Magnetic Resonance Imaging Findings of Cardiac Metastases: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024 Sep;27(3):e192-201 | Epub 9 September 2024
Magnetic Resonance Imaging Findings of Cardiac Metastases: A Pictorial Essay
Eda Cingoz1, Rana Gunoz Comert2, Mehmet Cingoz3, Memduh Dursun2
1 Department of Radiology, Istanbul Bagcilar Training and Research Hospital, Istanbul, Turkey
2 Department of Radiology, Istanbul University, Istanbul, Turkey
3 Department of Radiology, Basaksehir Cam and Sakura City Hospital, Istanbul, Turkey
Correspondence: Dr E Cingoz, Department of Radiology, Istanbul Bagcilar Training and Research Hospital, Istanbul, Turkey. Email: edacanipek@gmail.com
Submitted: 31 July 2023; Accepted: 19 October 2023.
Contributors: EC and MD designed the study. RGC acquired the data. MC analysed the data. EC, RGC and MC drafted the manuscript. MD
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Istanbul Medical Faculty Clinical Research Ethics Committee, Turkey (Ref No.: 2022/709).
The requirement for informed patient consent was waived by the Committee due to the retrospective design of the study. The data used in the
study were de-identified.
INTRODUCTION
Tumours metastatic to the heart may involve the
pericardium, epicardium, myocardium and/or
endocardium.[1] [2] These metastases are approximately
30 times more common than primary cardiac tumours.[3]
Cardiac metastases occur with an incidence of 1.5% to
20% according to postmortem statistics.[4]
Echocardiographic data have suggested an increase
in the incidence of cardiac metastases over the past 30
years due to increased life expectancy in patients with a
known malignancy who have benefitted from progress in
cancer treatment.[5]
Over 90% of cardiac metastases remain clinically
silent, explaining the lack of antemortem diagnosis.[2]
Tumours that most commonly involve the heart include
lung cancer, breast cancer, melanoma, and lymphoma,
reflecting the relatively high prevalence of these
malignancies in the population.[6] In all, 36% to 39% of
cardiac metastases originate from primary lung cancer, followed by 10% to 12% from breast cancer and 10%
to 21% from haematological malignancies.[1] [4] Tumours
such as melanoma have a much higher propensity (nearly
50%) to involve the heart.[1] [7] Following melanoma, the
tumours that tend to metastasise to the heart include
ovarian, gastric, renal, and pancreatic carcinomas.[1] [4]
Cardiac metastases may manifest a variety of appearances.
A mass stemming from the lung or mediastinum can
directly invade the heart. Additionally, tumour cells that
reach the heart via the pulmonary veins (haematogenous
spread) can manifest as a central mass.[8] Metastases may
present as pericardial effusion and nodularity, as well as
myocardial nodules.
Echocardiography is the most frequently used initial
modality for the diagnosis of any cardiac mass, though
there are some limitations regarding its diagnostic
capabilities. First, it is difficult to differentiate a
cardiac thrombus from an endocardial mass with echocardiography unless it is performed with contrast
imaging.[9] Moreover, metastases to the heart with
extracardiac extension cannot be evaluated solely by
using echocardiography. Cardiac magnetic resonance
imaging (MRI) provides a more comprehensive
anatomical evaluation by demonstrating the entire
thoracic cavity and serves as an excellent diagnostic tool
in patients with suspected cardiac metastases. The use of
contrast medium adds extra value to cardiac MRI images
since it may allow the distinction between a mass that
shows contrast enhancement versus a non-enhancing
thrombus. Cardiac MRI offers excellent soft tissue
contrast resolution, allowing the clinician to distinguish
between metastatic lesions and myocardial tissue. The
differentiation between benign and malignant tumour,
thrombus and blood may be provided by MRI with
relative ease.
This pictorial essay presents our experience and highlight
the diverse appearances of cardiac involvement by metastases.
CARDIOVASCULAR MAGNETIC RESONANCE PROTOCOL
A total of 1119 consecutive cardiac MRI studies that
were carried out at our institution from January 2015 to
March 2022 were reviewed and 22 cases of metastases
involving the heart were detected. These 22 patients
were aged 14 to 98 years, and their demographics as well
as features and locations of the lesions were recorded.
Cardiac MRI studies were performed using a 1.5T scanner
(Aera; Siemens, Erlangen, Germany) with phased
array coil systems. The protocol was the standardised
protocol as previously described by the Society for
Cardiovascular Magnetic Resonance,[10] which includes
steady-state free precession cine imaging, bright-blood
and dark-blood single-shot imaging, T1-weighted and
T2-weighted fast spin-echo imaging, and early and late
perfusion imaging during and after the administration of
contrast medium.[10] [11] [12]
IMAGING FINDINGS
All cardiac MRI images were evaluated by a radiologist
specialising in cardiac imaging with experience of
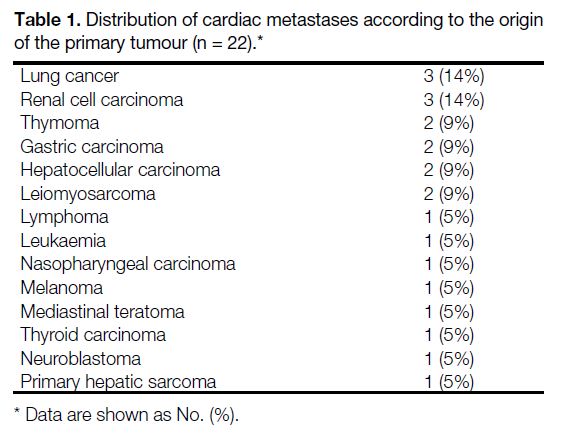
>20 years. Table 1 shows the origins of the primary
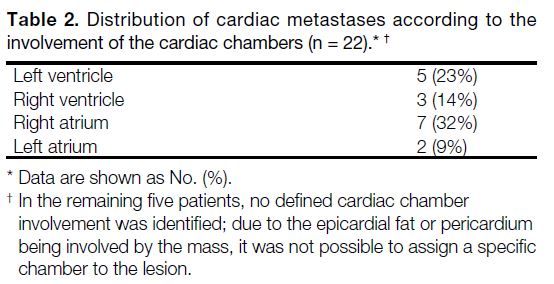
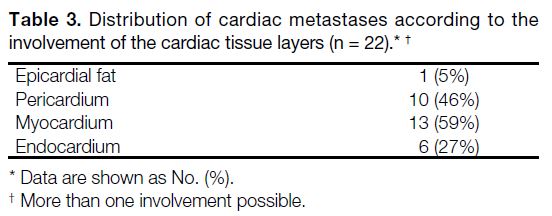
tumours, while Tables 2 and 3 show the sites of cardiac
involvement. Half of the patients were male, suggesting
an absence of gender predilection. The mean age of the
patients was 58.5 years, with a standard deviation of 21.1
years.
Table 1. Distribution of cardiac metastases according to the origin
of the primary tumour (n = 22).
Table 2. Distribution of cardiac metastases according to the
involvement of the cardiac chambers (n = 22).
Table 3. Distribution of cardiac metastases according to the
involvement of the cardiac tissue layers (n = 22).
Lymphatic Spread
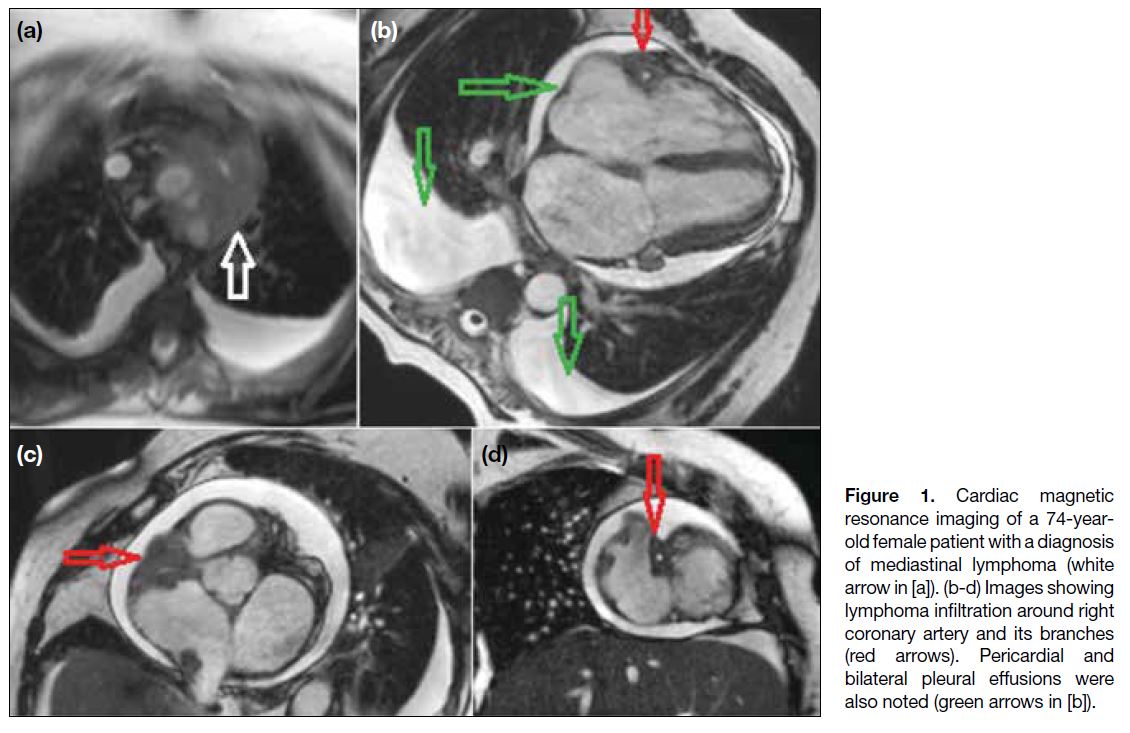
Figure 1 depicts metastatic involvement of pericardial
fat surrounding the right coronary artery from a
mediastinal lymphoma. Lymphatic drainage of the
pericardial space is by lymphatic channels located in
the pericardium that converge at the root of the aorta,
where these channels are most often obstructed, giving
rise to pericardial effusion.[8] Figure 2 shows an example
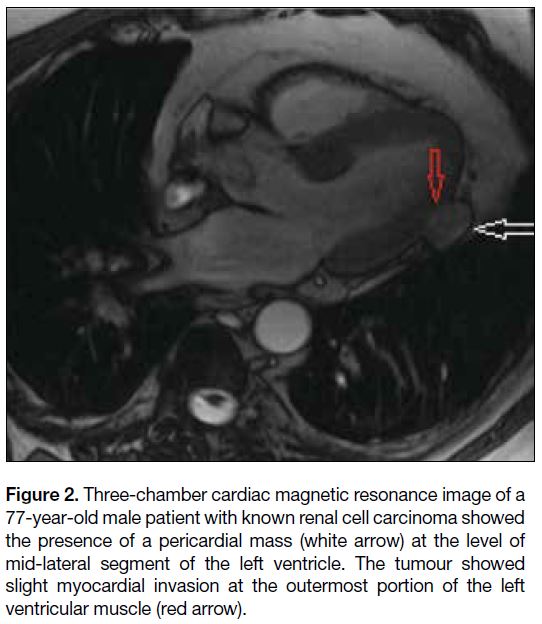
of pericardial metastasis from renal cell carcinoma.
Metastatic involvement of the pericardium gives rise
to pericarditis initially, followed by haemorrhagic
effusion.[13] The development of symptoms during the
progression of pericardial effusion depends on the rate
of accumulation of fluid. Although the accumulation
of large amounts of fluid over time may not cause
symptoms, rapid accumulation of small amounts of fluid may cause serious symptoms.[13] In addition to pericardial
effusion, deposits of malignant cells on the pericardium
may also result in constrictive pericarditis, leading to the
deterioration of heart function.[2]
Figure 1. Cardiac magnetic
resonance imaging of a 74-year-old female patient with a diagnosis of mediastinal lymphoma (white arrow in [a]). (b-d) Images showing lymphoma infiltration around right coronary artery and its branches (red arrows). Pericardial and bilateral pleural effusions were also noted (green arrows in [b]).
Figure 2. Three-chamber cardiac magnetic resonance image of a
77-year-old male patient with known renal cell carcinoma showed
the presence of a pericardial mass (white arrow) at the level of
mid-lateral segment of the left ventricle. The tumour showed
slight myocardial invasion at the outermost portion of the left
ventricular muscle (red arrow).
Haematogenous Spread
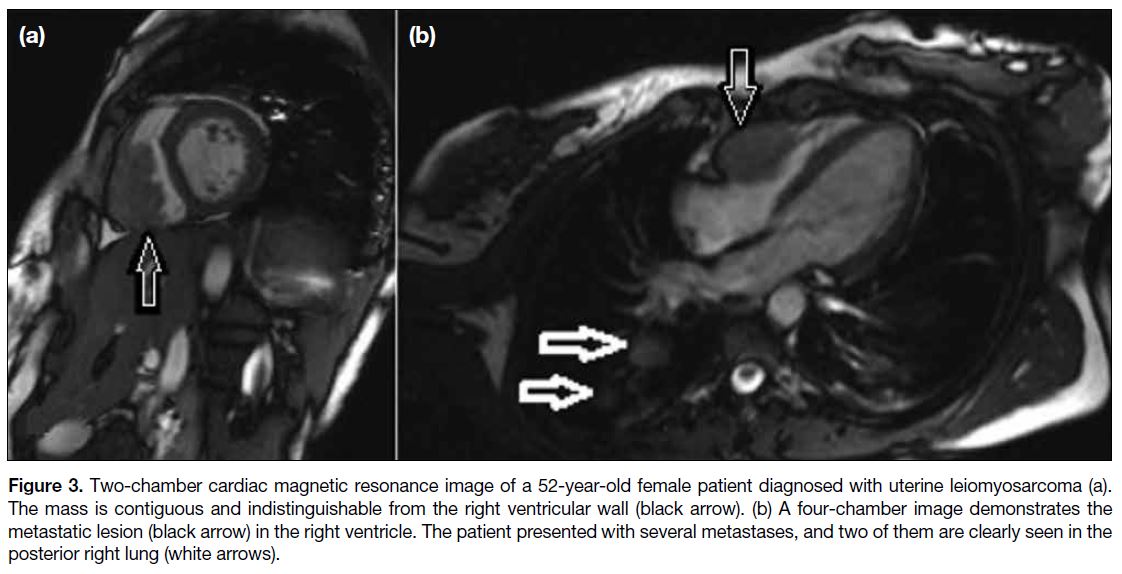
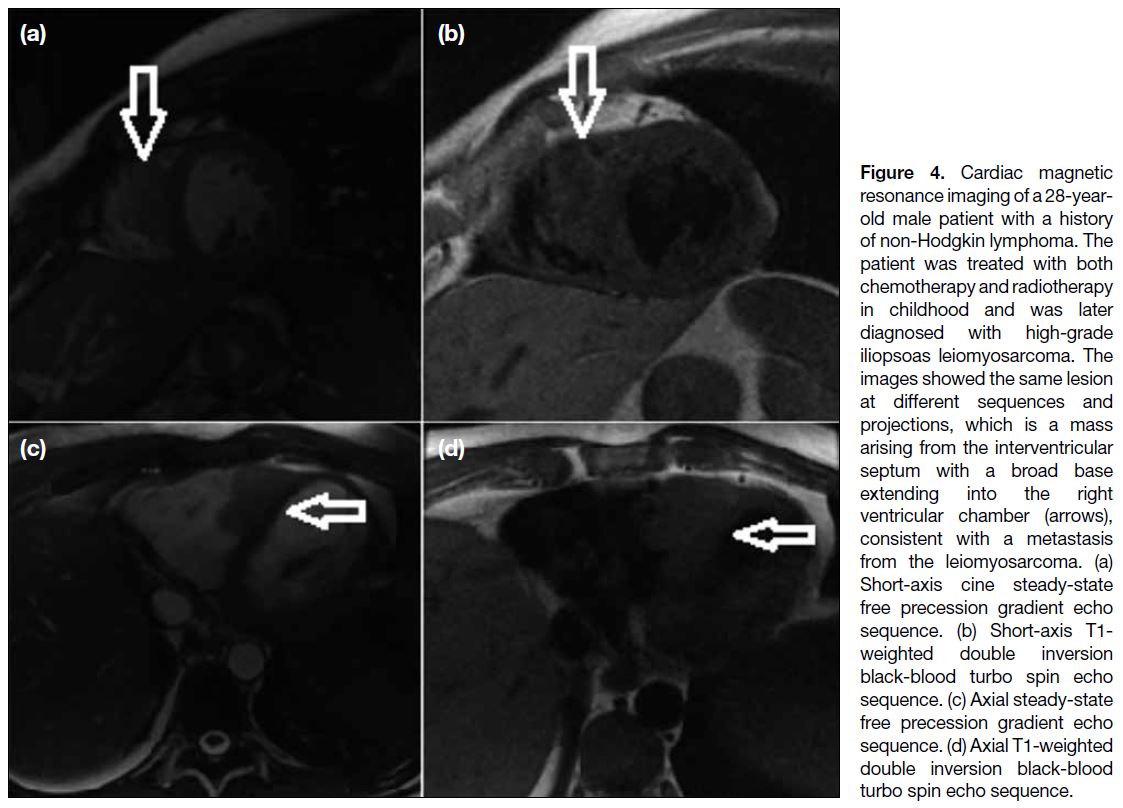
Figures 3 and 4 demonstrate the myocardial metastasis
of a uterine leiomyosarcoma and an iliopsoas muscle
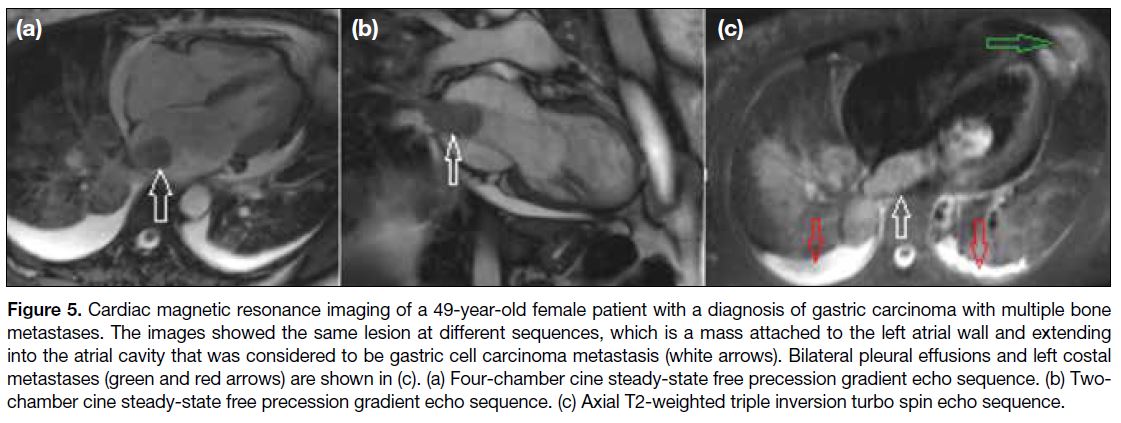
sarcoma, respectively. Figures 5 and 6 depict the
metastasis of gastric carcinoma to different chambers of
the heart. Figure 7 shows the myocardial involvement
of leukaemia that diffusely involved the left ventricular
myocardium. Figure 8 depicts a nasopharyngeal
carcinoma metastasis that caused left myocardial
involvement.
Figure 3. Two-chamber cardiac magnetic resonance image of a 52-year-old female patient diagnosed with uterine leiomyosarcoma (a).
The mass is contiguous and indistinguishable from the right ventricular wall (black arrow). (b) A four-chamber image demonstrates the
metastatic lesion (black arrow) in the right ventricle. The patient presented with several metastases, and two of them are clearly seen in the
posterior right lung (white arrows).
Figure 4. Cardiac magnetic
resonance imaging of a 28-year-old
male patient with a history
of non-Hodgkin lymphoma. The
patient was treated with both
chemotherapy and radiotherapy
in childhood and was later
diagnosed with high-grade
iliopsoas leiomyosarcoma. The
images showed the same lesion
at different sequences and
projections, which is a mass
arising from the interventricular
septum with a broad base
extending into the right
ventricular chamber (arrows),
consistent with a metastasis
from the leiomyosarcoma. (a)
Short-axis cine steady-state
free precession gradient echo
sequence. (b) Short-axis T1-weighted double inversion
black-blood turbo spin echo
sequence. (c) Axial steady-state
free precession gradient echo
sequence. (d) Axial T1-weighted
double inversion black-blood
turbo spin echo sequence.
Figure 5. Cardiac magnetic resonance imaging of a 49-year-old female patient with a diagnosis of gastric carcinoma with multiple bone
metastases. The images showed the same lesion at different sequences, which is a mass attached to the left atrial wall and extending
into the atrial cavity that was considered to be gastric cell carcinoma metastasis (white arrows). Bilateral pleural effusions and left costal
metastases (green and red arrows) are shown in (c). (a) Four-chamber cine steady-state free precession gradient echo sequence. (b) Two-chamber
cine steady-state free precession gradient echo sequence. (c) Axial T2-weighted triple inversion turbo spin echo sequence.
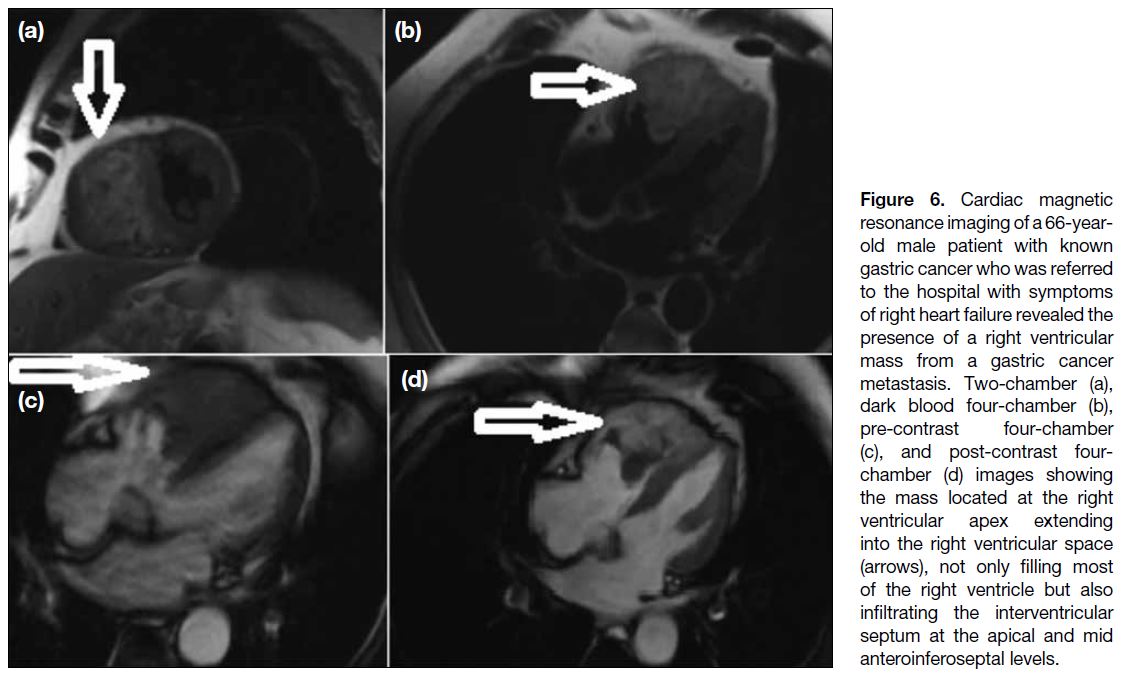
Figure 6. Cardiac magnetic
resonance imaging of a 66-year-old
male patient with known
gastric cancer who was referred
to the hospital with symptoms
of right heart failure revealed the
presence of a right ventricular
mass from a gastric cancer
metastasis. Two-chamber (a),
dark blood four-chamber (b),
pre-contrast four-chamber
(c), and post-contrast four-chamber
(d) images showing
the mass located at the right
ventricular apex extending
into the right ventricular space
(arrows), not only filling most
of the right ventricle but also
infiltrating the interventricular
septum at the apical and mid
anteroinferoseptal levels.
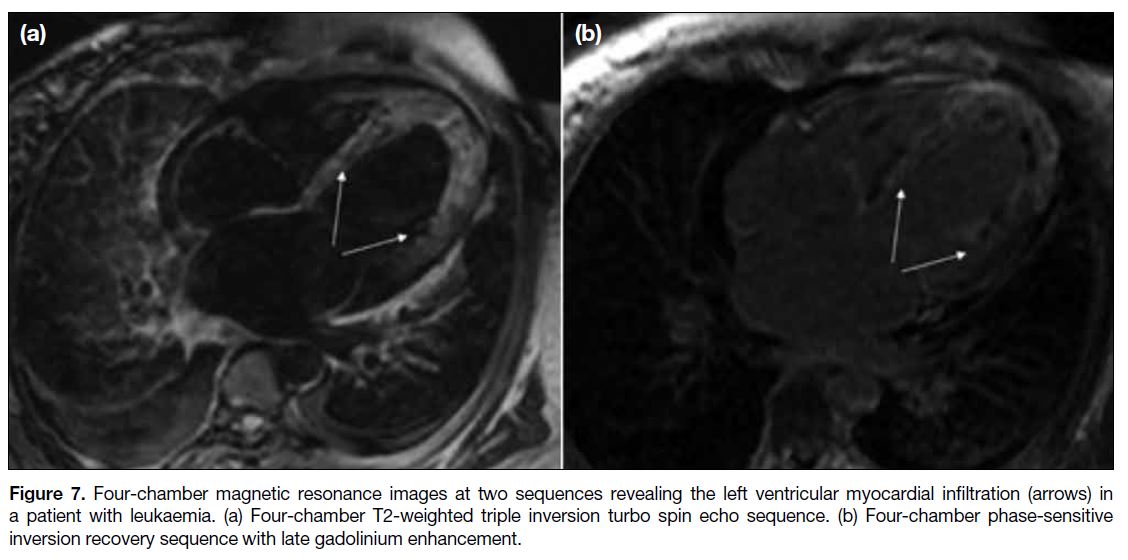
Figure 7. Four-chamber magnetic resonance images at two sequences revealing the left ventricular myocardial infiltration (arrows) in
a patient with leukaemia. (a) Four-chamber T2-weighted triple inversion turbo spin echo sequence. (b) Four-chamber phase-sensitive
inversion recovery sequence with late gadolinium enhancement.
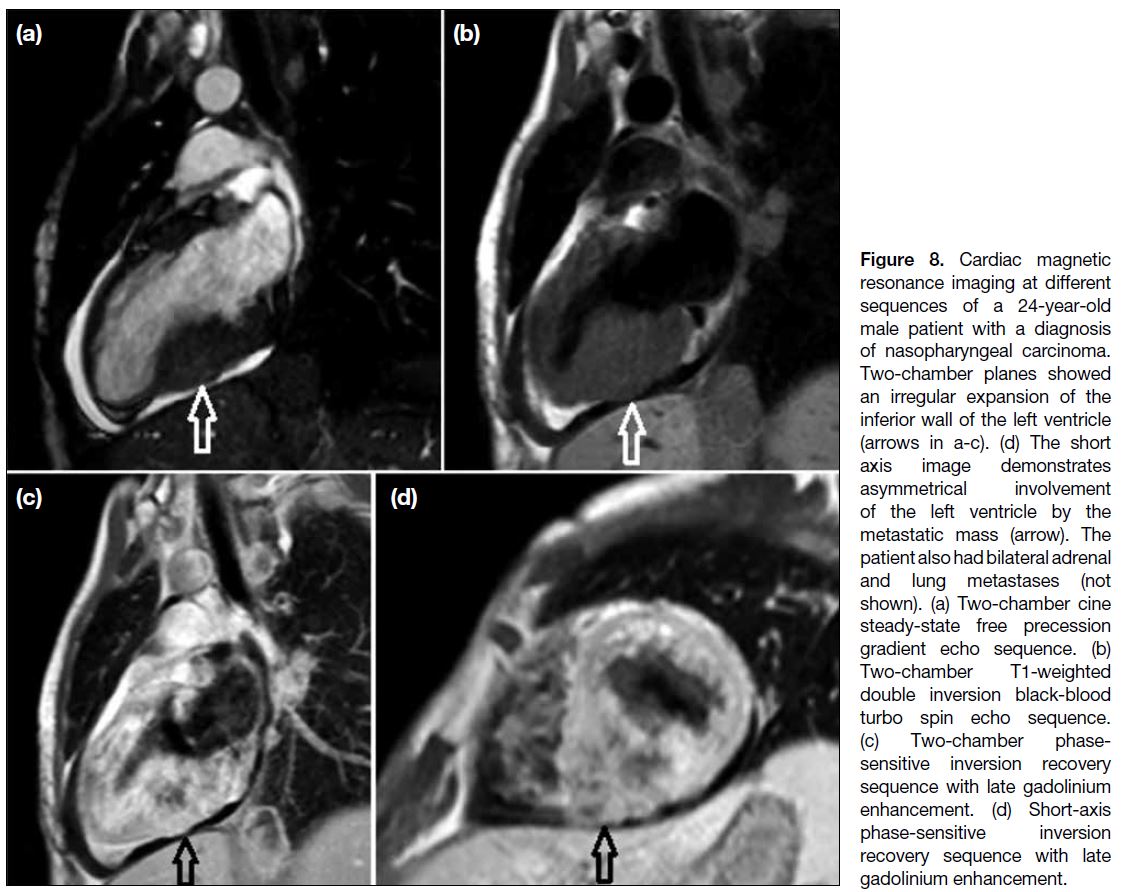
Figure 8. Cardiac magnetic
resonance imaging at different
sequences of a 24-year-old
male patient with a diagnosis
of nasopharyngeal carcinoma.
Two-chamber planes showed
an irregular expansion of the
inferior wall of the left ventricle
(arrows in a-c). (d) The short
axis image demonstrates
asymmetrical involvement
of the left ventricle by the
metastatic mass (arrow). The
patient also had bilateral adrenal
and lung metastases (not
shown). (a) Two-chamber cine
steady-state free precession
gradient echo sequence. (b)
Two-chamber T1-weighted
double inversion black-blood
turbo spin echo sequence.
(c) Two-chamber phase-sensitive
inversion recovery
sequence with late gadolinium
enhancement. (d) Short-axis
phase-sensitive inversion
recovery sequence with late
gadolinium enhancement.
Local Extension
Locally aggressive tumours can directly extend into the
pericardium and cause frank invasion.[2] This typically
occurs in patients with massive lung carcinomas;
however, oesophageal carcinomas and mediastinal lymphomas may also directly invade the heart due to
anatomical proximity.[9] Figures 9 and 10 show a central
primary lung carcinoma invading the pericardium and
myocardium. A large neuroblastoma in the thoracic
cavity invading the heart is shown in Figure 11. Similarly,
a mediastinal teratoma involving the pericardium is
shown in Figure 12. Large masses occurring in organs
close to the heart may involve the heart via anatomical
proximity as shown in Figure 13.
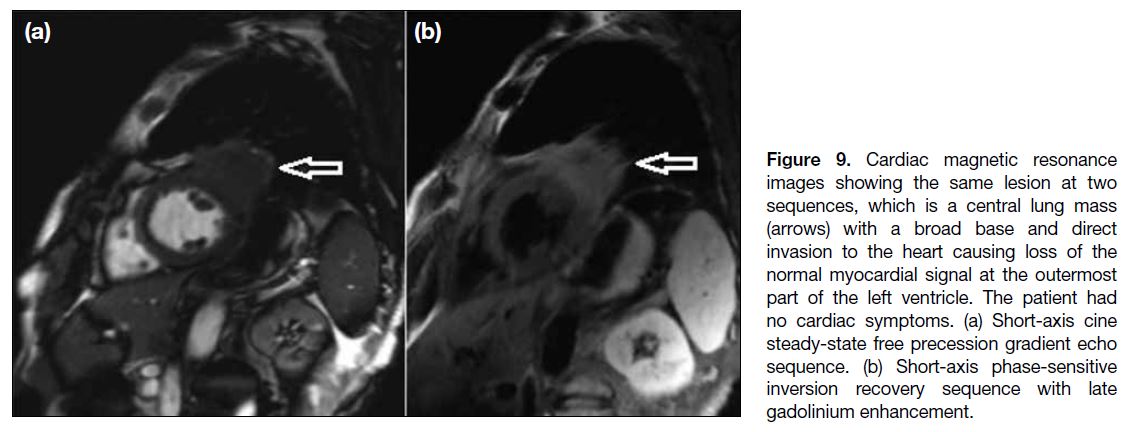
Figure 9. Cardiac magnetic resonance
images showing the same lesion at two
sequences, which is a central lung mass
(arrows) with a broad base and direct
invasion to the heart causing loss of the
normal myocardial signal at the outermost
part of the left ventricle. The patient had
no cardiac symptoms. (a) Short-axis cine
steady-state free precession gradient echo
sequence. (b) Short-axis phase-sensitive
inversion recovery sequence with late
gadolinium enhancement.
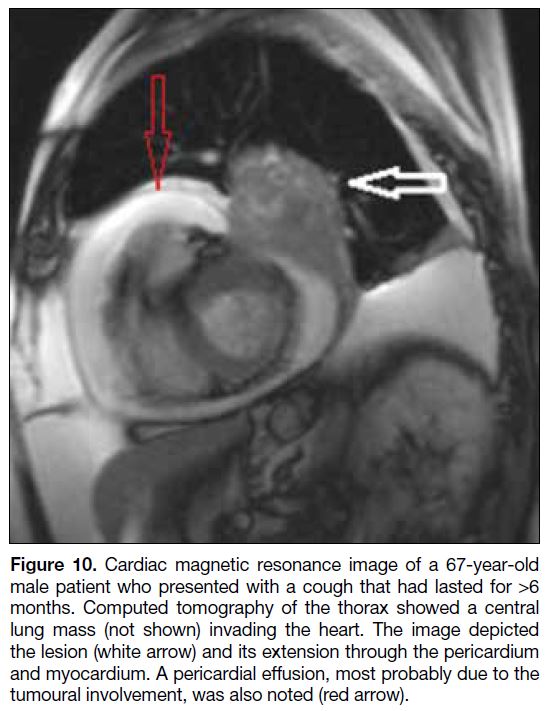
Figure 10. Cardiac magnetic resonance image of a 67-year-old
male patient who presented with a cough that had lasted for >6
months. Computed tomography of the thorax showed a central
lung mass (not shown) invading the heart. The image depicted
the lesion (white arrow) and its extension through the pericardium
and myocardium. A pericardial effusion, most probably due to the
tumoural involvement, was also noted (red arrow).
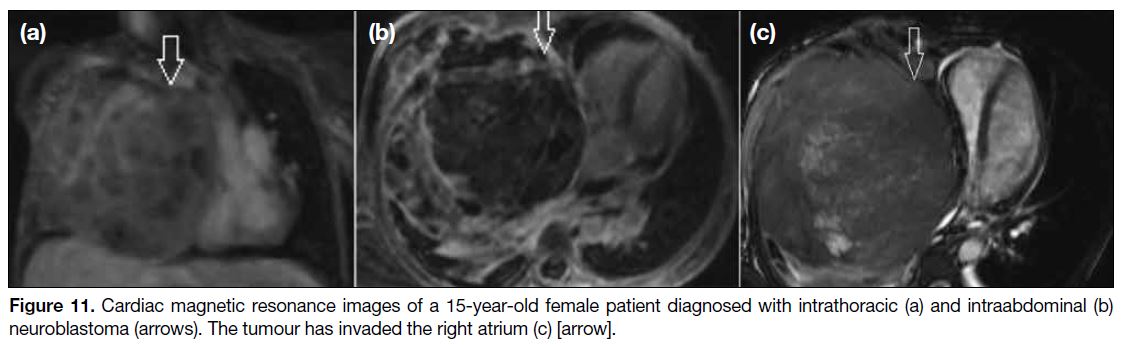
Figure 11. Cardiac magnetic resonance images of a 15-year-old female patient diagnosed with intrathoracic (a) and intraabdominal (b)
neuroblastoma (arrows). The tumour has invaded the right atrium (c) [arrow].
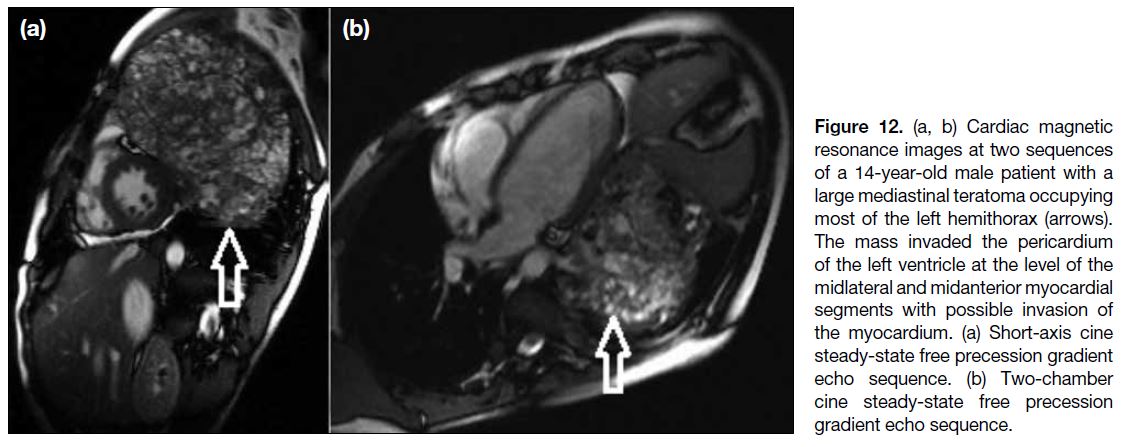
Figure 12. (a, b) Cardiac magnetic
resonance images at two sequences
of a 14-year-old male patient with a
large mediastinal teratoma occupying
most of the left hemithorax (arrows).
The mass invaded the pericardium
of the left ventricle at the level of the
midlateral and midanterior myocardial
segments with possible invasion of
the myocardium. (a) Short-axis cine
steady-state free precession gradient
echo sequence. (b) Two-chamber
cine steady-state free precession
gradient echo sequence.
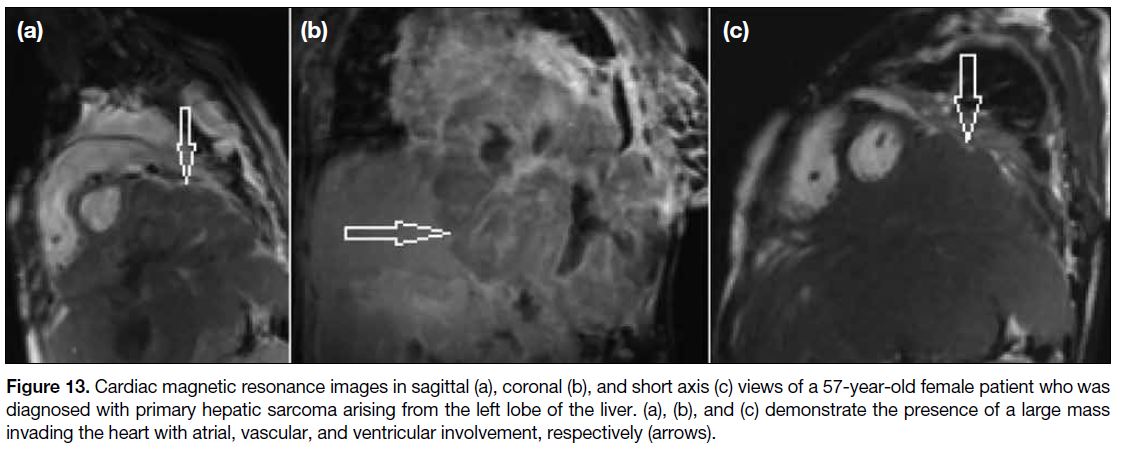
Figure 13. Cardiac magnetic resonance images in sagittal (a), coronal (b), and short axis (c) views of a 57-year-old female patient who was
diagnosed with primary hepatic sarcoma arising from the left lobe of the liver. (a), (b), and (c) demonstrate the presence of a large mass
invading the heart with atrial, vascular, and ventricular involvement, respectively (arrows).
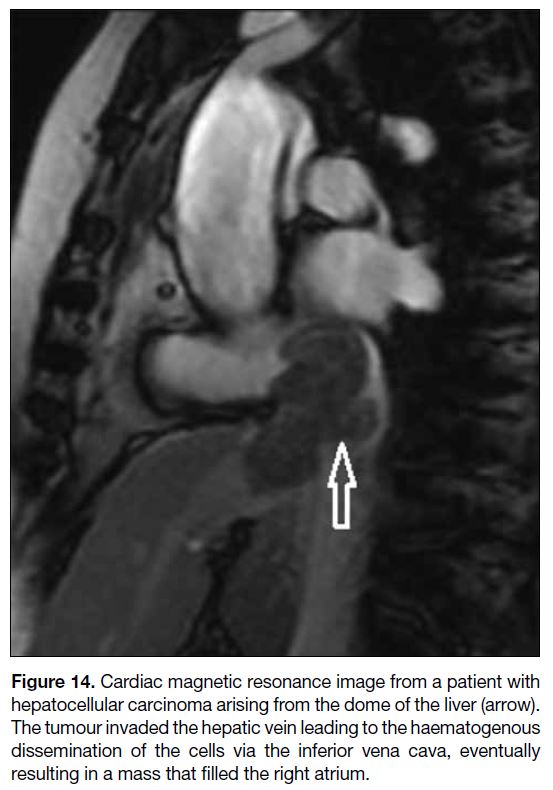
Some tumours, including renal cell carcinoma and
hepatocellular carcinoma, may extend into the inferior
vena cava (IVC), allowing for growth into the right
atrium via transvenous extension.[2] Figure 14 shows a
hepatocellular carcinoma causing cardiac metastasis via
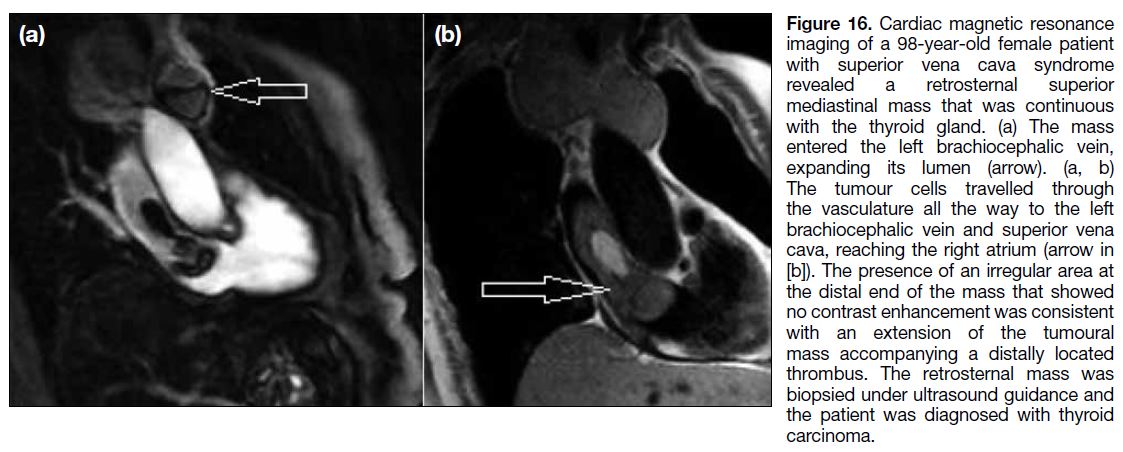
the IVC. The superior vena cava may also serve as a
transportation route for cancer cells to the heart, as seen
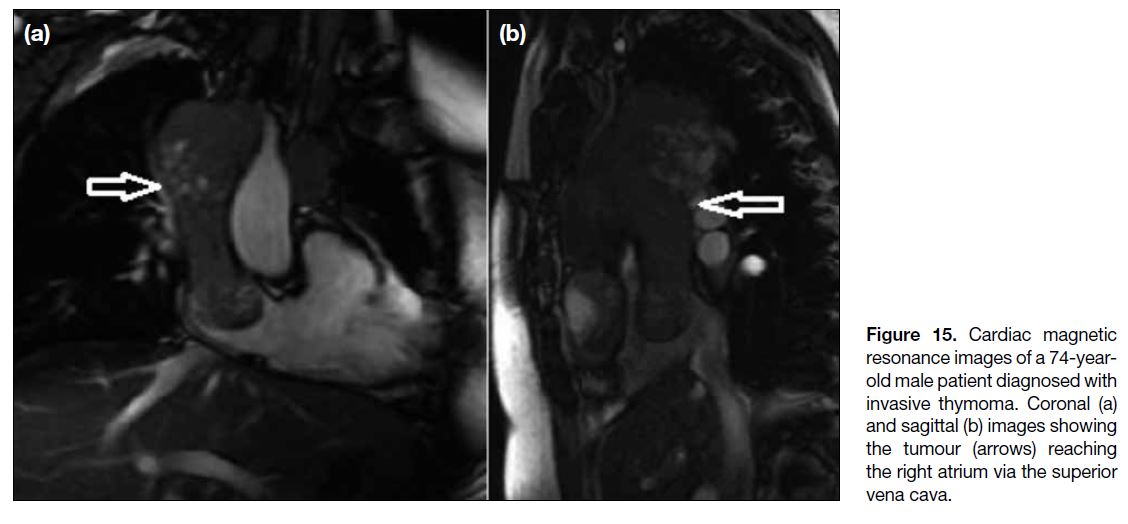
with thoracic and mediastinal tumours.[13] Figures 15 and
16 demonstrate a case of invasive thymoma and a thyroid carcinoma, respectively, in which the malignant tissue
arising from the thymus and thyroid gland reached the
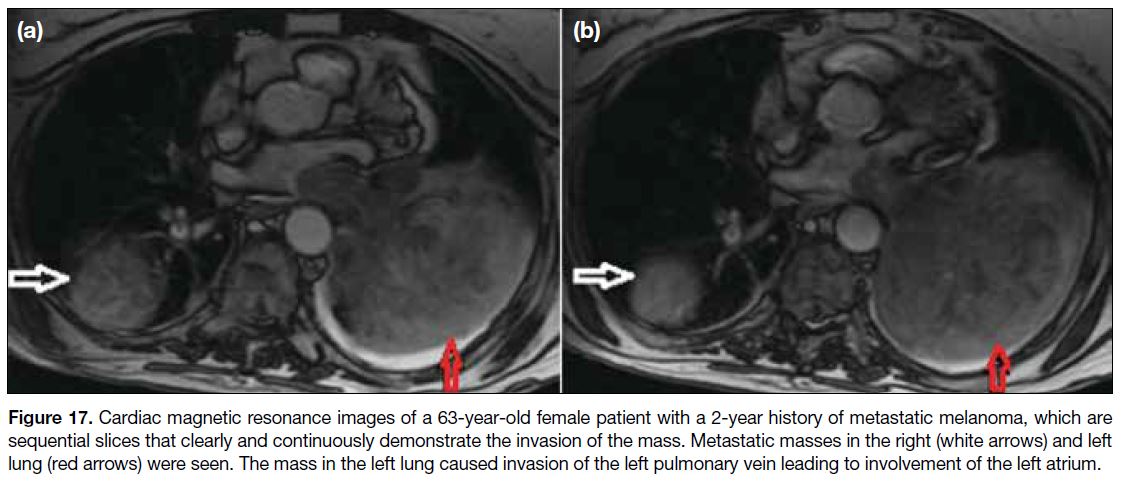
right atrium through the superior vena cava. Figure 17
shows left atrial metastatic involvement of a melanoma
case through the left pulmonary vein enabling the tumour
cells to reach the left atrium from the left lung mass.
Figure 14. Cardiac magnetic resonance image from a patient with
hepatocellular carcinoma arising from the dome of the liver (arrow).
The tumour invaded the hepatic vein leading to the haematogenous
dissemination of the cells via the inferior vena cava, eventually
resulting in a mass that filled the right atrium.
Figure 15. Cardiac magnetic
resonance images of a 74-year-old
male patient diagnosed with
invasive thymoma. Coronal (a)
and sagittal (b) images showing
the tumour (arrows) reaching
the right atrium via the superior
vena cava.
Figure 16. Cardiac magnetic resonance
imaging of a 98-year-old female patient
with superior vena cava syndrome
revealed a retrosternal superior
mediastinal mass that was continuous
with the thyroid gland. (a) The mass
entered the left brachiocephalic vein,
expanding its lumen (arrow). (a, b)
The tumour cells travelled through
the vasculature all the way to the left
brachiocephalic vein and superior vena
cava, reaching the right atrium (arrow in
[b]). The presence of an irregular area at
the distal end of the mass that showed
no contrast enhancement was consistent
with an extension of the tumoural
mass accompanying a distally located
thrombus. The retrosternal mass was
biopsied under ultrasound guidance and
the patient was diagnosed with thyroid
carcinoma.
Figure 17. Cardiac magnetic resonance images of a 63-year-old female patient with a 2-year history of metastatic melanoma, which are
sequential slices that clearly and continuously demonstrate the invasion of the mass. Metastatic masses in the right (white arrows) and left
lung (red arrows) were seen. The mass in the left lung caused invasion of the left pulmonary vein leading to involvement of the left atrium.
DISCUSSION
Metastatic dissemination to the heart from noncardiac
tumours may occur via the lymphatics, or via
haematogenous routes that include both arterial and
transvenous dissemination.[9] While lymphatic spread or
direct invasion targets the pericardium first, myocardial
or endocardial involvement is more common in
haematogenous metastases for anatomical reasons.[1] [2]
Metastatic cardiac tumours have a poor prognosis,
camouflaging themselves until a serious complication
develops. The symptoms are broad and range from mild
chest pain to cardiac rupture leading to sudden death.
Pericardial and myocardial metastases may especially
mimic acute coronary syndrome, and the onset of a
new cardiac symptom in any cancer patient should be approached with the suspicion of cardiac metastases.
Imaging findings of cardiac metastases are diverse.[14]
There was one melanoma patient with left atrial metastasis
in our cohort. It was demonstrated in a recent study of
23 patients with melanoma metastatic to the heart that
although all chambers may be involved, right ventricular
involvement was most common.[15] If hepatocellular
carcinoma metastasises to the heart, the route is usually
extension into the IVC, allowing for growth into the
right atrium via transvenous access as with our cases.[16] [17]
The two cases of uterine leiomyosarcoma in our patient
group showed metastasis to the ventricles. However, the
atria can also be involved.[18] Cardiac metastases from
renal cell carcinoma are not frequently encountered and
they may have varying imaging appearances.[19] [20] [21] A new cardiac symptom in a patient with a known renal cell
carcinoma should alert the clinician to a possible cardiac
metastasis. Although there were no examples in our
patient cohort, malignant neuroendocrine tumours and
benign uterine leiomyomas may also metastasise to the
heart.[22] [23] [24]
CONCLUSION
Cardiac metastases are far more common than previously
thought and should be taken into consideration in
oncology patients presenting with a new cardiac
symptom. The clinical scenario of cardiac metastases
includes a variety of signs and symptoms depending
on the anatomical site of the involvement. Although
echocardiography is the preferred initial diagnostic modality owing to its relatively easy accessibility
and availability, cardiac MRI may also provide a
comprehensive visualisation of both cardiac and
extracardiac involvement.
REFERENCES
1. Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases.
J Clin Pathol. 2007;60:27-34. Crossref
2. Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the
heart. Circulation. 2013;128:1790-4. Crossref
3. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38:261-2.
4. Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. Cardiac metastases. Int J Clin Oncol. 2008;13:369-
72. Crossref
5. Yusuf SW, Bathina JD, Qureshi S, Kaynak HE, Banchs J,
Trent JC, et al. Cardiac tumors in a tertiary care cancer hospital:
clinical features, echocardiographic findings, treatment and
outcomes. Heart Int. 2012;7:e4. Crossref
6. Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the
heart: review of 3314 consecutive autopsies. Am J Cardiovasc
Pathol. 1990;3:195-8.
7. Allen BC, Mohammed TL, Tan CD, Miller DV, Williamson EE,
Kirsch JS. Metastatic melanoma to the heart. Curr Probl Diagn
Radiol. 2012;41:159-64. Crossref
8. Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic
involvement of the heart and pericardium: CT and MR imaging.
Radiographics. 2001;21:439-49. Crossref
9. Mankad R, Herrmann J. Cardiac tumors: echo assessment. Echo
Res Pract. 2016;3:R65-77. Crossref
10. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD,
Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance
imaging (CMR) protocols: 2020 update. J Cardiovasc Magn
Reason. 2020;22:17. Crossref
11. Velangi PS, Choo C, Chen KA, Kazmirczak F, Nijjar PS,
Farzaneh-Far A, et al. Long-term embolic outcomes after detection
of left ventricular thrombus by late gadolinium enhancement
cardiovascular magnetic resonance imaging: a matched cohort
study. Circ Cardiovasc Imaging. 2019;12:e009723. Crossref
12. Hooks M, Okasha O, Velangi PS, Nijjar PS, Farzaneh-Far A,
Shenoy C. Left ventricular thrombus on cardiovascular magnetic
resonance imaging in non-ischaemic cardiomyopathy. Eur Heart
J Cardiovasc Imaging. 2021;22:1425-33. Crossref
13. Lewis MA, Hendrickson AW, Moynihan TJ. Oncologic emergencies: pathophysiology, presentation, diagnosis, and
treatment. CA Cancer J Clin. 2011;61:287-314. Crossref
14. Castro-Martín JJ, Di Silvestre-Alonso MA, Rivero-García M,
Muñoz-Rodríguez R, Izquierdo-Gómez MM, Baeza-Garzón F, et
al. Magnetic resonance imaging in the study of cardiac masses: a
case series. Medicina (Kaunas). 2023;59:705. Crossref
15. Balinski AM, Vasbinder AL, Kerndt CC, Catalan TC, Parry NP,
Rehman RA, et al. Metastatic melanoma of the heart: retrospective
cohort study and systematic review of prevalence, clinical
characteristics, and outcomes. Cancer Med. 2023;12:2356-67. Crossref
16. Amin S, Shahab A, Qazi AR, Yunus H, Saeed L, Khan AA, et al.
A rare incidence of hepatocellular carcinoma with tumor thrombus
extending to the right heart. Cureus. 2023;15:e43965. Crossref
17. Sung AD, Cheng S, Moslehi J, Scully EP, Prior JM, Loscalzo J.
Hepatocellular carcinoma with intracavitary cardiac involvement:
a case report and review of the literature. Am J Cardiol.
2008;102:643-5. Crossref
18. Patel T, Aswal P, Jakhetiya A, Meena V, Pandey A. A novel case
of left atrial and right lung mass turned out to be unconventional metastasis of uterine leiomyosarcoma with a review of literature.
Indian J Pathol Microbiol. 2023;66:601-4. Crossref
19. Ali A, Shah S, Furrukh M. Renal cell carcinoma metastasis to the left atrium. Tex Heart Inst J. 2022;49:e207452. Crossref
20. Li Fraine S, Coman D, Durand M, Laskine M. Renal cell carcinoma
with cardiac metastases. World J Oncol. 2021;12:124-6. Crossref
21. Romejko K, Rytel A, Rozmyslowicz T, Niemczyk S. Heart
metastases of clear cell renal cell carcinoma. Diagnostics (Basel).
2023;13:1600. Crossref
22. Arnfield EG, Tam L, Pattison DA, Younger J, Chikatamarla VA,
Wyld D, et al. Cardiac metastases from neuroendocrine neoplasms:
complementary role of SSTR PET/CT and cardiac MRI. J Nucl
Cardiol. 2023;30:2676-91. Crossref
23. El Ghannudi S, Ouvrard E, Mikail N, Leroy Freschini B,
Schindler TH, Imperiale A. Cutting-edge imaging of cardiac
metastases from neuroendocrine tumors: lesson from a case series.
Diagnostics (Basel). 2022;12:1182 Crossref
24. Li J, Zhu H, Hu SY, Ren SQ, Li XL. Case report: cardiac metastatic
leiomyoma in an Asian female. Front Surg. 2022;9:991558. Crossref