Wilcoxon signed-rank test.
Techniques
Patients with disturbing pain and recurrent haemarthrosis
(defined as three or more bleeding episodes in the
same joint over 6 months) despite medical treatment,
and with clinical or radiological evidence of synovitis,
were considered indicated for radiosynoviorthesis
and referred by haematologists.[10] Initial evaluation
was conducted by nuclear medicine physicians.
Contraindications included pregnancy, breastfeeding,
or local skin infection at the targeted joint.[14] Relative
contraindications included severe joint instability, bony
destruction, or significant cartilage loss. Preprocedural
imaging, including X-rays, ultrasound, and/or magnetic
resonance imaging, was used to assess the severity of
synovitis and arthropathy (Figure 1).
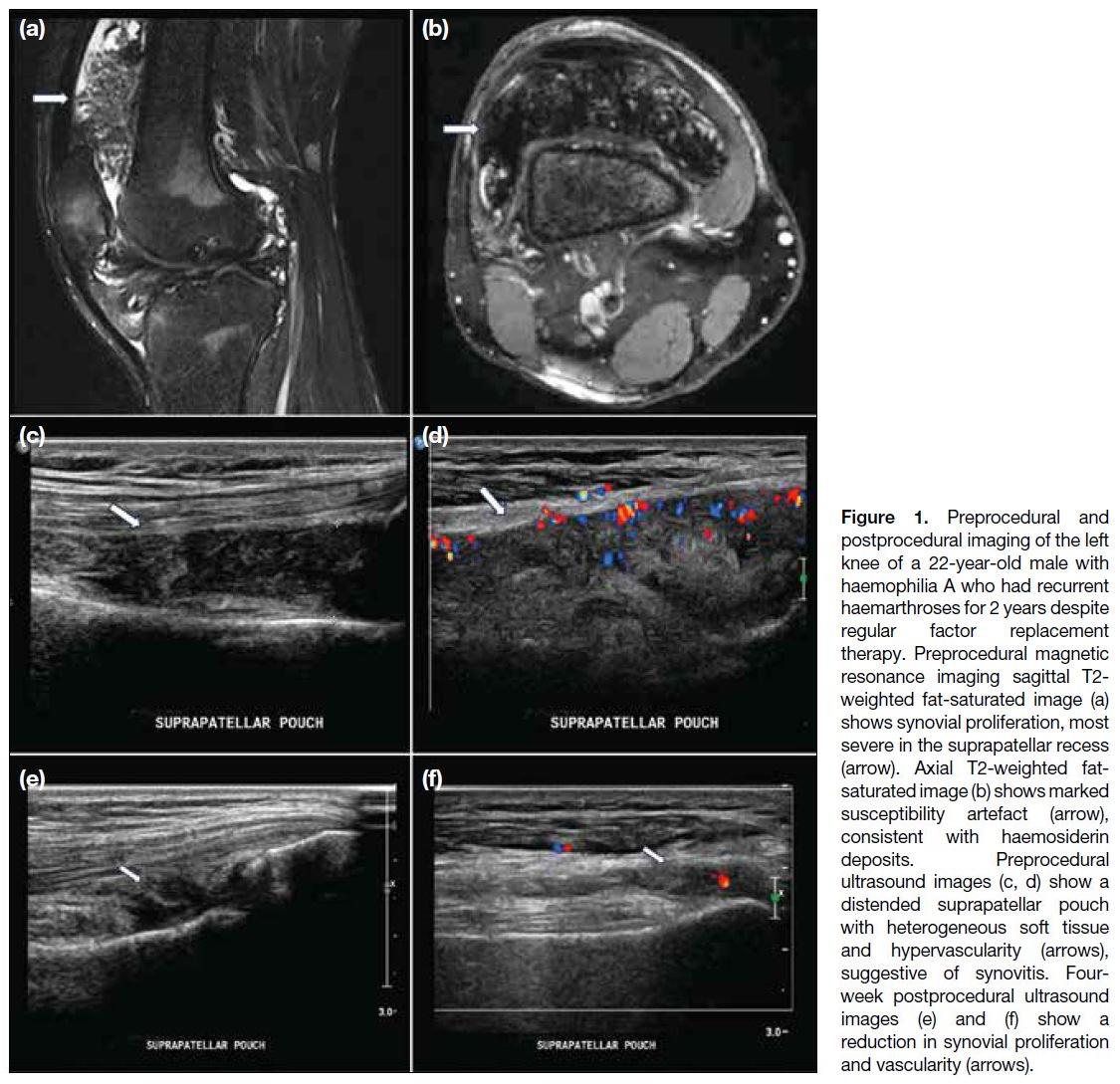
Figure 1. Preprocedural and
postprocedural imaging of the left knee of a 22-year-old male with haemophilia A who had recurrent haemarthroses for 2 years despite regular factor replacement therapy. Preprocedural magnetic resonance imaging sagittal T2-weighted fat-saturated image (a) shows synovial proliferation, most severe in the suprapatellar recess (arrow). Axial T2-weighted fat-saturated image (b) shows marked susceptibility artefact (arrow), consistent with haemosiderin deposits. Preprocedural ultrasound images (c, d) show a distended suprapatellar pouch with heterogeneous soft tissue and hypervascularity (arrows), suggestive of synovitis. Four-week postprocedural ultrasound images (e) and (f) show a reduction in synovial proliferation and vascularity (arrows).
The choice of radionuclides was based on the size of the
joint and required tissue penetration. Two beta-emitting
isotopes were used, namely, yttrium-90 (90Y) and
rhenium-186 (186Re).[15] These isotopes exhibit different
physical characteristics. 90Y, with a maximum beta
energy of 2.26 MeV and a mean tissue penetration of
3.6 mm, was used for knee joint. 186Re, with a maximum
beta energy of 0.98 MeV and a mean penetration of 1.2
mm, was employed for medium-sized joints including the hip, shoulder, elbow, and ankle. Doses ranged from
4.4 to 5.2 mCi (162.8-192.4 MBq) of 90Y for knees, 2.1
to 2.2 mCi (77.7-81.4 MBq) of 186Re for ankles, and
5.3 mCi (196.1 MBq) of 186Re for shoulders and hips.
All procedures were performed in ambulatory setting.
Under ultrasound or fluoroscopic guidance, the joint was
punctured, and contrast medium was injected to confirm
intra-articular location. The radionuclide was then
administered, along with a long-acting corticosteroid
such as triamcinolone acetonide, to reduce the risk of
radiation-induced synovitis and minimise leakage.[11] The
needle tract was flushed with saline during withdrawal to
prevent radiation necrosis of the puncture site.
After the procedure, the affected joint was immobilised
for 48 hours using a splint to reduce the risk of leakage
into surrounding tissues.[16] Bremsstrahlung imaging was
employed within 24 hours to confirm intra-articular
distribution of the radiopharmaceutical. Patients were
counselled on hygiene precautions due to urinary
excretion of the radionuclide. They were instructed to
flush the toilet twice after each use, wash their hands
thoroughly, avoid soiling underclothing or areas around
the toilet bowl, and wash any soiled garments separately.
Clinical follow-up was carried out by haematologists.
RESULTS
A total of 26 male patients, aged between 10 and 57
years (median, 35), underwent radiosynoviorthesis
during the study period (Table). The mean duration of
follow-up was 76 months (range, 10-116). Among them,
23 patients had haemophilia A, two had haemophilia B,
and one had von Willebrand disease. A total of 47 joints
were injected: 22 (47%) knees, 14 (30%) elbows, eight
(17%) ankles, two (4%) hips, and one (2%) shoulder.
Table. Demographics and clinical characteristics of patients (n = 26) and injected joints (n = 47).
Technical success was achieved in 46 (98%) out of the
47 joints. In one case, the right hip joint could not be
accessed due to advanced degenerative changes.
Improvement in symptoms, specifically pain
and swelling, was observed in 38 (83%) joints
(95% confidence interval [95% CI] = 69%-92%).
Eight (17%) joints showed no change in symptoms
(95% CI = 8%-31%), and no joint demonstrated
worsening. Three patients experienced partial symptom
relief after the first injection and subsequently underwent
a second injection 6 months later, after which all
reported further improvement. Although routine
follow-up imaging was not conducted for every patient, ultrasound in selected cases showed reduced synovial
proliferation and vascularity, indicating improvement
(Figure 1).
A reduction in bleeding frequency was noted in 42
(91%) joints (95% CI = 79%-98%), while four (9%)
joints showed no change (95% CI = 2%-21%). No
joint exhibited increased bleeding frequency. The mean
monthly bleeding frequency decreased from 2.2 episodes
(range, 0.5-6) before the procedure to 0.6 episode
(range, 0-4) afterwards (p = 0.005). Hospitalisations and
outpatient clinic visits due to haemarthrosis decreased from 60 to 31 in the year following the procedure (p = 0.01).
There were no major complications or procedure-related
mortality. There were no documented cases of bleeding,
infection, or necrosis.[17] [18] Minor complications or side-effects
were observed in eight cases. Six (13%) joints
showed postprocedural scintigraphic uptake in the liver
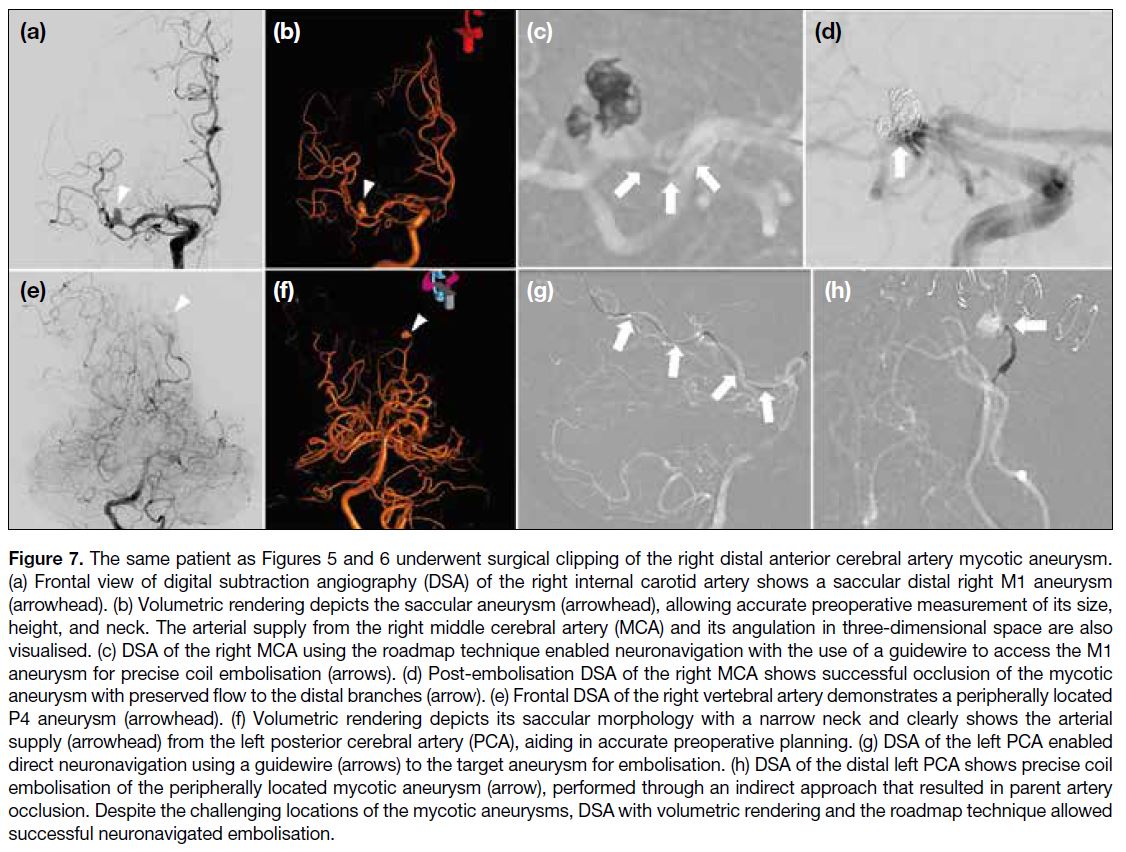
and spleen, suggestive of systemic absorption (95% CI = 5%-26%) [Figure 2]. Liver function tests during follow-up
were normal, and these patients still experienced
clinical improvement.
Figure 2. Scintigrams of a
24-year-old male with haemophilic
arthropathy of the right ankle
following radiosynoviorthesis
using rhenium-186. (a) Planar
images of the abdomen and pelvis
show tracer activity in the liver and
spleen. (b) Planar images of the
ankle show tracer activity within
the joint space of the right ankle,
confirming the intra-articular
location of the radionuclide.
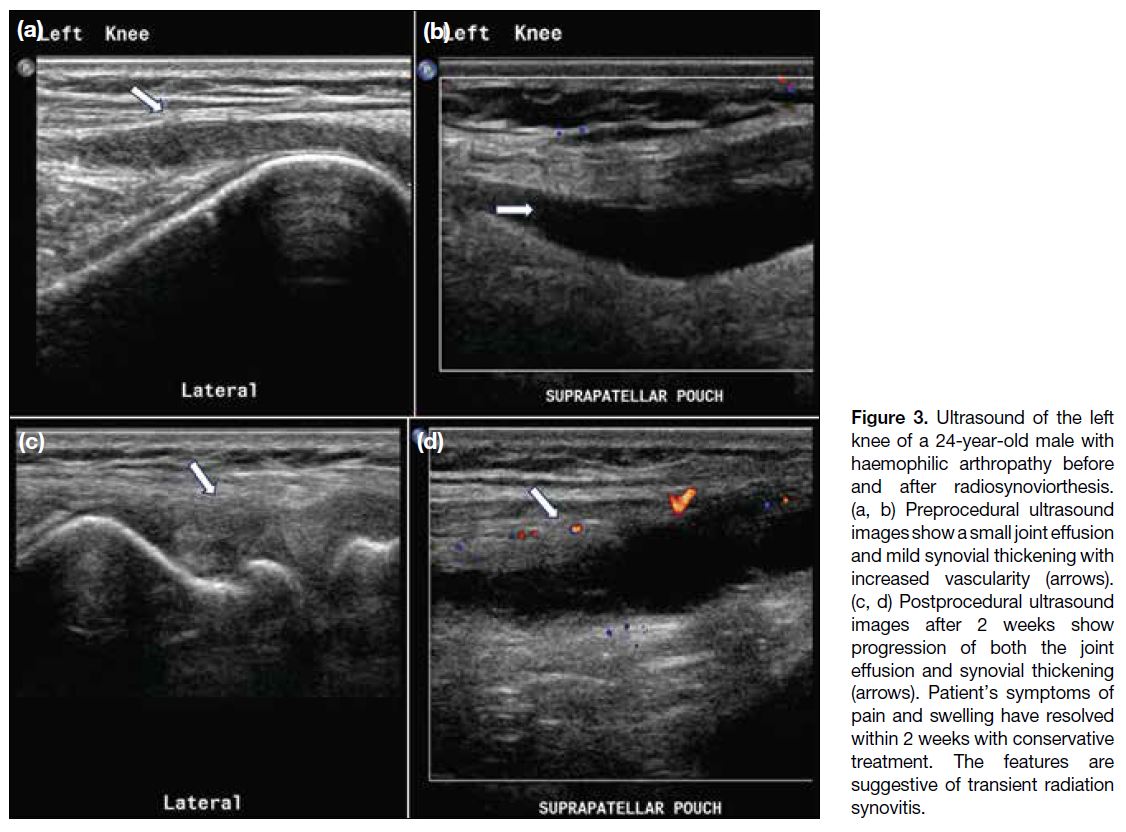
Two (4%) joints developed transient radiation
synovitis (95% CI=0.5%-15%), characterised by pain
and swelling shortly after the procedure. Ultrasound
confirmed increased joint effusion and synovitis (Figure 3). These symptoms resolved within 2 weeks following
conservative treatment with ice packs and nonsteroidal
anti-inflammatory drugs.
Figure 3. Ultrasound of the left
knee of a 24-year-old male with
haemophilic arthropathy before
and after radiosynoviorthesis.
(a, b) Preprocedural ultrasound
images show a small joint effusion
and mild synovial thickening with
increased vascularity (arrows).
(c, d) Postprocedural ultrasound
images after 2 weeks show
progression of both the joint
effusion and synovial thickening
(arrows). Patient’s symptoms of
pain and swelling have resolved
within 2 weeks with conservative
treatment. The features are
suggestive of transient radiation
synovitis.
DISCUSSION
This study demonstrated that most patients with bleeding
disorders and recurrent haemarthrosis responded well
to radiosynoviorthesis. Our findings are consistent with previous international studies. A systematic review
and a meta-analysis reported an overall response rate
of 72.5%.[19] Radiosynoviorthesis can be performed in
paediatric patients with appropriate selection and dosage
adjustment,[20] as shown by the successful treatment of
a 10-year-old in our cohort. It offers the advantages of
reduced hospital stays and lower costs compared with
surgical synovectomy. Moreover, it can be repeated up to
three times per joint, with intervals of at least 6 months.[21]
It can be difficult to perform intra-articular injection,
particularly in patients with severely deformed joints.
According to the literature, the best clinical improvement
was identified in patients with high inflammatory
activity in an early phase of arthropathy.[22] Therefore,
this procedure is expected to have maximal benefit in
patients in the earlier stages of arthropathy.
Radiosynoviorthesis is well tolerated, with a low
incidence of side-effects or complications. Extra-articular
activity was uncommon and was not accompanied by
clinically significant side-effects. Direct leakage out of
the joint space was rare, but systemic absorption could
occur due to uptake by the lymphatic circulation and,
subsequently, the bloodstream. This may be reduced by
immobilisation of the joints. Patients were encouraged
to increase their fluid intake and to void frequently.
Two patients experienced radiation-induced synovitis in
our review. It was a clinical manifestation of rapid and
extensive synovial tissue necrosis that can occur 6 to
48 hours after the procedure.[23] The joint pain, swelling,
and effusion are usually self-limiting and can be treated
conservatively by cooling the joint with ice packs and, if necessary, with anti-inflammatory drugs. Intra-articular
corticosteroid injection during the procedure can reduce
inflammation and decrease leakage of the radioisotope
through dilated capillaries of the synovium into the
systemic circulation.
Limitations
There are several limitations in this study. First, it
was retrospective in nature, with a relatively small
cohort size, and no control arm was available for
comparison. Nonetheless, this study still offered
results in our local population that were in line with
other studies demonstrating the efficacy and safety of
radiosynoviorthesis.[5] [6] [7] [10] [22] Another limitation was the
heterogeneous study population, with different joints
involved and varying severity of arthropathy. In general,
most patients still showed favourable clinical outcomes.
Subgroup analysis may be considered in the future with
a larger number of patients.
There was no objective pain scoring system in place to
provide a quantitative assessment of clinical symptoms,
nor was there a standardised magnetic resonance imaging
protocol to exclude patients with severe cartilage loss or
degenerative joint conditions that could contribute to
pain. A prospective study would be ideal for recruiting
patients and conducting assessments using an objective
scoring system for symptoms and a standard follow-up
protocol. Radiological investigations, such as ultrasound,
can be used to assess for synovitis and serve as another
objective parameter in evaluating outcomes. This
approach would also facilitate longitudinal comparisons
to investigate long-term efficacy and allow monitoring
of disease progression. Lastly, there may be potential
confounders such as the co-injection of steroid with the
radionuclides. The use of steroids may have caused a
period of analgesia and helped bridge the gap between
the administration of the radiopharmaceutical and the
onset of the effects of radiosynoviorthesis. However, such an effect was expected to be short term and unlikely
to persist beyond 3 months, when our clinical assessment
was conducted.
CONCLUSION
Radiosynoviorthesis is a safe and effective procedure
which can contribute to symptomatic improvement
and a reduction in bleeding frequency in patients with
haemophilic arthropathy. It should be considered as part
of a multidisciplinary management approach.
REFERENCES
1. van Galen KP, Mauser-Bunschoten EP, Leebeek FW. Hemophilic
arthropathy in patients with von Willebrand disease. Blood Rev.
2012;26:261-6. Crossref
2. Hoots WK, Rodriguez N, Boggio L, Valentino LA. Pathogenesis
of haemophilic synovitis: clinical aspects. Haemophilia. 2007;13
Suppl 3:4-9. Crossref
3. Oldenburg J. Optimal treatment strategies for hemophilia:
achievements and limitations of current prophylactic regimens.
Blood. 2015;125:2038-44. Crossref
4. Llinás A. The role of synovectomy in the management of a target
joint. Haemophilia. 2008;14 Suppl 3:177-80. Crossref
5. Querol-Giner M, Pérez-Alenda S, Aguilar Rodríguez M,
Carrasco JJ, Bonanad S, Querol F. Effect of radiosynoviorthesis
on the progression of arthropathy and haemarthrosis reduction in
haemophilic patients. Haemophilia. 2017;23:e497-503. Crossref
6. Desaulniers M, Paquette M, Dubreuil S, Senta H, Lavallée É,
Thorne JC, et al. Safety and efficacy of radiosynoviorthesis: a
prospective Canadian multicenter study. J Nucl Med. 2024;65:1095-100. Crossref
7. Kavakli K, Aydoğdu S, Omay SB, Duman Y, Taner M, Capaci K,
et al. Long-term evaluation of radioisotope synovectomy with
yttrium 90 for chronic synovitis in Turkish haemophiliacs: Izmir
experience. Haemophilia. 2006;12:28-35. Crossref
8. van Vulpen LF, Thomas S, Keny SA, Mohanty SS. Synovitis and
synovectomy in haemophilia. Haemophilia. 2021;27 Suppl 3:96-102. Crossref
9. Rodriguez-Merchan EC, Wiedel JD. General principles
and indications of synoviorthesis (medical synovectomy) in
haemophilia. Haemophilia. 2001;7 Suppl 2:6-10. Crossref
10. Kampen WU, Boddenberg-Pätzold B, Fischer M, Gabriel M, Klett R, Konijnenberg M, et al. The EANM guideline for
radiosynoviorthesis. Eur J Nucl Med Mol Imaging. 2022;49:681-708. Crossref
11. Fischer M, Mödder G. Radionuclide therapy of inflammatory joint diseases. Nucl Med Commun. 2002;23:829-31. Crossref
12. Hanley J, McKernan A, Creagh MD, Classey S, McLaughlin P,
Goddard N, et al. Guidelines for the management of acute joint
bleeds and chronic synovitis in haemophilia: A United Kingdom
Haemophilia Centre Doctors’ Organisation (UKHCDO) guideline.
Haemophilia. 2017;23:511-20. Crossref
13. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M,
Pipe SW, et al. WFH Guidelines for the Management of
Hemophilia, 3rd edition. Haemophilia. 2020;26 Suppl 6:1-158. Crossref
14. Chojnowski MM, Felis-Giemza A, Kobylecka M. Radionuclide
synovectomy—essentials for rheumatologists. Reumatologia.
2016;54:108-16. Crossref
15. Knut L. Radiosynovectomy in the therapeutic management of arthritis. World J Nucl Med. 2015;14:10-5. Crossref
16. Ahmad I, Nisar H. Dosimetry perspectives in radiation synovectomy. Phys Med. 2018;47:64-72. Crossref
17. Kampen WU, Matis E, Czech N, Soti Z, Gratz S, Henze E. Serious
complications after radiosynoviorthesis. Survey on frequency and
treatment modalities. Nuklearmedizin. 2006;45:262-8. Crossref
18. Infante-Rivard C, Rivard GE, Derome F, Cusson A, Winikoff R,
Chartrand R, et al. A retrospective cohort study of cancer incidence
among patients treated with radiosynoviorthesis. Haemophilia.
2012;18:805-9. Crossref
19. van der Zant FM, Boer RO, Moolenburgh JD, Jahangier ZN,
Bijlsma JW, Jacobs JW. Radiation synovectomy with (90)yttrium,
(186)rhenium and (169)erbium: a systematic literature review with
meta-analyses. Clin Exp Rheumatol. 2009;27:130-9.
20. Manco-Johnson MJ, Nuss R, Lear J, Wiedel J, Geraghty SJ,
Hacker MR, et al. 32P radiosynoviorthesis in children with
hemophilia. J Pediatr Hematol Oncol. 2002;24:534-9. Crossref
21. Clunie G, Fischer M; EANM. EANM procedure guidelines for radiosynovectomy. Eur J Nucl Med Mol Imaging. 2003;30:BP12-6. Crossref
22. Kresnik E, Mikosch P, Gallowitsch HJ, Jesenko R, Just H,
Kogler D, et al. Clinical outcome of radiosynoviorthesis: a
meta-analysis including 2190 treated joints. Nucl Med Commun.
2002;23:683-8. Crossref
23. Pirich C, Schwameis E, Bernecker P, Radauer M, Friedl M,
Lang S, et al. Influence of radiation synovectomy on articular
cartilage, synovial thickness and enhancement as evidenced by MRI
in patients with chronic synovitis. J Nucl Med. 1999;40:1277-84.
CASE REPORT
Intracranial Neuroendocrine Tumour of Unknown Origin Mimicking Neurocysticercosis: A Case Report
CASE REPORT
Hong Kong J Radiol 2025 Dec;28(4):e250-6 | Epub 10 December 2025
Intracranial Neuroendocrine Tumour of Unknown Origin
Mimicking Neurocysticercosis: A Case Report
CW Chan, KO Cheung, CY Cheung
Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr CW Chan, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: ccw147@ha.org.hk
Submitted: 3 December 2024; Accepted: 11 April 2025.
Contributors: All authors designed the study. CWC acquired and analysed the data and drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki and provided verbal consent for publication of this case
report, including the accompanying clinical images.
INTRODUCTION
Neuroendocrine tumours of the central nervous system
are relatively rare entities and most cases are metastases.
Primary intracranial neuroendocrine tumour is even rarer,
with fewer than a dozen cases reported worldwide.[1] [2] [3] [4] [5] [6] [7] [8]
Apart from a case report on a 5-year-old child,[9] all
reported primary cases have been in adults. The location
of lesions reported varies greatly. Reported extra-axial
locations include, but are not limited to, the
cerebellopontine angle, jugular foramen, cavernous
sinus, and skull base. Reported intra-axial locations include the left temporal and parietal lobes, as well as the left
cerebellum. We report a case of neuroendocrine tumour
of unknown origin with multiple intracranial metastases.
CASE PRESENTATION
A 56-year-old woman with good past health presented to
the accident and emergency department with dizziness
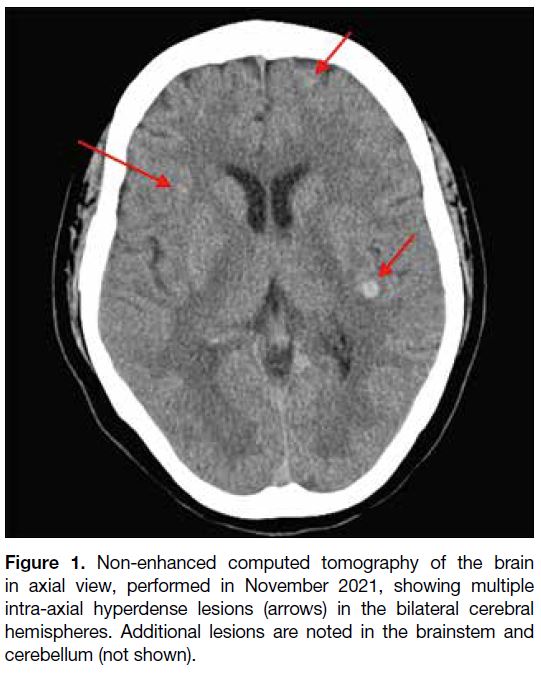
in October 2021. Initial computed tomography of the
brain revealed multiple hyperdense lesions scattered
across both cerebral hemispheres, the brainstem, and the
cerebellum (Figure 1). Whole-body positron emission
tomography–computed tomography (PET-CT) with 18F-fluorodeoxyglucose (18F-FDG) did not reveal any
primary malignancy elsewhere that could suggest brain
metastases. Contrast-enhanced magnetic resonance
imaging (MRI) of the brain was performed to
characterise the intracranial lesions (Figure 2). The
corresponding cerebral, brainstem, and cerebellar lesions
showed T1 hyperintense and T2 heterogeneous mixed
signals. Most lesions showed susceptibility artefacts,
while some showed a signal on the phase sequence
characteristic of calcification. Overall features were
suggestive of concurrent haemorrhagic and calcified
lesions. Some lesions also showed eccentric nodular
enhancement. One 7-mm lesion in the left frontal lobe demonstrated restricted diffusion and a suspicious eccentric scolex (Figure 2b). In view of the previously
negative whole-body PET-CT, the possibility of central
nervous system infection with neurocysticercosis in
different stages was considered a likely possibility. A
differential diagnosis of haemorrhagic/calcified brain
metastases appeared less likely. Serology testing for
Taenia solium was negative, but given the radiological
appearance of neurocysticercosis, the patient was
prescribed a course of albendazole and praziquantel, as
well as dexamethasone to minimise cerebral oedema.
Figure 1. Non-enhanced computed tomography of the brain
in axial view, performed in November 2021, showing multiple
intra-axial hyperdense lesions (arrows) in the bilateral cerebral
hemispheres. Additional lesions are noted in the brainstem and
cerebellum (not shown).
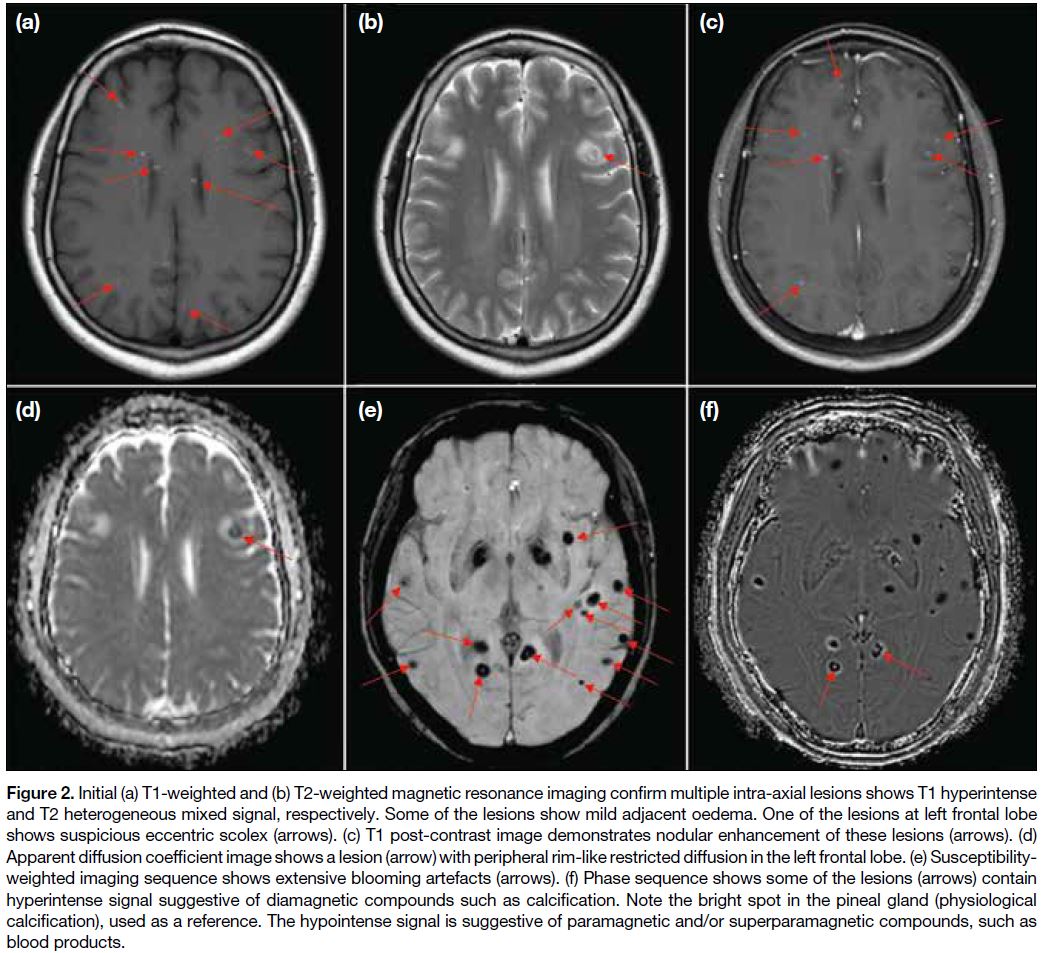
Figure 2. Initial (a) T1-weighted and (b) T2-weighted magnetic resonance imaging confirm multiple intra-axial lesions shows T1 hyperintense
and T2 heterogeneous mixed signal, respectively. Some of the lesions show mild adjacent oedema. One of the lesions at left frontal lobe
shows suspicious eccentric scolex (arrows). (c) T1-weighted post-contrast image demonstrates nodular enhancement of these lesions (arrows). (d)
Apparent diffusion coefficient image shows a lesion (arrow) with peripheral rim-like restricted diffusion in the left frontal lobe. (e) Susceptibility-weighted
imaging sequence shows extensive blooming artefacts (arrows). (f) Phase sequence shows some of the lesions (arrows) contain
hyperintense signal suggestive of diamagnetic compounds such as calcification. Note the bright spot in the pineal gland (physiological
calcification), used as a reference. The hypointense signal is suggestive of paramagnetic and/or superparamagnetic compounds, such as
blood products.
Multiple follow-up MRI scans of the brain were
performed. Initially, at 1-month post-treatment, some
lesions (particularly those at the frontal and temporal lobes) showed interval enlargement with an increase in
perilesional vasogenic oedema (Figure 3). These findings
were thought to be attributable to posttreatment change.
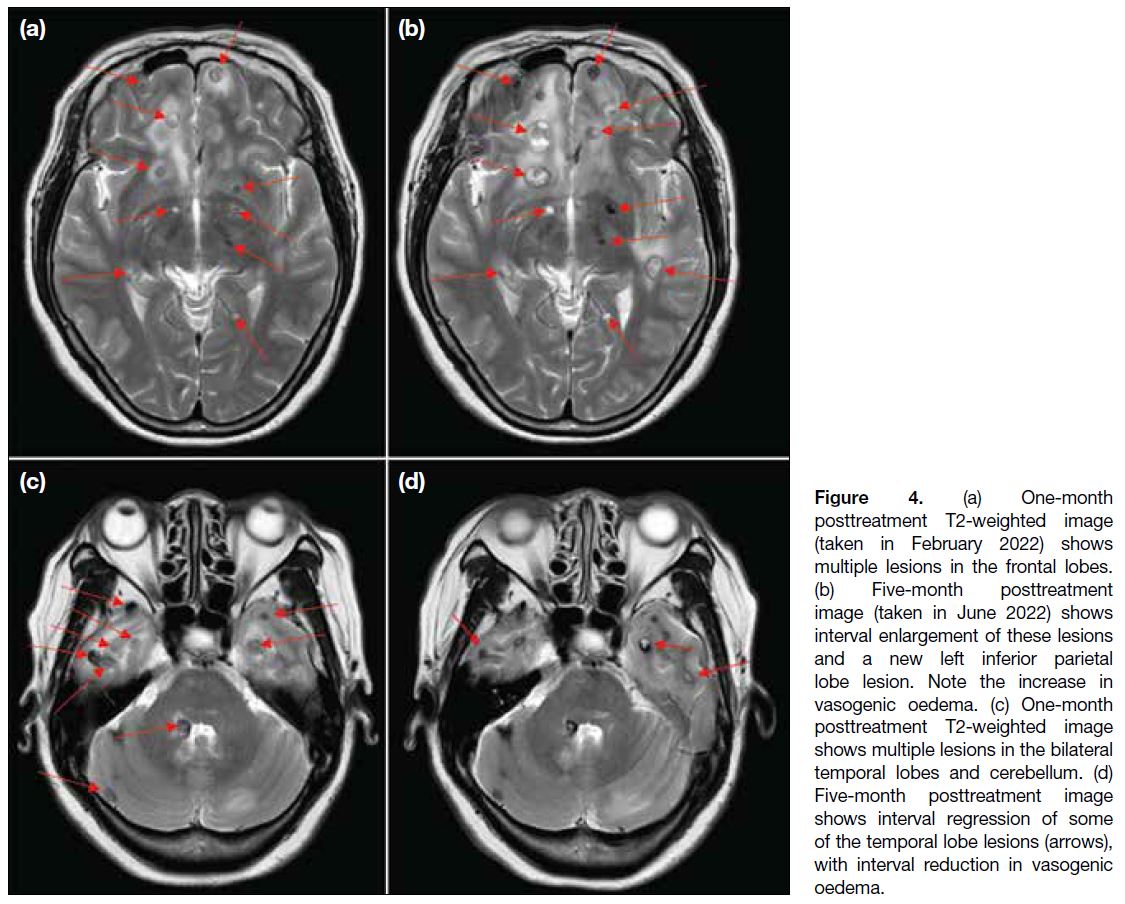
A scan at 5 months posttreatment revealed continued progression of some lesions in the bilateral frontal and left inferior parietal lobes (Figure 4a and b), while some lesions in the bilateral temporal lobes had regressed (Figure 4c and d). The overall picture favoured a mixed treatment response.
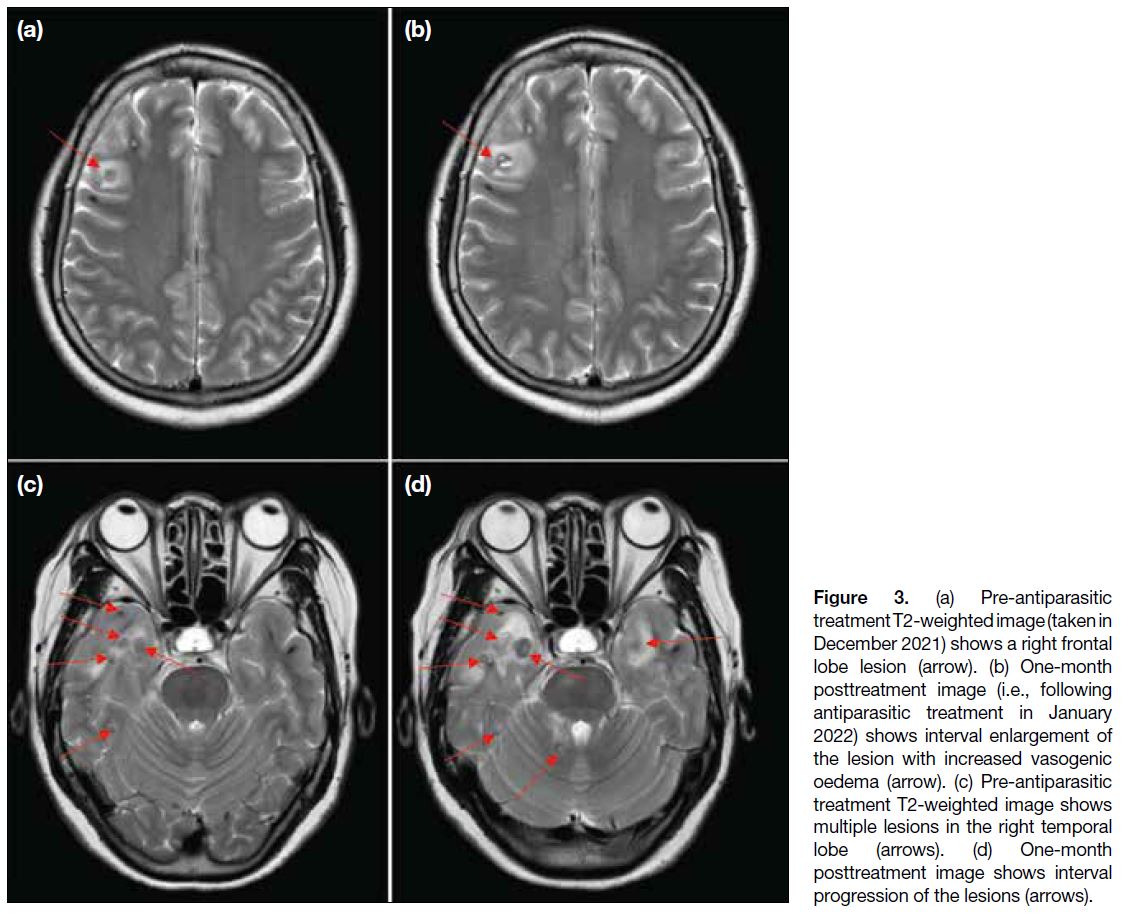
Figure 3. (a) Pre-antiparasitic
treatment T2-weighted image (taken in
December 2021) shows a right frontal
lobe lesion (arrow). (b) One-month
posttreatment image (i.e., following
antiparasitic treatment in January
2022) shows interval enlargement of
the lesion with increased vasogenic
oedema (arrow). (c) Pre-antiparasitic
treatment T2-weighted image shows
multiple lesions in the right temporal
lobe (arrows). (d) One-month
posttreatment image shows interval
progression of the lesions (arrows).
Figure 4. (a) One-month
posttreatment T2-weighted image
(taken in February 2022) shows
multiple lesions in the frontal lobes.
(b) Five-month posttreatment
image (taken in June 2022) shows
interval enlargement of these lesions
and a new left inferior parietal
lobe lesion. Note the increase in
vasogenic oedema. (c) One-month
posttreatment T2-weighted image
shows multiple lesions in the bilateral
temporal lobes and cerebellum (arrows). (d)
Five-month posttreatment image
shows interval regression of some
of the temporal lobe lesions (arrows),
with interval reduction in vasogenic
oedema.
A further course of antiparasitic treatment was given,
assuming the infection was unresolved. Nonetheless,
follow-up scan at 16 months after initiation of antiparasitic
treatment showed not only persistent lesions, but interval
enlargement of some (the largest at the left cerebellar
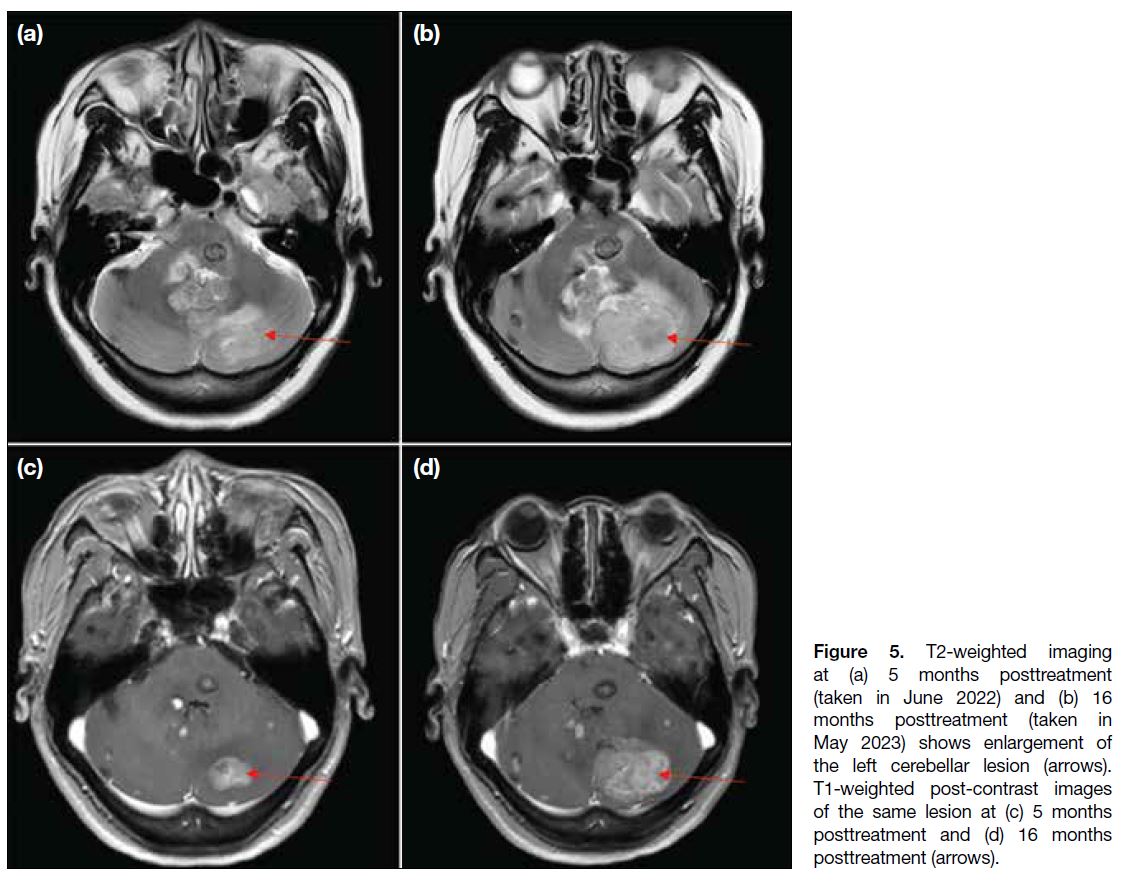
hemisphere; Figure 5), with developing obstructive
hydrocephalus. In view of the patient’s worsening
symptoms of increased intracranial pressure (headache, dizziness and vomiting), as well as imaging findings,
the neurosurgical team intervened and left posterior
craniotomy was performed for decompression and to
excise the left cerebellar lesion. An external ventricular
drain was placed. Intraoperative findings noted a large
intra-axial tumour at the left cerebellar hemisphere,
likely malignant. Pathology confirmed a grade 3
neuroendocrine tumour, with additional comment that
a metastatic lesion was likely. A repeated whole-body
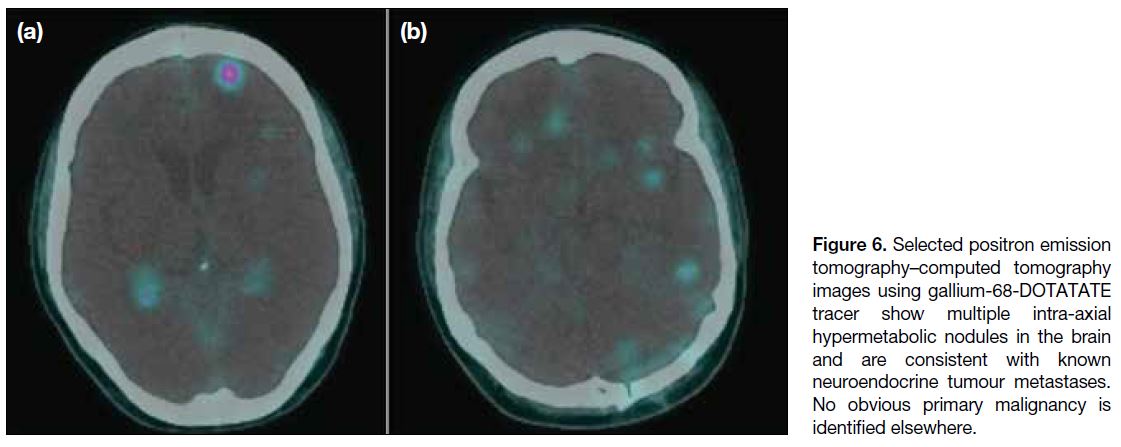
PET-CT with gallium-68-DOTA-tyr3-octreotate (Ga-68-DOTATATE) showed multiple hypermetabolic nodules
in the brain suggestive of known neuroendocrine tumour
(Figure 6), but still no obvious location for a primary
malignancy. A preliminary diagnosis was reached of
neuroendocrine tumour of unknown origin, with possible
primary within the brain. Postoperatively, the patient
underwent further follow-up MRI scans that revealed
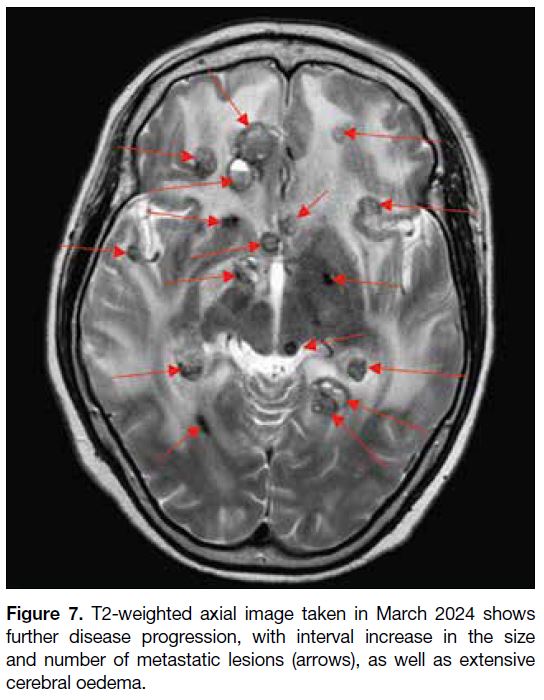
new suspicious drop metastasis at the C4 level, as well as significant progression of brain metastases and
worsening vasogenic oedema (Figure 7). The patient
was followed up by the neurosurgery and oncology
teams and underwent radiotherapy of the whole brain
and the cervical spinal cord as palliative care. At 35
months after the initial presentation, the patient died due
to a complication of pneumonia.
Figure 5. T2-weighted imaging
at (a) 5 months posttreatment
(taken in June 2022) and (b) 16
months posttreatment (taken in
May 2023) shows enlargement of
the left cerebellar lesion (arrows).
T1-weighted post-contrast images
of the same lesion at (c) 5 months
posttreatment and (d) 16 months
posttreatment (arrows).
Figure 6. Selected positron emission
tomography–computed tomography
images using gallium-68-DOTATATE
tracer show multiple intra-axial
hypermetabolic nodules in the brain
and are consistent with known
neuroendocrine tumour metastases.
No obvious primary malignancy is
identified elsewhere.
Figure 7. T2-weighted axial image taken in March 2024 shows
further disease progression, with interval increase in the size
and number of metastatic lesions (arrows), as well as extensive
cerebral oedema.
DISCUSSION
Neurocysticercosis and neuroendocrine tumour of the
brain are two distinct entities that require very
different treatment approaches. The patient’s presenting
signs and symptoms (such as headache, dizziness and
seizure) are often non-specific. Serology testing for
Taenia solium, while specific, is often not sensitive. A
negative serology test does not exclude the diagnosis
of neurocysticercosis; hence, it was reasonable for our patient to undergo a trial of antiparasitic treatment based
on radiological appearance alone.
Imaging plays an important role in guiding the diagnosis
as well as treatment in such difficult cases. Nonetheless,
as with our case, imaging also has its limitations and can
be misguided by disease mimics.
On MRI, neurocysticercosis has varied radiological
appearances depending on its four main stages.[10] [11]
During the vesicular stage, cysts with cerebrospinal
fluid (CSF) intensity are often seen, sometimes with an
eccentric scolex that may show enhancement. Typically,
no surrounding vasogenic oedema is seen at this stage.
Intraventricular cysts may be difficult to visualise, and
heavily T2-weighted sequences such as FIESTA (fast
imaging employing steady-state acquisition) may help delineate the walls and scolex of neurocysticercosis. In
addition, the cystic content may show a slightly lower
signal compared with CSF, making them stand out.[12] For
our case, the FIESTA sequence was not performed due
to limited resources.
During the colloidal vesicular stage, cysts will often
contain increased proteinaceous content, leading to T1
and fluid-attenuated inversion recovery hyperintense
signal relative to CSF. Thickening and enhancement of
the cyst wall, as well as surrounding oedema, may be
seen. Some lesions may also show restricted diffusion,[11]
as in our case, which further complicates the clinical picture.
During the granular nodular stage, the cystic component
will resolve, becoming a small enhancing nodule.
Contrast enhancement and perilesional oedema will
gradually decrease and eventually resolve in the final
nodular calcified stage, where calcified nodules are seen. Neuroendocrine tumour of the brain, whether primary
or secondary, can also have variable appearances
mimicking other diseases. Among the reported primary
cases, MRI appearances ranged from a
solid enhancing mass to a cystic mass with a peripheral
enhancing component.[1] [2] [3] [4] [5] [6] [7] [8] [9]
Spontaneous regression of up to one quarter of
neuroendocrine tumours has also been reported, albeit
most were extracranial in location, possibly due to host
immune response against neoantigens expressed by the
tumour.[13] This further increases diagnostic confusion, as
in our patient, and led us to interpret the regression of
lesions as a partial response to antiparasitic treatment.
Other imaging modalities such as PET scan may
offer more diagnostic clues, but 18F-FDG, which is
the most common tracer, may not show uptake in well-differentiated neuroendocrine tumours. On the
contrary, Ga-68-DOTATATE has a high sensitivity and
specificity in the detection of neuroendocrine tumours.[14]
Contrary to 18F-FDG which targets glucose metabolism,
Ga-68-DOTATATE targets somatostatin receptors that
are usually overexpressed by neuroendocrine tumours.
Nonetheless, this tracer is not yet widely available in our
region.
With hindsight, there are lessons to be learnt from
our patient and improvements to be made, especially
in her management. There was an 11-month period
(between 5 and 16 months posttreatment) with
no imaging follow-up or further workup. There were
already significantly enlarging lesions on the 5-month
posttreatment scan, and although present, regression of
the temporal lesions was subtle. More aggressive follow-up
imaging (e.g., within a few months) would have been
appropriate.
Furthermore, brain parenchymal haemorrhage, which
was already present on her initial MRI scan, is an
uncommon finding in neurocysticercosis. Alternative
differential diagnoses should have been considered,
especially in view of the suboptimal radiological
response to antiparasitic treatment. Given the vital
location of the enlarging lesions, further investigations
such as brain biopsy should also have been considered
and offered at an earlier stage.
Although treatment trials with antiparasitic drugs and
interval follow-up scans may provide a general idea of
the course of the disease, histological diagnosis including
excisional biopsy may be the only means by which to
confirm a diagnosis.
CONCLUSION
Neurocysticercosis in our region is uncommon, and
neuroendocrine tumour of the brain is even rarer. We
encountered an atypical presentation of a neuroendocrine
tumour of the brain mimicking neurocysticercosis. A
multidisciplinary approach involving the infectious diseases team, as well as neurosurgical and oncological
specialists, is necessary to reach definitive diagnosis.
REFERENCES
1. Caro-Osorio E, Perez-Ruano LA, Martinez HR, Rodriguez-Armendariz AG, Lopez-Sotomayor DM. Primary neuroendocrine
carcinoma of the cerebellopontine angle: a case report and literature
review. Cureus. 2022;14:e27564. Crossref
2. Porter DG, Chakrabarty A, McEvoy A, Bradford R. Intracranial
carcinoid without evidence of extracranial disease. Neuropathol
Appl Neurobiol. 2000;26:298-300. Crossref
3. Deshaies EM, Adamo MA, Qian J, DiRisio DA. A carcinoid tumor
mimicking an isolated intracranial meningioma. Case report. J
Neurosurg. 2004;101:858-60. Crossref
4. Ibrahim M, Yousef M, Bohnen N, Eisbruch A, Parmar H. Primary
carcinoid tumor of the skull base: case report and review of the
literature. J Neuroimaging. 2010;20:390-2. Crossref
5. Hakar M, Chandler JP, Bigio EH, Mao Q. Neuroendocrine
carcinoma of the pineal parenchyma. The first reported case. J Clin
Neurosci. 2017;35:68-70. Crossref
6. Liu H, Wang H, Qi X, Yu C. Primary intracranial neuroendocrine
tumor: two case reports. World J Surg Oncol. 2016;14:138. Crossref
7. Reed CT, Duma N, Halfdanarson T, Buckner J. Primary
neuroendocrine carcinoma of the brain. BMJ Case Rep. 2019;12:e230582. Crossref
8. Tamura R, Kuroshima Y, Nakamura Y. Primary neuroendocrine
tumor in brain. Case Rep Neurol Med. 2014;2014:295253. Crossref
9. Stepien N, Haberler C, Theurer S, Schmook M, Lütgendorf-Caucig
C, Müllauer L, et al. Unique finding of a primary central nervous
system neuroendocrine carcinoma in a 5-year-old child: a case
report. Front Neurosci. 2022;16:810645. Crossref
10. Teitelbaum GP, Otto RJ, Lin M, Watanabe AT, Stull MA, Manz HJ, et al. MR imaging of neurocysticercosis. AJR Am J
Roentgenol. 1989;153:857-66. Crossref
11. Santos GT, Leite CC, Machado LR, McKinney AM, Lucato LT.
Reduced diffusion in neurocysticercosis: circumstances of
appearance and possible natural history implications. AJNR Am J
Neuroradiol. 2013;34:310-6. Crossref
12. Neyaz Z, Patwari SS, Paliwal VK. Role of FIESTA and SWAN
sequences in diagnosis of intraventricular neurocysticercosis.
Neurol India. 2012;60:646-7. Crossref
13. Amoroso V, Agazzi GM, Roca E, Fazio N, Mosca A, Ravanelli M, et al. Regression of advanced neuroendocrine tumors among
patients receiving placebo. Endocr Relat Cancer. 2017;24:L13-6. Crossref
14. Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role
of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in
patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55:389-98. Crossref
PICTORIAL ESSAYS
Exploring the Power of Hybrid Intervention: Utility of an Angiography-Computed Tomography System in Interventional Radiology
PICTORIAL ESSAY CME
Hong Kong J Radiol 2025 Dec;28(4):e257-67 | Epub 10 December 2025
Exploring the Power of Hybrid Intervention: Utility of an Angiography–Computed Tomography System in Interventional Radiology
CL Wong1, KKF Fung2, HY Lo1, LH Yeung1, JC Ng1, KH Lee1, DHY Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr CL Wong, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China. Email: wcl094@ha.org.hk
Submitted: 9 October 2024; Accepted: 24 October 2024.
Contributors: All authors designed the study. CLW and DHYC acquired the data. All authors analysed the data. CLW and KKFF drafted the
manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Clinical Research Ethics Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-231-3). Informed patient consent was waived by the Board due to retrospective nature of the study.
INTRODUCTION
The use of cross-sectional imaging in interventional
radiology (IR) procedures is crucial for accurate target
identification, procedure planning, guidance, and
immediate therapy response monitoring.
Cone beam computed tomography (CBCT), integrated
into angiography systems with flat-panel detectors, has
been widely adopted for supplementary cross-sectional
imaging during IR procedures, in order to improve
procedural precision and safety.
Combined angiography-CT (angio-CT) systems integrate
a helical CT scanner and an angiography unit, placed on
the same rail with the same patient table. This allows
for seamless transition between CT and conventional
fluoroscopy/angiography, avoiding the need to move
the patient and its attendant risks. Besides achieving a
more efficient workflow, it also provides superior image
quality in terms of contrast resolution, noise, and artefact reduction, and a larger field of view compared to CBCT.[1]
In a study comparing the use of CBCT and angio-CT
for transarterial chemoembolisation (TACE), the overall
image quality of CT hepatic angiography in angio-CT
outperformed that of CBCT in identification of tumour
arterial feeders, reduction of streak and respiratory
artefacts, resulting in higher overall image quality.[2]
Through illustrative cases, we aim to demonstrate the
advantages of angio-CT in a wide range of vascular and
non-vascular interventions.
ANGIOGRAPHY–COMPUTED TOMOGRAPHY SYSTEM
The angio-CT (Nexaris Angio-CT; Siemens, Tubingen,
Germany) which includes a C-arm angiography system
and a helical CT scanner installed on the same rail
system (Figure 1). During procedures, the patient is
positioned with the target organ as close as possible to
the CT gantry. While the patient is lying on the IR table
for fluoroscopy or angiography, the C-arm can be moved aside to allow the CT gantry to enclose the patient during
the procedure, enabling acquisition of three-dimensional
(3D) image data that can be processed and analysed
immediately at the workstation. Immediate fusion of
3D angiography and fluoroscopy allows the operator to
navigate to the target during IR procedures such as radio-embolisation
and TACE, where precision is crucial.
Figure 1. Setting of angiography–computed tomography. The
operator can slide the computed tomography (CT) gantry around
the patient table (direction indicated by white arrow) for CT imaging
during fluoroscopy/angiography using the control panel next to the
patient table (circle).
IMAGE ACQUISITION AND
INJECTION PROTOCOL CLINICAL
APPLICATIONS
Transarterial Chemoembolisation
Improving Visualisation of Small Hepatocellular
Carcinoma
Angio-CT with hepatic arteriography outperforms
diagnostic CT in terms of hepatocellular carcinoma
(HCC) visualisation.[1] [3] It can sometimes detect small
HCCs that are not conspicuous in magnetic resonance
imaging (MRI) or digital subtraction angiography
(DSA),[4] as demonstrated in the case below.
A 59-year-old hepatitis B carrier with a history of
HCCs at segments 7/8 and 6 (Figure 2 a-c) treated with
microwave ablation. During follow-up, alpha-fetoprotein
level elevated up to 10 ng/mL. Contrast MRI of the liver
showed suspicious multifocal recurrence of HCCs and
the patient was referred for TACE. Angio-CT hepatic
arteriography demonstrated superior diagnostic power
compared to DSA and MRI in detection of subcentimeter HCC with faint arterial enhancement. All lesions
demonstrated lipiodol deposition on postprocedural
CT, with complete staining, a predictive factor for good
therapeutic response to TACE.[3] Alpha-fetoprotein level
in follow-up decreased to 7.9 ng/mL (Figure 2).
Figure 2. A 59-year-old patient. (a-c) Magnetic resonance imaging (MRI) of the liver with gadoxetic
acid contrast in the arterial phase shows suspicious multifocal
subcentimeter recurrent hepatocellular carcinoma in segments 6, 7
and 8 (arrows). The arrow in (c) indicates a faint arterial enhancing
focus at segment 6 near the liver edge. (d) Right hepatic artery digital
subtraction angiography shows no tumour blush in the MRI detected
lesions. (e-g) Angiography–computed tomography (Angio-CT) with intra-arterial contrast injection into the
right hepatic artery shows multiple arterial-phase enhancing lesions
corresponding to the MRI-detected tumours as seen in (a) to (c)
[arrowheads]. Chemoembolisation was performed. (h-j) Immediate
post–transarterial chemoembolisation plain CT on the angio-CT
system shows lipiodol uptake in target lesions (dashed arrows).
All lesions demonstrated lipiodol deposition on the postprocedural
scan, with complete staining seen in (i) and (j).
Improving Visualisation of Extrahepatic Arterial
Supply
For HCC cases with extrahepatic supply, studies suggest
that angio-CT arteriography offers superior diagnostic
capability compared to conventional triphasic CT liver
and DSA.[1] [4] Its use can increase sensitivity in detecting
and confirming parasitic supply, thereby guiding
additional treatment strategies.[3] [4]
A 77-year-old hepatitis B carrier with a history of left
hepatectomy for HCC was later found to have multifocal
recurrent HCCs. Multiple TACEs were performed via
different branches of the right hepatic artery, but the
patient was still found to have persistent right hepatic
lobe HCCs on follow-up CT scan (Figure 3).
Figure 3. A 77-year-old patient. (a, b) Contrast computed tomography (CT) of the liver in late arterial phase shows several faint enhancing
lesions in the right hepatic lobe suspicious for recurrent hepatocellular carcinoma (arrows). Contrast CT was unable to identify the
extrahepatic feeding artery. Given the location of these lesions, the right inferior phrenic artery was thought to be one of the common
extrahepatic supplies. (c, d) The right inferior phrenic artery was cannulated with a 1.7-Fr microcatheter (arrows) [Merit Pursue; Merit
Medical Systems, Warrington (PA), United States]. (d) Digital subtraction angiography shows no sizable tumour blush. (e, f) Angiography–computed tomography (angio-CT) with intra-arterial injection of the right inferior phrenic artery confirmed it supplying the right hepatic
lesions (arrowheads). A chemotherapeutic mixture was administered via microcatheter (arrow in [e]). (g, h) Postprocedural plain CT on the
angio-CT system performed after several additional sessions of transarterial chemoembolisation, shows dense lipiodol uptake (dashed
arrows) and a reduction in lesion size, as seen in (h).
Enhancing Treatment Efficacy of Drug-Eluting
Bead Transarterial Chemoembolisation
In drug-eluting bead (DEB)-TACE, angio-CT offers
additional benefits beyond its higher sensitivity for
detecting viable tumour components and feeding
arteries. Unlike conventional TACE using lipiodol,
DEB-TACE does not produce lipiodol staining to assess
immediate treatment response. Therefore, an immediate
postprocedural angio-CT with intra-arterial contrast
injection can help identify residual arterial enhancement
and guide further management, such as the need for
additional drug administration.
A 64-year-old patient had a large right hepatic lobe
HCC. The lesion was too large for resection or ablation.
Hence, he underwent several episodes of TACE.
However, the patient had poor response with suboptimal
tumour lipiodol staining and rapid lipiodol washout,
and was referred for DEB-TACE. Right hepatic artery
DSA showed three suspicious arterial feeders, which
were selectively cannulated with a microcatheter (2.8-Fr
Meri Maestro Swanneck microcatheter; Merit Medical
Systems, Inc, South Jordan [UT], United States). This
case demonstrated the ability of precise identification
of feeding arteries in DEB-TACE using angio-CT,
particularly for equivocal or indeterminate feeders in
DSA and preprocedural CT. It also enabled assessment
of immediate treatment response and detection of
residual lesions (Figure 4).
Figure 4. A 64-year-old patient. (a) Preprocedural contrast computed tomography of the liver in
late arterial phase, showing poor lipiodol staining at the anterior aspect of the lesion with a viable arterial enhancing component (dashed circle). (b) Target site 1 cannulated with microcatheter. Digital subtraction angiography (DSA) showed tumour blush (arrow). (c) The arterial component was confirmed with intra-arterial injection using angiography–computed tomography (angio-CT), showing arterial blush (arrowhead). Drug-eluting beads loaded with chemomixture were administered. (d) Immediate post–drug-eluting bead transarterial chemoembolisation (DEB-TACE) angio-CT with intra-arterial injection at the feeder showing reduction in the enhancing component (arrow). (e) DSA of Target site 2 shows no sizable tumour blush. (f) However, angio-CT with an intra-arterial injection shows arterially enhancing viable component (arrowhead). Drug-eluting beads loaded with chemomixture were administered. (g) Immediate post–DEB-TACE angio-CT with intra-arterial injection at feeder shows significant reduction in arterial enhancing component (arrow). (h, i) DSA of Target site 3 shows no sizable tumour blush, in concordance with intra-arterial injection angio-CT showing no arterial enhancing viable component. No drug was administered at this site.
Tumour Ablation
Improving Target Visualisation
Identifying target hepatic tumours with ultrasound for
ablation can be difficult due to cirrhotic liver or prior
treatment changes. Contrast CT significantly helps with
lesion identification and ablation probe placement. Some
operators also perform intraprocedural angio-CT with
intra-arterial injection for tumour identification and
ablation margin monitoring,[1] [4] improving the precision
of ablation and treatment response monitoring.
Increasing Ease for Artificial Ascites Creation
For creation of artificial ascites, an angio-catheter is
first inserted into the peritoneal space under ultrasound
guidance, followed by a guidewire, then exchanged to a
catheter for dextrose infusion. Using angio-CT, operators
can safely manipulate the guidewire and exchange to the
catheter under real-time fluoroscopy, confirm catheter
position on CT, and proceed to image-guided ablation.
All steps involving different imaging modalities can be
performed on the same table without moving the patient.
Radiofrequency Ablation of Liver Metastases
An 85-year-old patient with a history of colonic cancer
and prior liver metastases treated with ablations. Follow-up
CT showed several new liver metastases, and the
patient was referred for image-guided radiofrequency
ablation. On-table ultrasound identified several nodules
in the right hepatic lobe, but it was difficult to distinguish
them from prior ablation zones. Triphasic angio-CT liver
showed several liver metastatic lesions (Figure 5).
Figure 5. (a-c) An 85-year-old male patient. Three hypoenhancing nodules in segments 8 and 4a (circles), suggestive of liver metastases. (d-f)
For creation of artificial ascites, a 16-gauge angio-catheter (Becton Dickson, Franklin Lakes [NJ], US) was used to target
the perihepatic space under ultrasound guidance, which was then exchanged to a 6-Fr catheter (Boston Scientific, Marlborough [MA],
US) over a 0.035-inch guidewire (Terumo, Tokyo, Japan) under fluoroscopy. The catheter tip was confirmed with angiography–computed tomography (angio-CT), followed by infusion of 5% dextrose solution. (g, h) Each lesion was targeted with an ablation antenna
under ultrasound and computed tomography (CT) guidance (antenna tips indicated by circles), with a 12-minute ablative cycle performed. (i)
Postprocedural CT showing a hyperdense layer (arrow) in the artificial ascites and noted blood-stained fluid in drain. (j-l)
Immediate multiphasic images were acquired by angio-CT, showing no evidence of active bleeding or pseudoaneurysm. The patient’s vital
signs were stable and he was sent back to the ward for close observation.
Acute Haemorrhage Embolisation
Improving Detection of Bleeding Source
In cases of acute bleeding, angio-CT angiogram can
detect bleeding sources too small or slow to be identified
on CT with intravenous contrast.[1] With 3D reformatting,
the precise location of the bleeder can be accurately
determined.
Quicker Cessation of Bleeder
Unstable patients with active bleeding can be transferred
directly to angio-CT for urgent CT, followed by immediate embolisation on the same table. This
eliminates the need to move patients between the
diagnostic CT and IR suites, allowing quicker
haemorrhage control and improved outcomes.
Embolisation of Haemorrhagic Renal Tumour
A 70-year-old patient was incidentally found to have
an enhancing soft tissue mass at the lower pole of left
kidney. Ultrasound-guided biopsy was performed, but
the patient developed left flank pain with a haemoglobin
drop from 14.1 g/dL to 11.6 g/dL on day 1 post-biopsy.
Urgent CT of the kidney found intratumoural
haemorrhage and trace left haemoretroperitoneum.
Post-embolisation haemoglobin level remained stable,
with post-embolisation day 5 follow-up CT showing
no progression or active bleeding. The patient was later
discharged (Figure 6).
Figure 6. (a) A 70-year-old patient. Pre-contrast computed tomography (CT) of the kidneys shows a left renal lower pole mass with
internal hyperdensities (arrow), suggesting intratumoural haemorrhage. Arterial phase CT shows no pseudoaneurysm or active contrast
extravasation. (b) The left main renal artery was cannulated with a catheter (5-Fr C1 catheter; Cordis, Miami Lakes [FL], US) and
digital subtraction angiography (DSA) performed, showing a long curved tortuous inferior segmental artery to the left lower pole (arrow), with
dysplastic distal branches. (c-e) With angiography–computed tomography, this was confirmed to be the feeding artery (arrowheads) to the
haemorrhagic renal lesion, without pseudoaneurysm or active contrast extravasation. (f) The feeder was selectively cannulated with a 2.4-Fr
microcatheter (Maestro; Merit Medical Systems, Warrington [PA], US) with DSA confirming its supply
to the renal lesion (dashed arrow). Embolisation was performed with Embospheres 500-700 μm (Merit, Warrington [PA], US) until
stasis was achieved. (g) Check of left renal angiogram showing successful occlusion of the bleeding artery.
Endoleak Detection and Management
Diagnosis for Endoleak
Angio-CT combines the benefits of DSA and CT by integrating real-time flow dynamics with detailed cross-sectional
anatomy. This allows comprehensive and
accurate evaluation of the type and site of endoleak, as
demonstrated in the following case.
A 76-year-old male patient with infrarenal abdominal
aortic aneurysm and left common and internal iliac artery
aneurysms was managed with endovascular aneurysm
repair. A diagnostic aortogram was performed 1 year
after endovascular aneurysm repair to clarify the type
and site of endoleak. 5-Fr Multipurpose catheter (Merit
Medical, South Jordan [UT], United States) was then navigated to the left iliac limb and superior mesenteric
artery, with angio-CT angiogram performed to exclude
other endoleak sites. The patient was managed with
extension of the right iliac limb endograft (Figure 7).
Figure 7. A 76-year-old male patient.
(a) Initial computed tomography
aortogram shows endoleak within
posterior aspect of the distal
aortic aneurysmal sac (arrow).
It was originally thought to be a
type II endoleak from the median
sacral artery. (b-d) Upon follow-up
computed tomography aortography
increasing diameters of abdominal
aortic aneurysm and left common
iliac artery aneurysm were noted,
with increased endoleak within the
aortic aneurysmal sacs (arrowheads).
(e) An aortogram with intra-arterial
injection in the right iliac limb. There
was abnormal contrast leakage near
the distal abdominal aortic aneurysm
sac and the right common iliac
artery aneurysmal sac (arrow). (f)
Angiography–computed tomography
with intra-arterial contrast injection
via the right iliac limb shows an
endoleak originating from the right
iliac endograft, consistent with a type
IB endoleak (arrowhead).
Embolisation of Endoleak
Angio-CT is useful for endoleak treatment. A CT
aortogram with intra-arterial injection enables precise
localisation of the endoleak, followed by targeting
under combined CT and fluoroscopic guidance, and
embolisation under real-time fluoroscopy. Final
placement of embolic material and any immediate complications can be verified with angio-CT. The system
allows the entire multimodality process to be performed
on the same table.
An 86-year-old male patient with an infrarenal abdominal aortic aneurysm, bilateral common iliac artery and IIA
aneurysms was treated with endovascular aneurysm
repair, right IIA coil embolisation and left iliac bifurcation
device. The endoleak was targeted for balloon-assisted
percutaneous transluminal glue embolisation in the same session. With angio-CT enabling seamless, efficient
transition between CT angiogram and fluoroscopy, the
operator safely targeted the endoleak site for embolisation
without injuring adjacent organs or damaging the stent
(Figure 8).
Figure 8. An 86-year-old male patient. (a) Initial computed tomography aortogram showing increase in size of abdominal aortic aneurysm
sac and left IIA sac, with only mild endoleak suspected near the left internal iliac limb (arrow). (b, c) Aortogram and angiography–computed
tomography (angio-CT) respectively, with intra-arterial injection at common iliac limb at left iliac bifurcation device, confirming left internal
iliac limb endoleak (arrows). (d) Successful cannulation of the left internal iliac artery limb and deployment of a 10 mm × 40 mm balloon
(Mustang, Boston [MA], US) at the site of the endoleak with position confirmed with angio-CT. (e) Under combined computed
tomography (CT) and fluoroscopic guidance, a 17-gauge needle (arrow) [Gangi-SoftGuard; Apriomed, Uppsala, Sweden] was advanced towards the site
of the endoleak in the left internal iliac artery (IIA) via the right anterior abdominal wall, through which a 20-gauge Chiba needle (arrow)
(Cook Medical, Bloomington [IN], US) [arrowhead] was introduced to puncture the left IIA endoleak site. (f) The position of the Chiba needle
(arrow) was confirmed by CT and fluoroscopy with contrast injection. (g) A 10 mm × 40 mm 135-cm balloon (Mustang;
Boston Scientific, Marlborough [MA], US) was inflated to occlude the endoleak site whilst total of 1.5 mL of hutyl cyanoacrylate
glue (50% dilution with lipiodol) was injected under real time fluoroscopy. The balloon was then withdrawn. (h) Post-embolisation angiogram
shows satisfactory obliteration of the left internal iliac limb endoleak.
Other Cross-Modalities Applications
Percutaneous Embolisation of Pulmonary Vein
Pseudoaneurysm
The angio-CT system is valuable in complex IR cases
requiring precise target localisation with CT and
real-time fluoroscopic guidance, as illustrated in the
following case. An 88-year-old patient with multiple
co-morbidities and a history of Stanford type A aortic
dissection managed conservatively was admitted with
haemoptysis of 50 to 100 mL/day. Haemoglobin level
dropped from 10.8 g/dL to 7.7 g/dL despite on transamin
and repeated blood transfusions. He required oxygen
support at 2 L/min via mask. An urgent CT aortogram
was performed, and the patient was referred for
angiogram for lesion characterisation and subsequent
management. His condition improved, with haemoptysis
level reduced to 10 mL/day. Haemoglobin level remained stable at 7 to 8 g/dL and oxygen support was weaned off (Figure 9).
Figure 9. (a) An 88-year-old patient. Urgent computed tomography aortogram showing pulmonary consolidation and haemorrhage in the
left lower lobe, with a 0.9-cm enhancing lesion within the consolidation (arrow). It is closely abutting the left inferior pulmonary vein, raising
suspicion of a pulmonary venous pseudoaneurysm. Known Stanford type A aortic dissection was static with no mediastinal haematoma. (b,
c) Left pulmonary angiogram shows no corresponding lesion in the left lower lobe in pulmonary arterial and parenchymal phases. (d, e) An
enhancing nodule in the left lower lobe appeared after pulmonary arterial and parenchymal phases (arrowhead in [d]), consistent with the
computed tomography (CT) findings that it originated from the left inferior pulmonary vein rather than pulmonary artery. The location was
confirmed in angiography–computed tomography (circle in [e]) for embolisation planning. (f, g) Under combined fluoroscopic and CT guidance, a 20-gauge Chiba needle (Cook Medical, Bloomington [IN], US) was used to percutaneously access
the pseudoaneurysm sac. Under fluoroscopy, contrast injection confirmed needle positioning with opacification of the pseudoaneurysm
sac (arrow in [f]) and the outflowing pulmonary vein (arrowhead in [g]). (h, i) Two 4 mm × 10 cm embolisation coils (Concerto; Medtronic,
Minneapolis [MN], US) [circle in (h)] were successfully deployed under fluoroscopy, with coil position confirmed with CT (circle in [i]). (j) Further attempt coiling of aneurysm was not successful. 0.6 mL of hutyl cyanoacrylate glue (50% dilution
with lipiodol) was injected under fluoroscopy for complete sac embolisation. (k) Postprocedural CT shows complete
obliteration of the pseudoaneurysm by glue and coils without residual contrast opacification (circle).
This case demonstrates successful lesion targeting
using a percutaneous approach, where reliance on either
fluoroscopy or intermittent CT alone poses a high risk
of injury to vital internal organs. With the advantage
of angio-CT allowing seamless transition between
CT and fluoroscopy, the operator safely punctured the
target without harming surrounding organs, followed by
embolisation under real-time fluoroscopy.
Adrenal Venous Sampling
Recognition and cannulation of the right adrenal
vein is one of the most challenging aspects of adrenal
venous sampling (AVS). Common issues include
catheter dislodgement, incorrect or deep cannulation, or
anatomical variants like an accessory hepatic vein that
may dilute cortisol. In such cases, CT during AVS can
help delineate anatomy and confirm catheter position.[5]
Compared to CBCT, CT offers superior image quality
and faster acquisition, reducing the risk of catheter
dislodgement. A case illustration is presented below.
A 42-year-old female patient with primary
hyperaldosteronism and hypertension previously failed
AVS, as the right adrenal venous sample lacked sufficient
cortisol to meet the required selectivity index, despite
venography showing a typical spidery configuration
on retrospective review. The cause of failure was
indeterminate and she was referred for a second AVS.
Angio-CT right venogram was performed before and just
after right sampling to: (1) ensure correct cannulation of
the right adrenal vein; (2) ensure the catheter remained in situ during sampling; and (3) exclude anatomical
variants such as an accessory hepatic vein. Post-sampling
angio-CT confirmed catheter position. Sampling of
right adrenal veins was successful reaching a selectivity
index of 15. The patient was diagnosed with a left-sided
aldosterone-secreting tumour and is pending surgery
(Figure 10).
Figure 10. (a) A 42-year-old female patient. During second trial of adrenal venous sampling (AVS), the right adrenal vein was cannulated
with a catheter (Yashiro; Terumo, Tokyo, Japan). Right adrenal venogram shows the spidery configuration of the right adrenal vein, similar
to previous AVS. (b, c) Angiography–computed tomography right adrenal venogram before sampling shows catheter tip (arrows) was within
the right adrenal vein, with contrast opacification of the right adrenal gland (arrowheads). There was no accessory vein draining to the right
adrenal vein. The injection protocol via the 5-Fr Yashiro catheter was: 6 mL undiluted contrast at 1 mL/sec, with computed tomography
acquisition at 4 seconds after start of contrast injection.
Details of the contrast injection and image acquisition
protocols for commonly performed vascular procedures requiring CT acquisition with our angio-CT system are
provided in the Table.
Table. Contrast injection and image acquisition protocol in our unit for some common interventional radiology procedures that might require the use of angiography–computed tomography.
DISCUSSION
The above cases highlight the applications and
advantages of using the angio-CT system in various
IR procedures. Compared to CBCT previously used in
our unit, the image quality of CT hepatic angiogram in
angio-CT surpasses CBCT in visualisation of tumour,
identification of tumour arterial feeders, reduction of
streaking artefacts, wider field of view including the whole liver, fewer respiratory motion artefacts, and
higher overall subjective image quality[2] (Figure 11). It
also allows immediate postprocedural imaging to assess
treatment response, such as immediate lipiodol uptake
and presence of residual lesions, which are limited by
streaking and respiratory artefacts in CBCT.
Figure 11. (a) Cone beam computed
tomography (CBCT) of the liver in a patient
with large right hepatic tumour after
transarterial chemoembolisation (TACE). There
are marked streaking artifacts obscuring
assessment of the structures. (b)
Angiography–computed tomography
after TACE of the same patient. Compared to
CBCT,
it shows significant improvement
of image quality with less streaking
artifacts, fewer respiratory motion
artifacts and wider field of view.
For angio-CT hepatic arteriogram, a smaller amount of
contrast can be used for direct hepatic artery injection
compared to systemic intravenous injection.[4] [5] In DEB-TACE,
multiple contrast injections are typically required to verify target lesions. Therefore, using angio-CT may
help reducing fluid overload and contrast load, which
is beneficial to patients with liver cirrhosis with pre-existing
fluid status disturbances.
In suspected complications during or immediately after
IR, angio-CT can be promptly performed with multiphasic
studies to detect bleeding, without transferring the patient
to diagnostic CT. This allows immediate diagnosis and
treatment, such as urgent embolisation.
Apart from the above examples, angio-CT can improve
procedural outcomes in the following scenarios.
A common application is combined TACE and ablation
for liver cancers, where TACE is first performed first
to devascularise and stain the tumour, followed by
ablation in the same session. The ablation margin can
be monitored during and after with intra-arterial contrast
injection, improving margin visualisation.
In hypervascular soft tissue or bone tumours, such
as renal cell or thyroid carcinoma bone metastases,
embolisation can be done first under angiography to
reduce its vascularity, followed by CT-guided ablation
in the same session. This reduces haemorrhagic risks,
especially in hypervascular tumours.[5] [7]
In emergencies requiring urgent embolisation, such
as trauma or ruptured HCC, the patient can be directly
transferred to angio-CT for urgent CT angiogram and
embolisation. After reviewing images on the angio-CT workstation, the operator can proceed immediately
without transferring the patient from diagnostic
CT to IR suite. This is crucial when the patient is
haemodynamically unstable and also shortens scan-to-needle
time, potentially improving outcomes.
A major drawback of angio-CT is cost and space.
Depending on vendor and performance, angio-CT is
approximately 1.5 to 2 times more expensive than flat
panel CBCT.[8] It also requires more space compared with
C-arm CBCT, possibly needing re-design of the IR suite.
Radiation dose between angio-CT and CBCT remains
debated. A study on CT-guided lung biopsy showed
angio-CT delivered 1.2 to 1.7 times higher effective
dose than CBCT (mean: 15.77 mSv vs. 10.68 mSv).[9]
However, another study during TACE showed angio-CT
had 2.5 times lower effective dose than CBCT (median:
15.4 vs. 39.2 mSv).[10] Dose indices differ: angio-CT uses
dose-length product while CBCT uses dose-area product.
As these comparisons were based on estimated effective
doses using region-specific conversion factors and
phantom calculations, uncertainties must be considered
when interpreting dosage results.
The integration of angiography unit and dedicated CT
scanner into a hybrid angio-CT system is a revolutionary
technology for IR. By enabling detailed anatomical
characterisation and visualisation of critical structures,
angio-CT is a valuable tool to enhance the patient
outcome and reduce procedural risks in complex
interventional procedures.
REFERENCES
1. Taiji R, Lin EY, Lin YM, Yevich S, Avritscher R, Sheth RA, et al.
Combined angio-CT systems: a roadmap tool for precision
therapy in interventional oncology. Radiol Imaging Cancer.
2021;3:e210039. Crossref
2. Lin EY, Jones AK, Chintalapani G, Jeng ZS, Ensor J, Odisio BC.
Comparative analysis of intra-arterial cone-beam versus
conventional computed tomography during hepatic arteriography
for transarterial chemoembolization planning. Cardiovasc Intervent
Radiol. 2019;42:591-600. Crossref
3. Wang H, Han Y, Chen G, Jin L. Imaging biomarkers on angio-CT for predicting the efficacy of transarterial chemoembolization in
hepatocellular carcinoma. Quant Imaging Med Surg. 2023;13:4077-88. Crossref
4. van Tilborg AA, Scheffer HJ, Nielsen K, van Waesberghe JH,
Comans EF, van Kuijk C, et al. Transcatheter CT arterial
portography and CT hepatic arteriography for liver tumor
visualization during percutaneous ablation. J Vasc Interv Radiol.
2014;25:1101-11.e4. Crossref
5. Kobayashi K, Ozkan E, Tam A, Ensor J, Wallace MJ, Gupta S. Preoperative embolization of spinal tumors: variables affecting intraoperative blood loss after embolization. Acta Radiol.
2012;53:935-42. Crossref
6. Puijk RS, Nieuwenhuizen S, van den Bemd BA, Ruarus AH,
Geboers B, Vroomen LG, et al. Transcatheter CT hepatic
arteriography compared with conventional CT fluoroscopy
guidance in percutaneous thermal ablation to treat colorectal liver
metastases: a single-center comparative analysis of 2 historical
cohorts. J Vasc Interv Radiol. 2020;31:1772-83. Crossref
7. Owen RJ. Embolization of musculoskeletal bone tumors. Semin Intervent Radiol. 2010;27:111-23. Crossref
8. Tanaka T, Arai Y, Inaba Y, Inoue M, Nishiofuku H, Anai H, et al.
Current role of hybrid CT/angiography system compared with
C-arm cone beam CT for interventional oncology. Br J Radiol.
2014;87:20140126. Crossref
9. Strocchi S, Colli V, Conte L. Multidetector CT fluoroscopy
and cone-beam CT-guided percutaneous transthoracic biopsy:
comparison based on patient doses. Radiat Prot Dosimetry.
2012;151:162-5. Crossref
10. Piron L, Le Roy J, Cassinotto C, Delicque J, Belgour A, Allimant C,
et al. Radiation exposure during transarterial chemoembolization:
angio-CT versus cone-beam CT. Cardiovasc Intervent Radiol.
2019;42:1609-18. Crossref
Multimodality Imaging and Interventional Radiological Management of Neurological Complications of Infective Endocarditis
PICTORIAL ESSAY
Hong Kong J Radiol 2025 Dec;28(4):e268-75 | Epub 9 December 2025
Multimodality Imaging and Interventional Radiological
Management of Neurological Complications of Infective Endocarditis
EH Chan, HM Kwok, NY Pan, LF Cheng, JKF Ma
Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR, China
Correspondence: Dr EH Chan, Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR,
China. Email: eh278@ha.org.hk
Submitted: 2 September 2024; Accepted: 1 November 2024.
Contributors: All authors designed the study, acquired and analysed the data. EHC drafted the manuscript. All authors critically revised the
manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-012-4). The requirement for patient consent was waived by the Board due to retrospective nature of the study.
INTRODUCTION
Infective endocarditis (IE) affects 1.7 to 6.2 individuals
per 100,000 population per year and remains a
life-threatening condition.[1] Staphylococci are the
most frequent causative organisms.[1] [2] Neurological
complications are the most common and severe
extracardiac complications of IE[3] and have been reported
as the presenting symptom in up to 47% of cases.[4] These
complications are caused by cerebral septic embolisation
of endocardial vegetations. Patients with neurological
complications have significantly higher mortality
compared to those without (24% vs. 10%; p < 0.03).[3]
Neuroimaging leads to the identification of valvular
surgery indications in about 22% of patients with
symptoms of neurological complications of IE, and in
19% of asymptomatic IE patients.[2] Up to 82% of patients
have cerebral lesions on magnetic resonance imaging
(MRI) performed within 7 days after admission.[1]
MRI findings influence diagnostic classification and
other clinical decisions in 28% of patients, including
modification of medical or surgical treatment plans.[5]
According to the 2015 European Society of Cardiology guidelines,[6] the presence of cerebral emboli in patients
with left-sided valvular vegetations greater than 10
mm is an indication for urgent valve surgery to prevent
further embolisms. Familiarity with the neurological
imaging findings is essential for early diagnosis of this
complication of IE, allowing a window for early and
specific treatment, thereby reducing mortality. However,
the wide spectrum of presentations on neuroimaging
poses diagnostic challenges to radiologists, especially
when cerebral septic embolism is the first presentation.
This pictorial essay aims to review the spectrum of
presentations and the use of multimodality imaging to
increase awareness of the classic diagnostic imaging
findings of cerebral septic emboli secondary to IE, and to
highlight the role of interventional radiology in clinical
management.
Diagnosis
The diagnosis of IE is made according to the Modified
Duke criteria,[1] which include the presence of major
arterial emboli, mycotic aneurysm, and intracranial
haemorrhage as part of its minor criteria.
Computed tomography (CT) is the first-line imaging
study in patients with neurological symptoms as it is
readily available. MRI, including susceptibility-weighted
imaging (SWI) and diffusion-weighted imaging (DWI),
is required to detect more subtle findings such as cerebral
microbleeds and early infarcts. Further investigations
with computed tomographic angiography (CTA) or
magnetic resonance angiography (MRA) are useful for
detecting mycotic aneurysms, while digital subtraction
angiography (DSA) remains the gold standard and
should be performed in clinically suspicious cases with
negative CTA or MRA.[7] There is growing support for
performing screening MRI in patients with suspected
or confirmed IE, given the frequency of asymptomatic
findings and its usefulness in decision-making.
However, its cost-effectiveness and impact on mortality
reduction remain to be seen.[8]
Imaging Spectrum
Neurological complications of IE may present as cerebral
infarcts, micro- or macro-haemorrhage, abscess, and
meningitis. The pooled frequency of individual findings
on MRI is as follows: acute ischaemic lesions (61.9%),
cerebral microbleeds (52.9%), macro-haemorrhages
(24.7%), abscess or meningitis (9.5%), and intracranial
mycotic aneurysm (6.2%).[8] Accurate identification of these lesions allows early diagnosis of IE
complications and individualised management strategies.
Ischaemic Stroke
Ischaemic stroke is the most common neurological
manifestation of IE. It can result from embolisation
of endocardial vegetations, leading to occlusion of
intracerebral arteries.[3] The incidence of cerebral
ischaemia is correlated with left-sided endocarditis
(especially involving the anterior mitral valve leaflet),
larger endocardial vegetation size (>10 mm), mobile
vegetations, and Staphylococcus aureus infection.[3]
Disseminated ischaemic lesions may result from multiple
emboli occurring over a short period or fragmentation of
an embolus in the heart or aorta. The presence of multiple
cortical and subcortical cerebral infarcts of varying
ages within different vascular territories (especially
watershed areas) or bihemispheric involvement suggests
the diagnosis of septic emboli[4] (Figure 1). Large emboli
tend to cause cortical infarction in the middle cerebral
artery (MCA) territory, while smaller emboli often lodge
distally in terminal cortical branches of the anterior
cerebral artery and MCA, resulting in small peripheral
infarcts at the grey-white matter junction.[9] It is worth
noting that isolated brainstem strokes are rarely caused
by cardioembolism.
Figure 1. A 69-year-old woman presented with fever and confusion 3 days after dental surgery. (a) Axial T2-weighted magnetic resonance
imaging (MRI) shows multiple foci of hyperintense signals involving the bilateral high frontal and parietal cortices (asterisks). (b) Axial diffusion-weighted
imaging (DWI) with a high b-value revealed corresponding hyperintense signal (arrowheads) with low signal on apparent diffusion
coefficient map (ADC) [not shown], suggestive of restricted diffusion. (c) Axial T2-weighted MRI shows hyperintense signals involving the
bilateral basal ganglia, capsular regions, and thalami (asterisks). (d) Axial DWI with a high b-value shows patchy areas of hyperintense signal
(arrowheads), with low signal on the ADC map (not shown), suggestive of restricted diffusion. These findings were suggestive of acute
infarcts. The distribution pattern raised suspicion for an embolic shower, involving both deep perforating arteries and cortical branches.
Consequently, an echocardiogram was performed and revealed a ventricular septal defect and tricuspid valve vegetation, consistent with
a paradoxical embolism. The patient was managed conservatively with intravenous antibiotics and showed good neurological recovery.
DWI is useful for assessing the temporal relationship of
ischaemic lesions. Acute infarcts appear as hyperintense
on DWI and hypointense on apparent diffusion coefficient mapping. Over time, the apparent diffusion coefficient
signal increases and pseudonormalises in about 1 week,
signal increases and pseudonormalises in about 1 week,
while the DWI signal decreases and pseudonormalises in
about 2 weeks.[10]
Cerebral Abscesses and Meningitis
Cerebral abscesses and meningitis are uncommon
neurological manifestations of IE, occurring in up to
9.5% of patients.[8]
Typically, multiple abscesses appear in the MCA
territory at the grey-white matter junction, often with
vasogenic oedema and associated mass effect or haemorrhage.[3] On CT, cerebral abscesses are usually
hypodense with ring enhancement, but MRI is more
sensitive. Classic MRI features include lesions that are
hypointense on T1-weighted images and hyperintense
on T2-weighted images, with ring enhancement and
central restricted diffusion (Figure 2). A dual rim sign,
two concentric rims surrounding the abscess cavity,
where the outer rim is hypointense and the inner is
relatively hyperintense, may be visible on SWI or T2-weighted imaging. Cerebral abscesses may also arise
near mycotic aneurysms (Figures 3 and 4). The presence
of leptomeningeal enhancement on MRI or CT can
suggest concomitant meningitis.
Figure 2. A 56-year-old woman presented with fever and a bilateral lower extremity rash. Physical examination revealed a pansystolic
murmur with radiation to the left axilla and splinter haemorrhages. Echocardiography demonstrated mitral valve regurgitation and prolapse
with vegetation. Blood culture yielded Streptococcus sanguinis. (a) Axial post-contrast T1-weighted image shows a small ring-enhancing
lesion in the right basal ganglia (arrowhead). The lesion is hyperintense on T2-weighted image (not shown). There is associated focal leptomeningeal
enhancement in the adjacent right frontal lobe cortex (arrow), suggestive of leptomeningitis. (b) Axial diffusion-weighted imaging with a
high b-value demonstrates a focal central hyperintense signal (arrow) within the previous right basal ganglia ring-enhancing lesion. (c) The
corresponding apparent diffusion coefficient map shows hypointense signal (arrow). These findings were indicative of restricted diffusion
and therefore consistent with an abscess. (d) Axial gradient echo sequence reveals a focus of susceptibility artefact in the left occipital lobe
(arrowhead), consistent with a microbleed. All findings resolved with conservative management with a 6-week course of broad-spectrum
antibiotics. Mitral valve replacement was proposed but declined by the patient.
Figure 3. A 27-year-old woman presented with fever, left-sided weakness, and slurred speech. (a) Initial axial non-contrast computed
tomography (CT) of the brain shows a small focus of hypodensity in the right temporoparietal region (arrow). (b) Follow-up axial non-contrast
CT 1 day later demonstrates rapid enlargement of the hypodensity in the right temporoparietal region (arrow), with a small acute
haemorrhagic focus (arrowhead). (c) Axial computed tomography angiography (CTA) shows a small contrast-enhancing focus at the
proximal M2 segment of the right middle cerebral artery (arrowhead) within the infarct, suggestive of an aneurysm. (d) Volumetric rendering
of the CTA shows the saccular morphology of the proximal right M2 aneurysm (arrowhead), consistent with a mycotic aneurysm.
Figure 4. Same patient as in Figure 3. (a) Axial post-contrast T1-weighted magnetic resonance image shows a ring-enhancing lesion in the
right temporoparietal region (arrow), with a central non-enhancing area and a peripheral enhancing focus (arrowhead). (b) Axial diffusion-weighted
image with a high b-value demonstrates central hyperintense signal (arrow). (c) The apparent diffusion coefficient map shows
corresponding hypointense signal (arrow). Overall findings are suggestive of a cerebral abscess with a mycotic aneurysm. Subsequent
echocardiography revealed mitral valve regurgitation and prolapse with vegetation. Blood culture was negative. The patient underwent burr
hole drainage of the abscess, and pus culture yielded Staphylococcus aureus. She was subsequently treated with a course of intravenous
vancomycin.
Cerebral Haemorrhages
Macrohaemorrhage usually results from haemorrhagic
transformation of ischaemic stroke, progression of
microhaemorrhages, or rupture of mycotic aneurysms.
Haemorrhagic transformation occurs more frequently in
embolic strokes (51%-71%) than in non-embolic strokes
(2%-21%)[11] and may present as petechial haemorrhage
or large parenchymal haematomas.[9] Cerebral ischaemic
lesions of varying ages across multiple vascular
territories and different haemorrhagic patterns would
raise suspicion for cardiac emboli. In the context of
underlying IE, cerebral septic embolism is a likely
diagnosis (Figure 5).
Septic emboli damage the endothelium and disrupt the
blood-brain barrier, resulting in inflammatory vasculitis
and small vessel rupture, often leading to cerebral
microbleeds or even intracerebral haemorrhage. One
study found that cerebral microbleeds in 57% of patients
with IE.[4] These microbleeds appear as hypointense foci
on T2* imaging or SWI MRI often in the cortex, and less
frequently in subcortical white matter, basal ganglia, or
posterior fossa.[4]
Figure 5. A 62-year-old man with known mitral valve regurgitation. (a) Axial diffusion-weighted imaging of the brain with a high b-value
shows a focal hyperintense signal in the left frontal lobe (arrowhead), with corresponding hypointensity on the apparent diffusion coefficient
map (not shown). (b) Associated susceptibility artefact is noted in the same region (arrowhead), suggestive of haemorrhagic transformation.
(c) Axial unenhanced computed tomography (CT) of the brain 1 month later shows a new haemorrhagic infarction in the right occipital lobe
with mild perilesional oedema (arrow). (d) Axial computed tomography angiography (CTA) of the brain shows a tiny contrast-enhancing
focus within the haemorrhagic infarct, suggestive of an aneurysm (arrowhead), while the left frontal infarct shows signs of chronicity (arrow).
(e) Axial non-contrast CT of the brain 1 day later shows a new right frontal lobe infarct, as well as new subarachnoid haemorrhage in
the right frontal region (arrowhead) and suprasellar cistern (arrow). (f) Axial non-contrast CT of the brain 3 days later reveals a new right parieto-occipital
lobe infarct (arrow). (g) Axial non-contrast CT of the brain 1 month later shows a new haemorrhagic infarct in the high left parietal
lobe (arrow). (h) Axial CTA of the brain shows a tiny contrast-enhancing focus (arrowhead) within the haemorrhagic infarct suggestive
of aneurysm. The presence of multiple haemorrhagic infarcts of different timing, some with associated aneurysms, is highly suggestive
of cerebral septic emboli with mycotic aneurysms. Subsequent echocardiography revealed mild mitral and tricuspid regurgitation with
vegetations on both mitral valve leaflets. Blood cultures yielded Rothia dentocariosa. A diagnosis of infective endocarditis was established and the patient was treated with intravenous antibiotics.
Mycotic Aneurysms
Cerebral septic emboli can trigger inflammation and
weakening of vessel walls, forming mycotic aneurysms.[7] These aneurysms are found in about 6.2% of patients with
IE and may shrink, enlarge, or develop de novo within
1 week to 3 months of starting antibiotics.[12] Mycotic
aneurysms have a 2% to 10% risk of rupture regardless
of their size and are associated with a high mortality
rate of 80%.[7] About 22% of IE patients presenting with
intracerebral haemorrhage have mycotic aneurysms
which should be promptly identified.[13] CTA or MRA
should be performed to confirm the diagnosis (Figures 5 and 6), followed by DSA for clear delineation of the
number, size and location of the mycotic aneurysms and
surgical or endovascular planning.
Figure 6. Same patient as in Figure 5. (a) Axial plain computed tomography of the brain 2 weeks after antibiotic therapy shows a new,
large haemorrhagic infarct in the right frontal lobe with mass effect (arrow). (b) Axial unenhanced computed tomography at a lower level
from the same study demonstrates a preexisting haematoma with intraventricular extension (arrow). (c) Volumetric rendering of computed
tomography angiography reveals a saccular aneurysm arising from a distal branch of the right anterior cerebral artery (arrowhead), saccular
aneurysms of the right middle cerebral artery (asterisk), and a left posterior cerebral artery aneurysm (arrow). The peripheral location and
saccular morphology of these aneurysms are highly suggestive of mycotic origin.
CTA and MRA have low sensitivity for small (<5 mm)
or distal mycotic aneurysms.[1] Aneurysms near the skull
base may be overlooked on CTA, while those in low-flow
areas may be missed on time-of-flight MRA.[14] In
cases with clinical suspicion of mycotic aneurysm but
negative CTA or MRA, DSA should be performed.[1]
Features favouring mycotic aneurysms include
multiplicity, saccular shape, distal location (such as
MCA segments 2 to 4 or posterior cerebral artery), size
or morphological changes on consecutive angiograms,
presence of other intra- or extra-cranial mycotic
aneurysms, adjacent arterial occlusion or stenosis, and
cerebral infarction at the aneurysm site[13] (Figure 6).
Management
Neurological complications from IE are life-threatening
and require multidisciplinary management, involving
neurosurgeons, radiologists, cardiologists, and
microbiologists. Empirical intravenous antibiotic therapy
is promptly administered and later tailored according
to culture sensitivity results. Valvular replacement
combined with antibiotics yield better outcomes than
antibiotics alone in left-sided endocarditis.[15]
Radiologists play a key role in both diagnosis and guiding
treatment by accurately reporting the type and severity
of each lesion. Surgical drainage can be considered in
cases of cerebral abscesses with significant mass effect.
Antiplatelet drugs and anticoagulants are contraindicated in both ischaemic stroke and macrohaemorrhage caused
by septic embolism due to the high risk of bleeding.[3]
Cardiac surgery should be postponed for at least 4 weeks
after a clinically significant intracranial haemorrhage
or large ischaemic infarct.[3] Mycotic aneurysms should
be excluded before open heart surgery for valvular
replacement requiring anticoagulation to reduce bleeding
risk.[15]
Interventional radiologists play an evolving role of
in treating mycotic aneurysms in collaboration with
neurosurgeons. Techniques include preoperative CTA or
MRA with volumetric rendering, road-map technique for
neuro-navigation, and cone beam CTA for postprocedural
monitoring. Given the unpredictable nature of mycotic aneurysms and the weak correlation between size and
rupture risk, surgical or endovascular treatment should
be considered for unruptured aneurysms that enlarge
or do not regress on follow-up imaging.[7] [14] Ruptured or
symptomatic mycotic aneurysms also require surgical
or endovascular intervention.[14] A surgical approach
is indicated when an aneurysm exerts mass effect[14] or
supplies an eloquent brain region.[1] However, clipping
may be difficult due to a wide or absent aneurysmal neck
and fragile vessels.[3]
An endovascular approach is indicated for those unfit for
surgery due to cardiac disease.[3] It can be divided into
direct or indirect approaches. An indirect approach with
parent artery occlusion is the endovascular treatment of
choice, especially for distally located aneurysms and
circumferential vessel involvement. However, parent
artery sacrifice is not possible at times and the direct
approach may remain the only viable option. The direct approach using coils or liquid embolic agents allows
precise control of the aneurysm while preserving distal
flow from the parent artery. Endovascular coiling may be
a safer option with higher occlusion and lower procedure-related
complication rates[7] (Figure 7). Detachable
coils allow precise deployment and better durability
compared with liquid embolic agents. They are preferred
in proximal aneurysms, while liquid embolic agents
are more suitable for distal aneurysms not accessible
by microcatheter. Intracranial flow diverters can be
used to divert turbulent blood flow from the aneurysm
and preserve laminar blood flow in the main vessel
and its side branches. With reduced blood flow to the
aneurysm and gradual vessel remodelling, this results
in progressive aneurysmal sac thrombosis[16] (Figure 8). It is important to note that mycotic aneurysms may
grow after simple coiling, while the parent artery may
thrombose after flow diverter placement in the setting
of infection.
Figure 7. The same patient as Figures 5 and 6 underwent surgical clipping of the right distal anterior cerebral artery mycotic aneurysm.
(a) Frontal view of digital subtraction angiography (DSA) of the right internal carotid artery shows a saccular distal right M1 aneurysm
(arrowhead). (b) Volumetric rendering depicts the saccular aneurysm (arrowhead), allowing accurate preoperative measurement of its size,
height, and neck. The arterial supply from the right middle cerebral artery (MCA) and its angulation in three-dimensional space are also
visualised. (c) DSA of the right MCA using the roadmap technique enabled neuronavigation with the use of a guidewire to access the M1
aneurysm for precise coil embolisation (arrows). (d) Post-embolisation DSA of the right MCA shows successful occlusion of the mycotic
aneurysm with preserved flow to the distal branches (arrow). (e) Frontal DSA of the right vertebral artery demonstrates a peripherally located
P4 aneurysm (arrowhead). (f) Volumetric rendering depicts its saccular morphology with a narrow neck and clearly shows the arterial
supply (arrowhead) from the left posterior cerebral artery (PCA), aiding in accurate preoperative planning. (g) DSA of the left PCA enabled
direct neuronavigation using a guidewire (arrows) to the target aneurysm for embolisation. (h) DSA of the distal left PCA shows precise coil
embolisation of the peripherally located mycotic aneurysm (arrow), performed through an indirect approach that resulted in parent artery
occlusion. Despite the challenging locations of the mycotic aneurysms, DSA with volumetric rendering and the roadmap technique allowed
successful neuronavigated embolisation.
Figure 8. Same patient as Figures 3 and 4. Follow-up computed tomography angiography (CTA) 2 months later showed a persistent
M2 mycotic aneurysm (not shown). (a) Oblique projection of digital subtraction angiography (DSA) of the right internal carotid artery
(ICA) shows a lobulated M2 mycotic aneurysm arising from the middle cerebral artery (arrowhead). (b) DSA with the roadmap technique
enabled neuronavigation for precise embolisation of the mycotic aneurysm (arrow). (c) Post-embolisation DSA of the right ICA shows the
successfully embolised right M2 aneurysm (arrow), with preservation of distal flow. (d) Follow-up coronal cone beam CTA 2 months post-embolisation
shows a new saccular aneurysm adjacent to the previously embolised aneurysm (arrow). (e) Oblique projection DSA of the
right ICA confirms the presence of the new narrow-neck mycotic aneurysm arising from the medial wall of the M2 segment (arrow). (f) Coil
embolisation of the second aneurysm was performed, and a flow diverter was deployed across the aneurysmal neck. Follow-up sagittal
cone beam CTA 1.5 years later shows the flow diverter in situ (arrow), with successfully embolised aneurysm (arrowhead). The stent remains
patent and the distal branches are preserved.
CONCLUSION
Neurological complications secondary to IE require
prompt recognition of its typical presentations and
imaging manifestations to facilitate early diagnosis
of neurological complications and their subsequent
treatment, including possible radiological intervention.
REFERENCES
1. Ferro JM, Fonseca AC. Infective endocarditis. Handb Clin Neurol. 2014;119:75-91. Crossref
2. Papadimitriou-Olivgeris M, Guery B, Ianculescu N, Dunet V,
Messaoudi Y, Pistocchi S, et al. Role of cerebral imaging on
diagnosis and management in patients with suspected infective
endocarditis. Clin Infect Dis. 2023;77:371-9. Crossref
3. Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic
complications in infective endocarditis: identification, management,
and impact on cardiac surgery. Neurohospitalist. 2014;4:213-22. Crossref
4. Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, et al.
Brain MRI findings in neurologically asymptomatic patients with
infective endocarditis. AJNR Am J Neuroradiol. 2013;34:1579-84. Crossref
5. Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, et al.
Effect of early cerebral magnetic resonance imaging on clinical
decisions in infective endocarditis: a prospective study. Ann Intern
Med. 2010;152:497-504, W175. Crossref
6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP,
Del Zotti F, et al. 2015 ESC Guidelines for the management of
infective endocarditis: The Task Force for the Management of
Infective Endocarditis of the European Society of Cardiology
(ESC). Endorsed by: European Association for Cardio-Thoracic
Surgery (EACTS), the European Association of Nuclear Medicine
(EANM). Eur Heart J. 2015;36:3075-128. Crossref
7. Zanaty M, Chalouhi N, Starke RM, Tjoumakaris S, Gonzalez LF, Hasan D, et al. Endovascular treatment of cerebral mycotic
aneurysm: a review of the literature and single center experience.
Biomed Res Int. 2013;2013:151643. Crossref
8. Ahn Y, Joo L, Suh CH, Kim S, Shim WH, Kim SJ, et al. Impact
of brain MRI on the diagnosis of infective endocarditis and
treatment decisions: systematic review and meta-analysis. AJR
Am J Roentgenol. 2022;218:958-68. Crossref
9. Zakhari N, Castillo M, Torres C. Unusual cerebral emboli. Neuroimaging Clin N Am. 2016;26:147-63. Crossref
10. Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific
MR imaging findings that are useful in dating ischemic stroke.
Radiographics. 2012;32:1285-97. Crossref
11. Cho IJ, Kim JS, Chang HJ, Kim YJ, Lee SC, Choi JH, et al.
Prediction of hemorrhagic transformation following embolic
stroke in patients with prosthetic valve endocarditis. J Cardiovasc
Ultrasound. 2013;21:123-9. Crossref
12. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G, et al. Endovascular treatment of cerebral mycotic aneurysms.
Radiology. 2002;222:389-96. Crossref
13. Hui FK, Bain M, Obuchowski NA, Gordon S, Spiotta AM,
Moskowitz S, et al. Mycotic aneurysm detection rates with cerebral
angiography in patients with infective endocarditis. J Neurointerv
Surg. 2015;7:449-52. Crossref
14. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious
aneurysm): current options for diagnosis and management.
Neurocrit Care. 2009;11:120-9. Crossref
15. Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R,
Kotilainen P. Neurologic manifestations of infective endocarditis:
a 17-year experience in a teaching hospital in Finland. Arch Intern
Med. 2000;160:2781-7. Crossref
16. Ravindran K, Casabella AM, Cebral J, Brinjikji W, Kallmes DF,
Kadirvel R. Mechanism of action and biology of flow diverters
in the treatment of intracranial aneurysms. Neurosurgery.
2020;86(Suppl 1):S13-9. Crossref
Vascular Abnormalities in the Breast: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2025 Dec;28(4):e276-83 | Epub 10 December 2025
Vascular Abnormalities in the Breast: A Pictorial Essay
SH Lee, S Yang, GHC Wong
Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr SH Lee, Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China. Email: lsh689@ha.org.hk
Submitted: 15 October 2024; Accepted: 5 February 2025.
Contributors: SHL designed the study. SHL and GHCW acquired the data. SHL and SY analysed the data. SHL draft the manuscript. SY
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-186-2). A waiver of patient consent and acknowledgment forms was approved by the Board due to the retrospective nature of the study.
INTRODUCTION
Vascular lesions are not uncommonly seen in the
breast. They can range from benign haemangiomas
to aggressive angiosarcomas. Benign lesions and
vascular malformations are usually asymptomatic when
small in size and may only be incidentally found on
imaging. Malignant angiosarcomas are extremely rare
and aggressive, often presenting with disseminated
metastases on diagnosis. This pictorial essay aims to
illustrate the common imaging features of vascular
lesions, with the cases identified in the database of a
local tertiary hospital in Hong Kong from 2008 to 2024.
BENIGN VASCULAR LESIONS
Haemangioma
Haemangiomas are commonly found in the hepatobiliary
and musculoskeletal systems, as well as in the breasts,
where they are usually small in size and asymptomatic,
usually presenting as an incidental imaging finding. It
has been reported that haemangiomas are found in 1.2%
of mastectomy specimens and 11% of post-mortem
specimens (from a forensic population) of the female
breast.[1] Some patients might present with blue skin
discolouration when the lesion is large and superficial.
On mammography, haemangiomas are usually equal
dense to the breast tissue, and oval in shape with
circumscribed or microlobulated margins (Figure 1).[2] Intralesional microcalcifications may be found
occasionally[2], which may lead to the need for imaging
surveillance or core biopsy.
Figure 1. Cavernous haemangioma. (a) Planar mammographic
and (b) tomosynthesis images of the right breast (mediolateral
view) show a 1.5-cm oval, isodense mass with microlobulated
borders in the inferoposterior region, more clearly visualised on
tomosynthesis (arrows).
Sonographically, they are similar to their shape on
mammography and are oriented in a parallel manner
(Figures 2 and 3).[2] Their echogenicity is variable,[2] and
they may exhibit non-specific vascular flow.[3] Overall,
there are no definitive imaging features to suggest
benignity or malignancy. Histologically, these lesions
may present as a proliferation of variably sized, ectatic
blood vessels separated by fibrotic stroma.
Figure 2. Cavernous haemangioma (same case as Figure 1). Ultrasound of the right breast. At the 6-7 o’clock position, 6 cm inferior to the nipple, (a, b) a 1.4 × 0.6 × 1.4 cm3 oval, inhomogeneous, slightly hypoechoic mass (arrows) is seen, oriented parallel to the surrounding parenchyma, with microlobulated margins. (c) Mild intralesional vascularity is noted (arrow).
Figure 3. Another case of haemangioma. Ultrasound of the right breast (arrows in [a] and [c]). At the 11-12 o’clock position, 2 cm from the nipple, a 0.8
× 0.4 × 0.7 cm3 oval, hypoechoic mass is seen, oriented parallel to the tissue planes, with microlobulated margins. Internal vascularity is
demonstrated on Doppler ultrasound (arrows in [b] and [d]).
Management of benign haemangiomas remains
controversial due to the sampling error of core biopsy
samples and difficulty in clearly delineating the borders
of the haemangiomas confidently on core samples. A
recent review had explored the possibility of clinical
and radiological surveillance in cases of radiologically
pathologically concordant haemangiomas, but surgical
excision remains the mainstay of management.[4]
Superficial Venous Thrombophlebitis:
Mondor’s Disease
Mondor’s disease is a rare disease characterised by
inflammation and thrombosis of the superficial venous
structures in the breast. This disease is found in less
than 1% of the population.[5] Clinically, patients present
with skin swelling or tender cord-like palpable masses.
On mammography, elongated equal density tubular
structures are usually found in the upper outer quadrant
where the lateral thoracic veins are located (Figure 4).[6]
Ultrasound should be performed to exclude underlying
breast malignancy since dilated ducts could mimic
Mondor’s disease mammographically.[6]
Figure 4. Mondor’s
disease. Mammogram
of the right breast
(mediolateral oblique
view). A few linear
equal density tubular
lesions with a beaded
appearance (arrows) are
identified in the upper
quadrant of the right
breast.
On ultrasound, Mondor’s disease is seen as a tubular
hypoechoic structure with a beaded appearance, which
is the classical finding of a thrombosed vein, but not of
a dilated duct which usually with smooth wall (Figure 5).[6] It is also longer in extent and will not be connected
to the nipple areolar complex.[6] Similar to venous
thrombosis in the body elsewhere, the distended vein is
not compressible by the ultrasound probe and there is
absence of Doppler signal.[5]
Figure 5. Mondor’s disease. Ultrasound of the right breast. At the 10 o’clock position near the axilla, (a) an elongated tubular
lesion with a beaded appearance is visualised (arrow). (b) No
Doppler signal is detected within the structure (arrow).
Mondor’s disease does not require a pathological
diagnosis when clinical and radiological findings
are concordant. No specific treatment is needed for
the disease since it will resolve spontaneously in 1 to 2 months’ time.[5] Nonsteroidal anti-inflammatory
drugs may be considered in symptomatic cases if not
contraindicated.[5]
VASCULAR MALFORMATIONS
High Flow: Arteriovenous Malformation
Arteriovenous malformations (AVMs) are exceedingly
rare in the breast, with only scant case reports. No specific
mammographic features are found in AVMs. Similar
to other benign vascular entities, they may present on
mammography as an equal density mass with a round
shape and circumscribed margins (Figure 6). They may
also contain benign-appearing calcifications, indicating
the presence of phleboliths.[7]
Figure 6. Arteriovenous malformation. (a) Craniocaudal and (b)
mediolateral oblique views of the mammogram of the left breast.
A 0.6-cm equal density round mass is identified in the upper outer
quadrant of the left breast (arrows).
On ultrasound, they are again non-specific. In our case,
the lesion presented as an oval heterogeneous hypoechoic
mass with parallel orientation to the skin surface. No
posterior acoustic enhancement was demonstrated. Doppler ultrasound may be a non-invasive technique
to establish the diagnosis since it can demonstrate the
mixture of arterial and venous blood flow within the
lesion (Figure 7).[8]
Figure 7. Arteriovenous malformation. Ultrasound of the left breast. At the 2 o’clock position, 3 cm from the nipple, a 0.6 × 0.4 × 0.5 cm3 oval, heterogeneous, hypoechoic mass is visualised with parallel orientation and no posterior acoustic shadowing. It demonstrates
internal arterial and venous flow, with communication to adjacent breast parenchymal vasculature (arrows).
Magnetic resonance imaging (MRI) can demonstrate a
tangle of dilated blood vessels with progressive contrast
enhancement of the lesion.[9] Blooming artefacts indicate
the presence of phleboliths.[9]
The management of AVMs is dependent on the clinical
symptoms. For asymptomatic and small masses, as in our
case, conservative management should be considered. In
case of palpable symptomatic lesions, embolisation or
surgical excision would be the options.[9]
Slow Flow: Venous and Venolymphatic
Malformations
Venous and venolymphatic malformations are slow-flow
vascular lesions, which may be asymptomatic.
Some patients present with bluish skin discolouration
and even with enlarged breast volume when the lesion
is more sizable.
Ultrasound can be a good initial tool to establish the
diagnosis. These lesions are identified as an area of
hypoechogenicity within the breast. Doppler ultrasound
on this hypoechoic area will demonstrate the venous
flow pattern on spectral technique (Figure 8). Other
sonographic findings include echogenic foci indicating
phleboliths and absence of colour uptake due to
thrombosis or lymphatic components.[10]
Figure 8. Venous malformation. Ultrasound of the left anterior chest wall. A hypoechoic area with tubular structures demonstrating
venous flow patterns is observed (arrows). The total extent of involvement measures at least 4.3 cm along its greatest dimension.
Venous malformations can present as T2-weighted
hyperintense tubular structures. Variable unenhanced
T1-weighted signal has been described depending
on whether these structures contain thrombosis.[10]
Enhancement could be seen on T1-weighted images
after gadolinium injection (Figure 9). If phleboliths
are present, susceptibility artefacts may also be seen.[11]
For the lymphatic component, MRI might demonstrate
septal enhancement in the background of non-enhancing
lymphatic fluid.[12]
Figure 9. Venolymphatic malformation. Magnetic resonance images of the left chest wall. (a) T2-weighted coronal image with fat saturation and (b) T1-weighted axial image with fat saturation after gadolinium injection. Multiple clusters of lobulated T2-weighted hyperintense structures (arrow in [a]) with post-contrast enhancement are seen in the left breast (arrow in [b]).
If patients are symptomatic or there is cosmetic concern,
sclerotherapy, laser therapy, or surgical resection
are options.[11] In our case, sclerotherapy with sodium
tetradecyl sulphate foam was performed under the
guidance of direct puncture venography (Figure 10),
with resulting clinical improvement.
Figure 10. Venolymphatic malformation. Direct puncture venograms demonstrate (a) a slow-flow vascular malformation (arrow) and (b) the appearance following injection of an alcohol mixture (arrow).
MALIGNANT VASCULAR LESIONS
Angiosarcoma
Angiosarcoma is an exceedingly rare cause of primary
breast tumour, with the literature-quoted incidence
rate <0.05%.13 There are no specific mammographic
findings.[13]
On ultrasound, they show a variable echogenic pattern,
usually with hypervascularity on Doppler.[13] However,
as mentioned previously, haemangiomas may also
demonstrate hypervascularity. Hence, this feature is not
specific.
In our case, similar to other breast tumours, angiosarcomas
may enhance after contrast administration on computed
tomography scan (Figure 11).
Figure 11. Angiosarcoma.
Computed tomography scan of
the thorax. (a) The left breast implant
capsule (arrow) is seen
adjacent to a heterogeneous soft
tissue mass with (b) predominantly
peripheral enhancement (arrow).
On MRI scan, they showed variable signal intensity
on T1-weighted and T2-weighted images. High T1-weighted signal suggests the presence of haemorrhagic
products or venous lakes.[13] Elevated T2-weighted signals indicate the aggressiveness of the lesion
with cystic degeneration and tumour necrosis.[14]
They enhance, often at the periphery only, after
gadolinium administration (Figure 12).[14] The kinetic
characteristics of the tumours depend on their
grade.[13]
Figure 12. Angiosarcoma.
(a) Magnetic resonance imaging of
both breasts showing bilateral
breast implants (thin arrows).
Magnetic resonance images of
the left breast. (b) T1-weighted, (c)
T2-weighted, and (d) fat-saturated
T1-weighted post-gadolinium
sequences. An irregular,
infiltrative solid mass is seen at
the superolateral aspect of the left
breast implant, showing peripheral
contrast enhancement (thin
arrow), particularly in the upper
outer quadrant. Non-enhancing
T1-weighted hyperintense and
T2-weighted hypointense foci
suggest tumoural haemorrhage
(thin arrows), while non-enhancing
T1-weighted hypointense and T2-weighted hyperintense foci are
consistent with tumour necrosis
(red arrows).
Aggressive surgery remains the mainstay of treatment,
while there is still no consensus on adjuvant treatment.[15]
Angiosarcomas carry a poor prognosis, with the
majority of the cases in our centre showing disseminated
hypervascular metastases or contralateral breast
metastases despite radical surgery performed after
diagnosis (Figure 13).
Figure 13. Metastatic angiosarcoma. Computed tomography
pulmonary angiogram. Left breast mastectomy with myocutaneous
flap reconstruction for previous angiosarcoma. New bilateral lung
nodules are noted, along with cutaneous and subcutaneous
metastases involving the reconstructed left breast and the right
breast.
CONCLUSION
Vascular lesions have been increasingly discovered due
to increased health screening. We should be aware of
the typical imaging features of vascular malformations
to avoid unnecessary biopsies. While histopathological
results are still required to establish the diagnosis in
haemangiomas and angiosarcoma—with surgical
excision remaining the mainstay of management—if the
imaging features of haemangiomas are benign, such as
oval/lobulated shape with well-circumscribed margins,
imaging surveillance could be performed after needle
biopsy.[2]
REFERENCES
1. Kim KM, Kim JY, Kim SH, Jeong MJ, Kim SH, Kim JH,
et al. Breast cavernous hemangioma with increased size on
ultrasonography: a case report. J Korean Soc Radiol. 2018;79:311-4. Crossref
2. Mesurolle B, Sygal V, Lalonde L, Lisbona A, Dufresne MP,
Gagnon JH, et al. Sonographic and mammographic appearances
of breast hemangioma. AJR Am J Roentgenol. 2008;191:W17-22. Crossref
3. Glazebrook KN, Morton MJ, Reynolds C. Vascular tumors of the breast: mammographic, sonographic, and MRI appearances. AJR Am J Roentgenol. 2005;184:331-8. Crossref
4. Azam R, Mrkonjic M, Gupta A, Gladdy R, Covelli AM.
Mesenchymal tumors of the breast: fibroblastic/myofibroblastic
lesions and other lesions. Curr Oncol. 2023;30:4437-82. Crossref
5. Amano M, Shimizu T. Mondor’s disease: a review of the literature. Intern Med. 2018;57:2607-12. Crossref
6. Shetty MK, Watson AB. Mondor’s disease of the breast: sonographic and mammographic findings. AJR Am J Roentgenol.
2001;177:893-6. Crossref
7. Padmanabhan V, Dev B, Garg M, Gnanavel H. Arteriovenous malformation of the breast; report a case. Arch Breast Cancer.
2024;11:96-100. Crossref
8. O’Beirn E, Elliott J, Neary C, McLaughlin R. 32 Congenital arteriovenous malformation of the breast associated with giant
hairy naevus: a case report. Br J Surg. 2021;108(Suppl 6):znab259-64. Crossref
9. Swy E, Wahab R, Mahoney M, Vijapura C. Multimodality imaging review of breast vascular lesions. Clin Radiol. 2022;77:255-63. Crossref
10. Gautam R, Dixit R, Pradhan GS. Venous malformation in the breast: imaging features to avoid unnecessary biopsies or surgery. J Radiol Case Rep. 2023;17:1-8. Crossref
11. Vijapura C, Wahab R. Multiple venous malformations involving
the breast. J Breast Imaging. 2022;4:550-2. Crossref
12. Marín C, J Galindo P, Guzmán F, Ferri B, Parrilla P. Lymphovascular
malformation of the breast: differential diagnosis and a case report
[in English, Spanish]. Cir Esp (Engl Ed). 2019;97:541-3. Crossref
13. Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190:533-8. Crossref
14. Wu WH, Ji QL, Li ZZ, Wang QN, Liu SY, Yu JF. Mammography and MRI manifestations of breast angiosarcoma. BMC Womens
Health. 2019;19:73. Crossref
15. Bordoni D, Bolletta E, Falco G, Cadenelli P, Rocco N, Tessone A, et al. Primary angiosarcoma of the breast. Int J Surg Case Rep.
2016;20S(Suppl):12-5. Crossref