Evolution in Image-guided Preoperative Breast Lesion Localisation
PERSPECTIVE
Hong Kong J Radiol 2025 Mar;28(1):e35-43| Epub 12 March 2025
Evolution in Image-guided Preoperative Breast Lesion Localisation
T Wong, SC Woo, WY Fung, CM Chau, JKF Ma
Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr T Wong, Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China. Email: gloria_wong@live.com
Submitted: 16 January 2024; Accepted: 10 July 2024.
Contributors: All authors designed the study. TW, SCW and WYF acquired and analysed the data. TW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, TW was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref Nos.: CIRB-2025-028-1 and IRB-2025-034). The requirement for patient consent was waived by the Board due to the retrospective nature of the study.
Supplementary Material: The supplementary material was provided by the authors and some information may not have been peer reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by the Hong Kong College of Radiologists. The Hong Kong College of Radiologists disclaims all liability and responsibility arising from any reliance placed on the content.
Abstract
Successful surgical excision of nonpalpable breast lesions requires precise image-guided localisation. Wire
localisation is the most well-established technique, but it has many disadvantages related to its external part.
Intraoperative ultrasound may obviate the need for additional localisation device placement, but that requires
sonography training of surgeons. Radioguided occult lesion localisation has been available for two decades, yet it
is not available in every centre due to the lack of support from the nuclear medicine unit. Like wire localisation, the
procedure must be performed shortly before the surgery, which poses scheduling challenges to imaging and surgery
departments. Non-radioguided wireless devices have been developed to overcome these drawbacks. Their most
prominent advantages are the feasibility of being placed before the day of surgery, which provides logistic flexibility,
and their lack of an external component resulting in no restriction on surgical approach. Evidence of the success
of this technique is growing. This article provides an overview of the commonly used image-guided breast lesion
localisation techniques in Hong Kong, highlighting the merits and limitations of each technique. Future research
directions for the novel wireless devices are also discussed.
Key Words: Breast neoplasms; Radiography; Surgery
中文摘要
影像導引術前乳腺病灶定位進展
黃婷、鄔潔欣、馮惠鈺、周智敏、馬嘉輝
成功手術切除不可觸及的乳腺病灶需要精確的影像導引定位。導線定位是最成熟的技術,但它有許多與其外部部件相關的缺點。術中超音波不一定需要放置額外定位裝置,但需要外科醫生接受超音波檢查訓練。放射引導隱匿性病灶定位已有二十年歷史,但由於需依賴核醫學科支持,並非每個中心都可以使用。而且,與導線定位一樣,該程序必須在手術前不久進行,這為影像和手術部門的日程安排帶來挑戰。已開發的非放射引導的無線設備可克服這些缺點。它們最大的優勢是可以在手術前任何一天放置,提供了靈活性,而且它們沒有限制手術方法的外部組件。越來越多證據表明這項技術取得了成功。本文概述了香港常用的影像引導乳腺病變定位技術,強調了每種技術的優缺點,同時討論新型無線設備的未來研究方向。
INTRODUCTION
Advances in breast imaging and increasing breast cancer
awareness have led to a rise in the detection of small
breast lesions.[1] Together with effective neoadjuvant
chemotherapy that allows good tumour shrinkage, there
is an increasing need for image-guided localisation of
nonpalpable breast masses.[2] Accurate localisation is of
paramount importance for successful surgical excision.
Wire localisation is a conventional breast localisation
method. However, as part of the wire is external, it can
cause patient discomfort, wire dislodgement, and may
have an impact on the surgical approach.[1] [2] [3] [4] [5] [6] [7] [8] Intraoperative
ultrasound may omit the need for preoperative
localisation, requiring surgeons to be competent with
sonography skills. It is limited to sonographically visible
targets (either the lesion itself or a sonographically
visible marker). Radioguided occult lesion localisation
(ROLL) requires support from nuclear medicine for
the preparation of tracer and scintigraphy. Despite the
absence of a protruding component, it must be performed
on the same day or a day before the operation with the
accompanying lack of scheduling flexibility, similar to
wire placement.[3]
Non-radioactive wireless localisation devices, such as
magnetic seeds, radar reflectors, and radiofrequency
identification (RFID) tags, have emerged recently to
address these limitations.[1] [2] [3] [4] [5] [6] [7] [8] Since they are approved for
long-term implantation, the scheduling of their placement
and the operation can be decoupled. Such flexibility
can be particularly useful during the pandemic era, as appointments can be rearranged easily for infection
control reasons.
This article provides an overview of the common
techniques used for image-guided breast localisation in
Hong Kong, with a review of the local experience in
utilising wireless localisation and a brief discussion of
future research directions.
WIRE LOCALISATION
Wire localisation is a well-established technique, which
was first described in 1966.[1] It is considered the gold
standard and the reference standard for research in other
localisation techniques.[2] The procedure is performed on
the same day, or uncommonly a day before surgery.[1] [3]
A 3-to-15–cm wire is deployed via a 16-to-20–gauge needle delivery system, with the external part taped to
the skin to secure its position.[1] [4] [5] It can be placed under
mammographic, sonographic, or magnetic resonance
imaging (MRI) guidance.[1] Different configurations of
the distal anchoring wire end are available, commonly in
hook, barb or pigtail shape.[4] Some wires have a thicker
portion to give surgeons a better tactile sensation when
approaching the target.[1] It is low in cost, with reported
technical success rates of 65% to 100% and specimen
margin clearance rate of 58% to 84%.[6] Multiple wires
can be placed with minimal distance restriction between
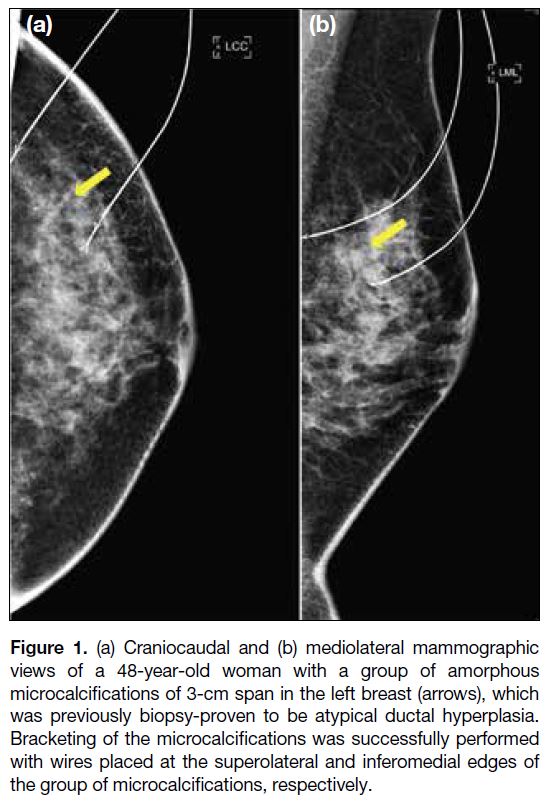
wires (Figure 1).[4]
Figure 1. (a) Craniocaudal and (b) mediolateral mammographic
views of a 48-year-old woman with a group of amorphous
microcalcifications of 3-cm span in the left breast (arrows), which
was previously biopsy-proven to be atypical ductal hyperplasia.
Bracketing of the microcalcifications was successfully performed
with wires placed at the superolateral and inferomedial edges of
the group of microcalcifications, respectively.
Wire localisation has a number of drawbacks. The
protruding part of the wire can cause patient discomfort, dislodgement, migration, kinking, transection, or
fragmentation.[1] [4] [6] The surgical incision site is
constrained by the wire entry site and healthy tissue
along the wire course is inevitably excised during wire
retrieval, potentially causing poorer cosmesis.[1] [4] Wire
placement on the day of surgery prolongs presurgical
fasting and increases the risk of vasovagal syncope.[1] [4]
The bundling of the wire placement and operation
schedules also can impair workflow efficiency.[4]
INTRAOPERATIVE ULTRASOUND
Intraoperative ultrasound was first described in the
literature in 1988.[2] [3] It is performed using a multi-frequency
sonographic probe (7-18 MHz) inside a
sterile probe cover, which is introduced into the breast
incision site.[3] The target can be the lesion itself or a
sonographically visible clip marker, which is either of a
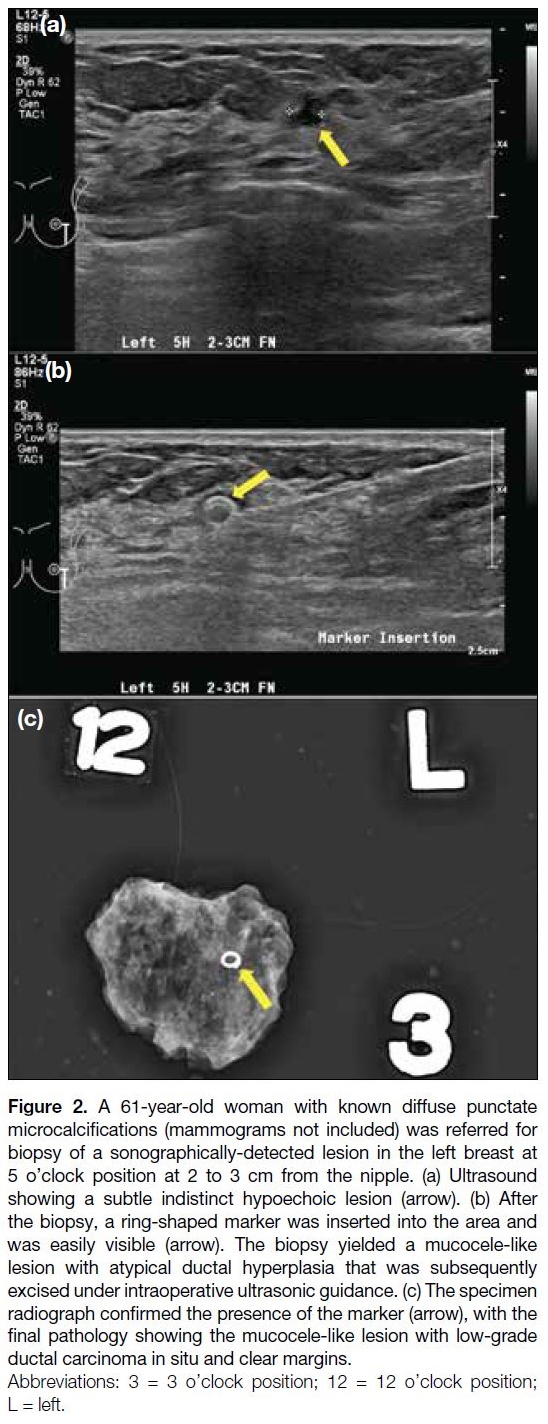
special shape (Figure 2) or associated with bioabsorbable
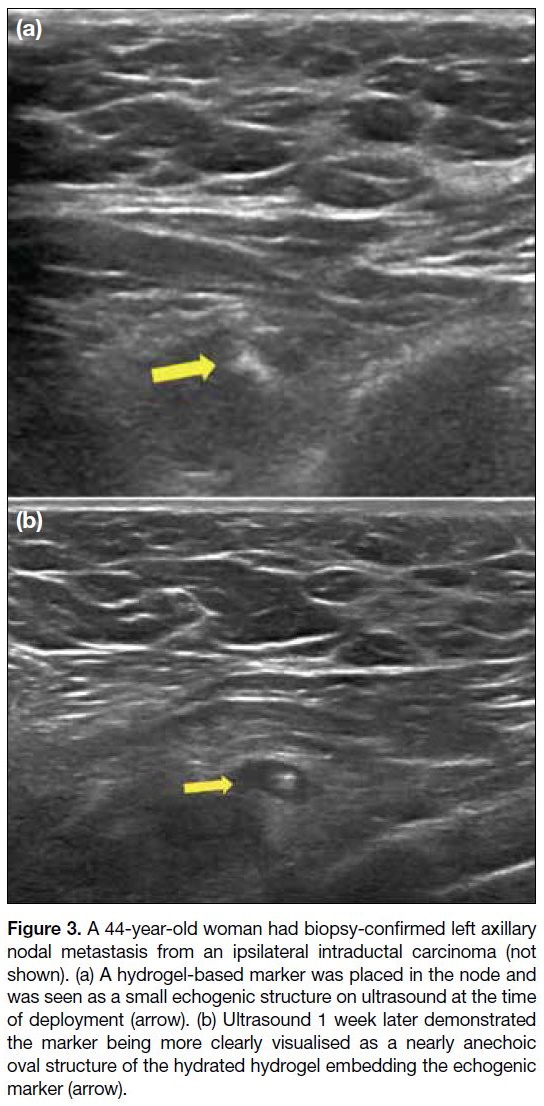
material (Figure 3), to make it easily visible with ultrasound.
Figure 2. A 61-year-old woman with known diffuse punctate
microcalcifications (mammograms not included) was referred for
biopsy of a sonographically-detected lesion in the left breast at
5 o’clock position at 2 to 3 cm from the nipple. (a) Ultrasound
showing a subtle indistinct hypoechoic lesion (arrow). (b) After
the biopsy, a ring-shaped marker was inserted into the area and
was easily visible (arrow). The biopsy yielded a mucocele-like
lesion with atypical ductal hyperplasia that was subsequently
excised under intraoperative ultrasonic guidance. (c) The specimen
radiograph confirmed the presence of the marker (arrow), with the
final pathology showing the mucocele-like lesion with low-grade
ductal carcinoma in situ and clear margins.
Figure 3. A 44-year-old woman had biopsy-confirmed left axillary
nodal metastasis from an ipsilateral intraductal carcinoma (not
shown). (a) A hydrogel-based marker was placed in the node and
was seen as a small echogenic structure on ultrasound at the time
of deployment (arrow). (b) Ultrasound 1 week later demonstrated
the marker being more clearly visualised as a nearly anechoic
oval structure of the hydrated hydrogel embedding the echogenic
marker (arrow).
The major advantage of this technique is the feasibility
of continuous intraoperative assessment of the margin,
thereby allowing the resection of less surrounding
healthy breast tissue with possible better cosmesis.[3] It
is reported to have better negative margin rates when
compared with wire localisation, thus reducing the
chance of re-excision due to close or involved margins.[3]
If the target itself is visible on ultrasound, no additional
image-guided device placement is necessary. This can
reduce patient anxiety and relieve the workload burden
of the radiology department.
This method requires surgeons to be well-trained in
breast ultrasound and requires an ultrasound machine
in the operating theatre.[2] [3] Multiple lesions in the
vicinity may cause confusion with the actual target. A
sonographically visible clip marker or a skin marker can
be placed under sonographic guidance in the radiology
department before the operation to aid the surgeons
identifying the target. Sonographically visible clip
markers can also be inserted under mammographic or
MRI guidance to localise lesions which are initially
sonographically occult. However, the reported risk of
clip marker migration >1 cm from target is 2% to 28%.[6]
OTHER WIRELESS TECHNIQUES
The other commonly available intraoperative wireless
techniques in Hong Kong include ROLL and non-radioguided
devices such as radar reflectors (Savi Scout;
Merit Medical, Aliso Viejo [CA], US), magnetic seeds
(Magseed; Endomag, Cambridge, United Kingdom),
and RFID tags (LOCalizer; Hologic, Santa Clara [CA],
US). Their mechanism is based on the transmission of
electromagnetic waves set at a specific wavelength.[4] [5]
Each system is composed of a probe that detects the
signal from the radioactive tracer or the wireless marker
and a console that emits real-time audio and visual
information to guide the surgeon.[4] [5] They have no impact
on the surgical incision site and provide a point signal
source that allows continuous reorientation to locate the
target, enabling less non-targeted tissue to be excised and
potentially better comesis.[1] [4] [6]
All of these techniques require a high startup cost
for purchasing the console and the probe, and more
expensive recurrent expenses for purchasing the tracer
or the single-use marker.[4] They cannot be performed
under MRI guidance as part of the equipment is MRI
incompatiable.[1] [3] [7]
Radioguided Occult Lesion Localisation
ROLL was first introduced in Europe in 1999.[3] An
occult lesion can be located with a gamma probe after
injecting 99mtechnetium-labelled radioisotope into it
under mammographic or sonographic guidance.[3]
ROLL shows technical success (93%-100%) and
margin clearance (60%-100%) comparable with
wire localisation.[6] It can map the sentinel lymph
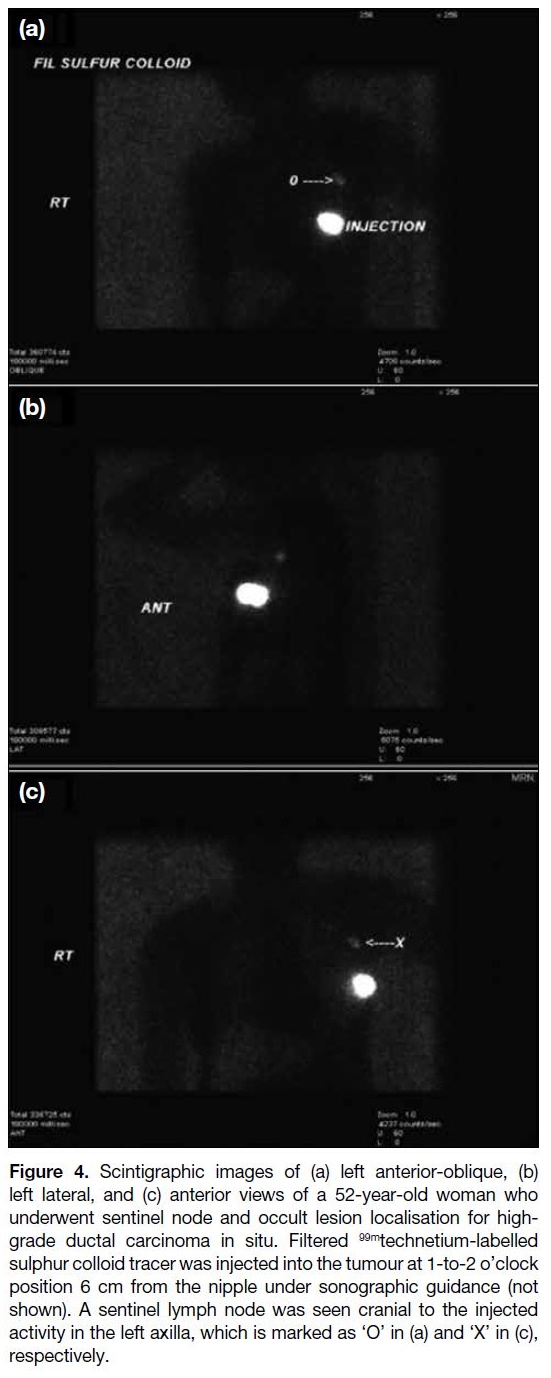
node simultaneously (sentinel node and occult lesion
localisation [SNOLL]) [Figure 4].[3] Due to tracer
decay, the surgery must be performed within a time
limit after tracer injection.[3] There is a risk of accidental intraductal injection that leads to conversion to wire
localisation, especially when targeting the more duct-abundant
subareolar region.[9] The use of a radionuclide requires support from the nuclear medicine department
and involves radiation safety precautions. Another
disadvantage is the radiation exposure to patients and
staff, although the dose is very low.[10] [11] A single-centre
study found it to be feasible in localising two targets in
the ipsilateral breast in eight patients (double-ROLL
technique),[10] but the technique is not commonly described in the literature.
Figure 4. Scintigraphic images of (a) left anterior-oblique, (b)
left lateral, and (c) anterior views of a 52-year-old woman who
underwent sentinel node and occult lesion localisation for high-grade
ductal carcinoma in situ. Filtered 99mtechnetium-labelled
sulphur colloid tracer was injected into the tumour at 1-to-2 o’clock
position 6 cm from the nipple under sonographic guidance (not
shown). A sentinel lymph node was seen cranial to the injected
activity in the left axilla, which is marked as ‘O’ in (a) and ‘X’ in (c),
respectively.
Non-radioguided Localisation
These new techniques involve markers that are approved
for long-term implantation.[7] This allows decoupling of
the day of marker placement and the day of surgery,
leading to more flexibility for the involved departments
and the patients.[1] [2] [3] [4] [5] [7] [8] Without the need for same-day
localisation in the radiology department, the patient can
undergo operation as the first case of the schedule. These
localisation systems do not involve any radioactivity.
Some of them are licensed to localise lymph nodes for
targeted axillary dissection as well.[4]
Although multiple markers can be used in the same
breast, they should be at least 2 cm apart in order to be
detected separately.[4] Placing two devices in the same
location of the breast with one in anterior depth and one
in posterior depth is not recommended as the signal from
the superimposed devices may be mistaken as one single
source in the supine patient intraoperatively.[4] [5] The
marker cannot be repositioned once deployed and has a
depth limit for its detection.[1] [2] [4] [5] [8]
Radar Reflectors
Radar localisation was the first US Food and Drug
Administration (FDA)–approved (2014) non-radioguided
wireless system.[8] It uses a radar reflector
12 mm in length, including a body with an infrared
receptor and two nitinol antennae on either side,
deployed with a 16-gauge delivery needle.[1] [2] [3] [4] A new
mini 8-mm–long design became available recently.[12]
The console sends micro-impulse radar signals to the
handpiece probe, which emits infrared light to activate
the reflector.[4] [8] The reflector reflects the radar signal back
to the handpiece, which is interpreted and presented by
the console as audible and numerical information about
the reflector proximity, with an accuracy of ± 1 mm.[4] [8]
This method can localise lesions up to 6 cm deep.[1] [4]
The marker is placed under sonographic or
mammographic guidance and can be used to localise
lesions in the breast, lymph nodes, or soft tissue.[3] It
achieves 85% to 93% surgical margin clearance.[4] Its distinctive feature of minimal susceptibility artefact
may be useful in patients that require MRI to monitor
response to neoadjuvant chemotherapy (Figure 5).[2]
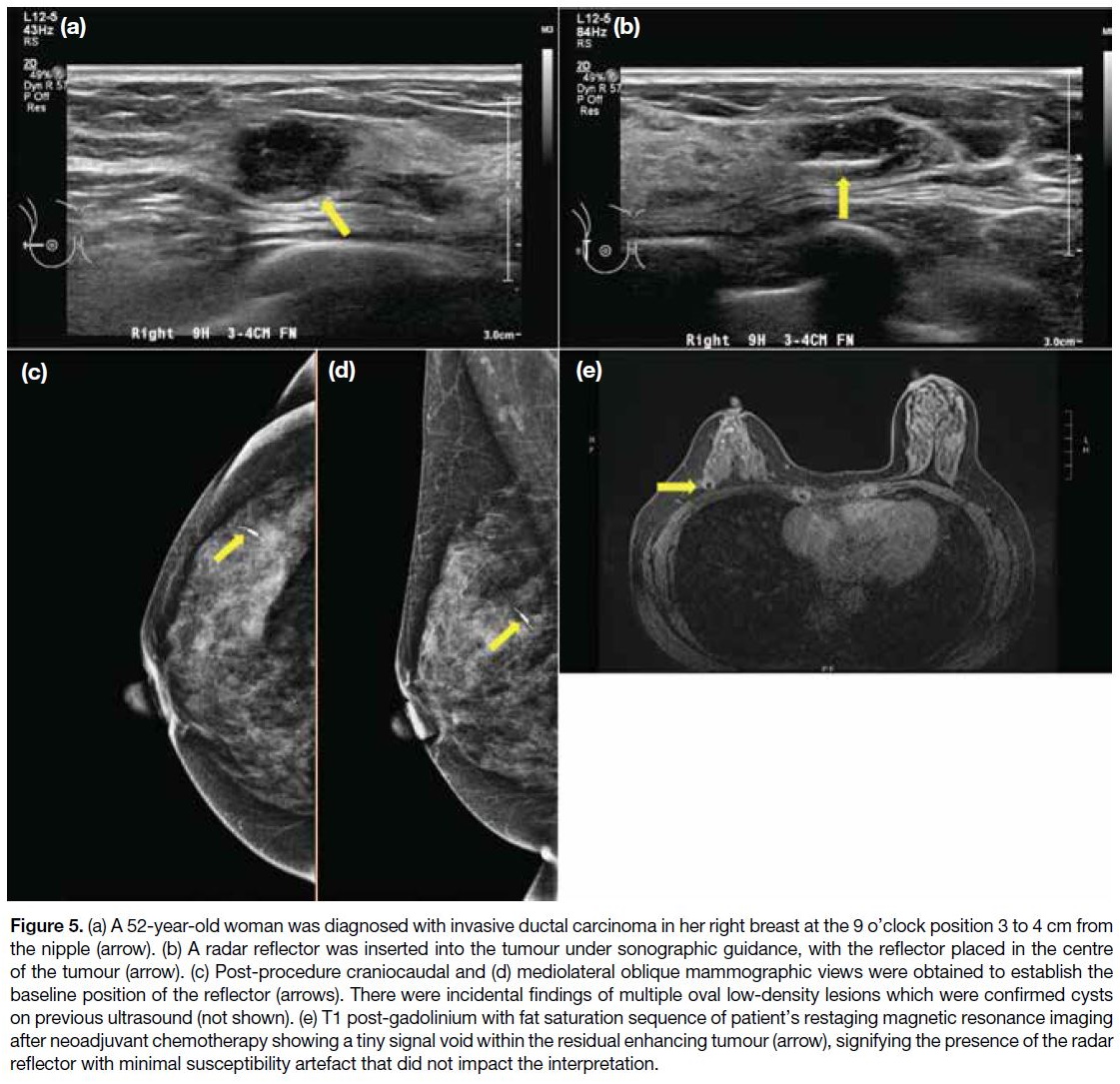
Figure 5. (a) A 52-year-old woman was diagnosed with invasive ductal carcinoma in her right breast at the 9 o’clock position 3 to 4 cm
from the nipple (arrow). (b) A radar reflector was inserted into the tumour under sonographic guidance, with the reflector placed in the
centre of the tumour (arrow). (c) Post-procedure craniocaudal and (d) mediolateral oblique mammographic views were obtained to establish
the baseline position of the reflector (arrows). There were incidental findings of multiple oval low-density lesions which were confirmed cysts on
previous ultrasound (not shown). (e) T1 post-gadolinium with fat saturation sequence of patient’s restaging magnetic resonance imaging
after neoadjuvant chemotherapy showing a tiny signal void within the residual enhancing tumour (arrow), signifying the presence of the radar
reflector with minimal susceptibility artefact that did not impact the interpretation.
Since the antennae are made of nitinol, there is risk
of nickel allergy.[1] [4] Dampened signal can occur when
dense objects such as haematomas, wires, or calcified
masses are located between the reflector and the
probe.[4] Halogen lights in the operating theatre must be
shielded or directed away from the surgical site.[4] Marker
deactivation may occur through direct contact with
electrocautery or antenna transection during dissection.[3] [8] The micro-impulse radar signal may interfere with
cardiac implants, hence caution is warranted in patients
with such devices.[13]
Magnetic Seeds
Approved by the FDA in 2016,[1] the magnetic seed
system uses a 5 × 1 mm2 stainless steel seed, which is
retained in an 18-gauge sterile introducer needle with
a terminal plug.[1] [4] The probe transiently magnetises
the seed and detects its magnetic field for real-time
localisation.[1] [4] The seed has a depth detection range up to 3 to 4 cm from the skin.[1] [4] [5]
The seeds are approved to be deployed into the breast and axilla under sonographic or mammographic guidance
(Figure 6).[3] The reported margin clearance rate is 78.1%
to 83%.[4] The same console and probe can be used for
sentinel lymph node mapping using a superparamagnetic
iron oxide tracer, resulting in an entirely magnetic
technique.[4] The magnetic seeds are made of low-nickel
stainless steel, reducing concern for nickel allergy.[1]
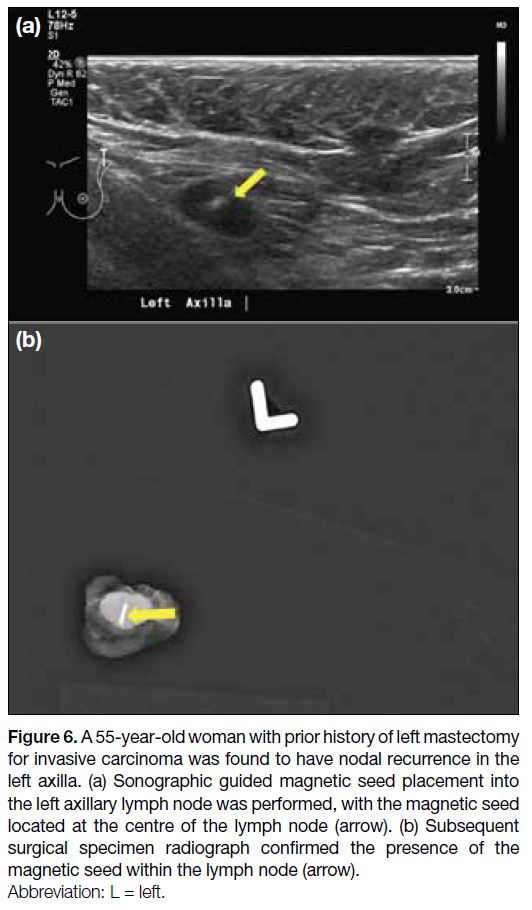
Figure 6. A 55-year-old woman with prior history of left mastectomy
for invasive carcinoma was found to have nodal recurrence in the
left axilla. (a) Sonographic guided magnetic seed placement into
the left axillary lymph node was performed, with the magnetic seed
located at the centre of the lymph node (arrow). (b) Subsequent
surgical specimen radiograph confirmed the presence of the
magnetic seed within the lymph node (arrow).
These seeds are contraindicated in patients with
pacemakers or implanted chest wall devices.[4] The
minimally absorbable terminal plug is made of beeswax,
which may cause allergic or foreign body reaction.[4]
Non-ferromagnetic surgical instruments need to be used
to avoid interference, incurring an additional cost.[4] [5] The
magnetic seed casts a 4-to-6–cm susceptibility artefact
on MRI, thus is not preferred in patients who require
subsequent breast MRI.[1] [2] [4] [5]
Radiofrequency Identification Tags
This device received FDA approval in 2017.[3] [4] The
tag consists of a ferrite rod wrapped in copper and
a microprocessor, which are enclosed within an
antimigratory polypropylene cap.[4] It involves the use
of radio waves for signal transmission.[4] The reusable
portable loop reader can be used directly on the skin to
detect tags up to 6 cm deep.[1] [4] The 8-mm single-used
pencil-sized probe can detect tags up to 3 cm deep.[4]
The tag can be inserted under sonographic or
mammographic guidance (Figure 7). Limited high-quality
published data are available for RFID tags given
its shorter history.[3] One study reported 97% margin
clearance.[4] Each tag has a unique identification number
displayed on the reader, allowing easy intraoperative
differentiation of multiple tags in the same breast.[1] [2] [4]
The pencil-sized probe allows a smaller incision without
obscuring the surgeon’s visualisation.[4]
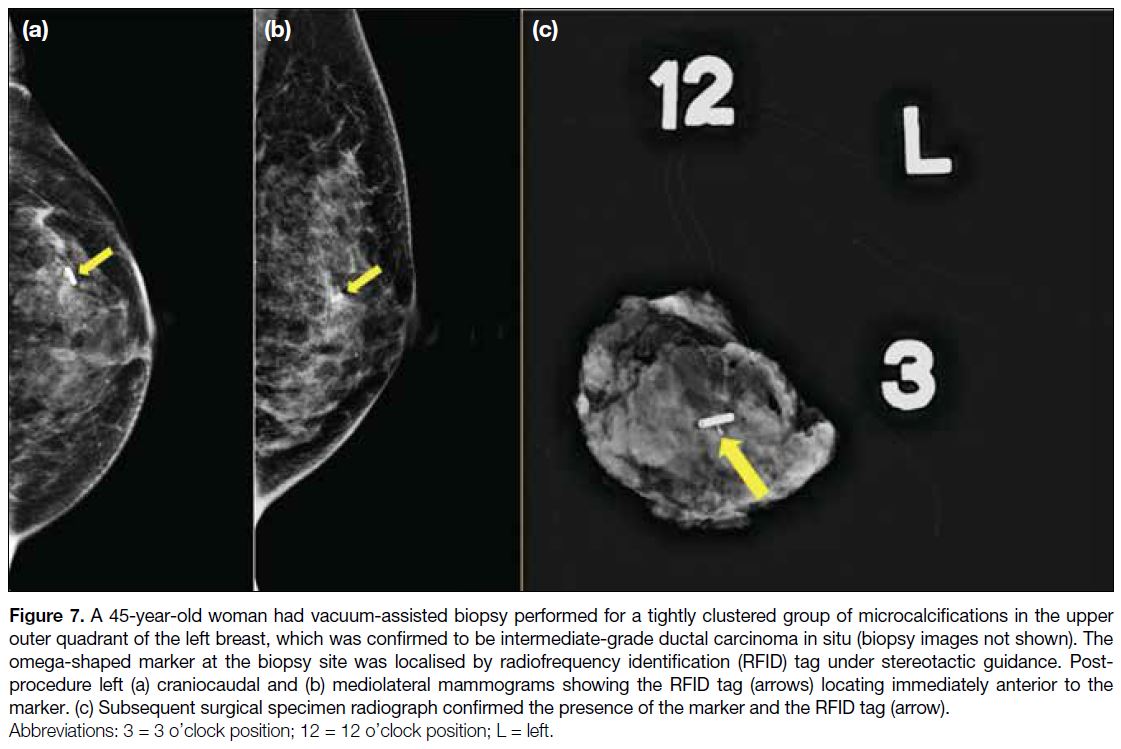
Figure 7. A 45-year-old woman had vacuum-assisted biopsy performed for a tightly clustered group of microcalcifications in the upper
outer quadrant of the left breast, which was confirmed to be intermediate-grade ductal carcinoma in situ (biopsy images not shown). The
omega-shaped marker at the biopsy site was localised by radiofrequency identification (RFID) tag under stereotactic guidance. Post-procedure
left (a) craniocaudal and (b) mediolateral mammograms showing the RFID tag (arrows) locating immediately anterior to the
marker. (c) Subsequent surgical specimen radiograph confirmed the presence of the marker and the RFID tag (arrow).
As the tag is sizable (11 × 2 mm2) and is deployed via a
12-gauge applicator, it may be more prone to migration
during intraoperative manipulation due to a wider
deployment tract.[3] Its size may also pose a challenge
during deployment in hard masses or through dense
breasts.[3] The associated 2-cm MRI artefact may limit
subsequent MRI breast evaluation.[1] Its use in axillary
lymph nodes is off-label currently, and caution should
be taken in patients with defibrillators or pacemakers due
to potential interference by the radiofrequency signal.[3] [4]
The above common techniques for image-guided breast
localisation in Hong Kong are summarised in the online supplementary Table.
LOCAL EXPERIENCE WITH WIRE-FREE
LOCALISATION AND FUTURE RESEARCH DIRECTIONS
ROLL was introduced in Hong Kong in the early 2000s,
but is not available in some centres due to the absence
of a nuclear medicine department. Compared to wire
localisation, it has a shorter procedure time and similar
specimen margin clearance and re-excision rates.[11]
Poorer stereotactic-guided localisation is associated with
targeting invasive carcinomas, possibly related to their
fibrotic and hard texture.[14] Small breasts are also more
susceptible to suboptimal stereotactic-guided ROLL
as a minor discrepancy in z-axis (depth) can lead to a
significant difference after release of breast compression.[11]
This limitation may be more concerning in the Chinese
population due to their tendency to have thin breasts.[15]
The first available non-radioguided wireless device in
Hong Kong was the magnetic seed, which was introduced
in 2019.[16] Its feasibility for schedule decoupling, and
its safety and efficacy in terms of technical success,
complications, margin clearance, and need for re-excision
have been demonstrated.[16] It has been found non-inferior
to ROLL and wire localisation.[17] [18] Its successful use in
papillary lesions and in targeting multiple lesions are
illustrated.[16] [17] [19] Related to the shorter history, there is
only one published article about the initial experience
with radar reflectors in Hong Kong,[13] while there is none
for RFID tags. Radar reflectors are found feasible to be
placed before the day of surgery, and to be safe, with a
high technical success rate.[13] More research is needed to
establish local long-term data.
Although these devices have a depth limitation for
detection, it may not be a significant concern in Chinese
women who tend to have thinner breasts.[13] [15] [16] The
migration risk should theoretically be lower in Asian
women given their higher frequency of dense breasts,
but accordion-effect–related migration of magnetic seeds
and radar reflectors is still observed.[13] [16] [18] Moreover, the large size of a RFID tag may make its placement in a
dense breast technically more difficult.[3] Prospective
studies with larger sample size are needed to validate
these postulations in our population.
The non-radioactive wireless markers are approved for
long-term implantation, therefore assuming a potential
role in replacing ordinary clip markers in highly
suspicious masses at the time of biopsy. Although the
cost is high, this may eliminate the need for another
localisation procedure, which reduces the operational
cost. A full cost analysis is required. Future local
research should also evaluate specimen weight, surgical
cosmesis, and patient’s and operator’s satisfaction.
CONCLUSION
A number of presurgical breast lesion localisation
techniques are currently available in Hong Kong,
each having its own advantages and disadvantages.
The novel devices eliminate some drawbacks of the
traditional methods, but large-scale studies are needed
to provide more evidence in our population given their
epidemiologically different breast characteristics. The decision to choose the best suitable localisation method
should be a joint consensus by radiologists and surgeons
after thorough consideration of each individual patient’s
condition and the features of the target lesion(s).
REFERENCES
1. Cheang E, Ha R, Thornton CM, Mango VL. Innovations in image-guided preoperative breast lesion localization. Br J Radiol. 2018;91:20170740. Crossref
2. Norman C, Lafaurie G, Uhercik M, Kasem A, Sinha P. Novel wire-free
techniques for localization of impalpable breast lesions—a
review of current options. Breast J. 2021;27:141-8. Crossref
3. Banys-Paluchowski M, Kühn T, Masannat Y, Rubio I, de
Boniface J, Ditsch N, et al. Localization techniques for non-palpable
breast lesions: current status, knowledge gaps, and rationale for the
MELODY study (EUBREAST-4/iBRA-NET, NCT 05559411).
Cancers (Basel). 2023;15:1173. Crossref
4. Kapoor MM, Patel MM, Scoggins ME. The wire and beyond:
recent advances in breast imaging preoperative needle localization.
Radiographics. 2019;39:1886-906. Crossref
5. Hayes MK. Update on preoperative breast localization. Radiol Clin
North Am. 2017;55:591-603. Crossref
6. Fusco R, Petrillo A, Catalano O, Sansone M, Granata V, Filice S,
et al. Procedures for location of non-palpable breast lesions: a
systematic review for the radiologist. Breast Cancer. 2014;21:522-31. Crossref
7. Guirguis MS, Adrada BE, Scoggins ME, Moseley TW,
Moseley TW, Le-Petross HC, et al. The challenging image-guided
preoperative breast localization: a modality-based approach. AJR
Am J Roentgenol. 2022;218:423-34. Crossref
8. Jeffries DO, Dossett LA, Jorns JM. Localization for breast surgery:
the next generation. Arch Pathol Lab Med. 2017;141:1324-9. Crossref
9. Au AK, Wan AY, Leung BS, Lo SS, Wong WW, Khoo JL. Efficacy
of radioguided occult lesion localisation: how well are we doing?
Hong Kong J Radiol. 2016;19:269-78. Crossref
10. Aydogan F, Ozben V, Yilmaz MH, Celik V, Uras C, Ferahman M,
et al. Simultaneous excision of ipsilateral nonpalpable multiple
breast lesions using radioguided occult lesion localization. Breast.
2011;20:241-5. Crossref
11. Chu TY, Lui CY, Hung WK, Kei SK, Choi CL, Lam HS.
Localisation of occult breast lesion: a comparative analysis
of hookwire and radioguided procedures. Hong Kong Med J.
2010;16:367-72.
12. Merit Medical. SCOUT Mini Reflector Datasheet. Available from:
https://www.merit.com/merit-oncology/localization/breast-soft-tissue-localization/scout-radar-localization/scout-mini-reflector/. Accessed 7 Jan 2024.
13. Woo SC, Wong T, Chau CM, Fung WY, Chan RL, Yung AW, et al.
Radar localisation of non-palpable breast lesions in a Chinese
population: a pilot study. Hong Kong J Radiol. 2022;25:192-203. Crossref
14. Wong WL, Wong LK, Fung EP, Kwok KM, Mak WS, Lam HS.
Factors affecting accuracy of stereotactic radioisotope-guided
occult lesion localisation for breast lesions. Hong Kong J Radiol.
2021;24:279-86. Crossref
15. Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30:959-65. Crossref
16. Fung WY, Wong T, Chau CM, Yu EL, Chan TS, Chan RL, et al.
Safety and efficacy of magnetic seed localisation of non-palpable
breast lesions: pilot study in a Chinese population. Hong Kong
Med J. 2020;26:500-9. Crossref
17. Tsui HL, Fung EP, Kwok KM, Wong LK, Lo LW, Mak WS. Magnetic marker wireless localisation versus radioguided
localisation of nonpalpable breast lesions. Hong Kong J Radiol.
2021;24:247-56. Crossref
18. Yang S, Wong YT, Leong PW, Li AO, Kwok KY, Chow DL, et al. Image-guided localisation of nonpalpable breast lesions: a comparative analysis of magnetic seeds and hookwires in an Asian population. Hong Kong J Radiol. 2022;25:204-13. Crossref
19. Law MK, Ma LW, Lai AY, Chau PL, Au AK, Ling YH, et al. Magseed localisation of non-palpable papillary lesions: a pictorial essay. Hong Kong J Radiol. 2022;25:156-62. Crossref