The Different Faces of Osler-Weber-Rendu Syndrome on Radiological Imaging: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024 Sep;27(3):e183-91 | Epub 12 September 2024
The Different Faces of Osler-Weber-Rendu Syndrome on Radiological Imaging: A Pictorial Essay
Rajoo Ramachandran1, Nishita Goyal1, Sheelaa Chinnappan1, Harini Gnanavel1, Jagadeesan Dhanasekaran2, Rajeswaran Rangasami1
1 Department of Radiodiagnosis, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
2 Department of Intervention Radiology, Sri Ramachandra Institute of Higher Education and Research,
Chennai, India
Correspondence: Dr N Goyal, Department of Radiodiagnosis, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Email: drgoyalnishita@gmail.com
Submitted: 4 September 2022; Accepted: 9 May 2023.
Contributors: All authors designed the study and acquired the data. R Ramachandran, NG, SC, HG and JD analysed the data. All authors drafted the manuscript and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Publication Oversight Committee of Sri Ramachandra Institute of Higher Education and Research, India [Ref No.: 11026.docx(D110578897)]. The patients were treated in accordance with the Declaration of Helsinki and informed verbal consent was obtained from the patients.
INTRODUCTION
Osler-Weber-Rendu syndrome (OWRS), also known
as hereditary haemorrhagic telangiectasia (HHT), is
characterised by multiple mucocutaneous telangiectasias
and multiorgan arteriovenous malformations (AVMs)
due to abnormal vascular remodelling traceable to a
genetic defect in a binding protein for transforming
growth factor. The telangiectasias and AVMs have
a propensity to bleed due to their fragile nature.
The multisystemic involvement of OWRS makes it
imperative for clinicians and radiologists to identify
the different clinical manifestations and radiological
features of this syndrome. In this pictorial essay, we give
an overview of the radiological features found in patients
with OWRS and the role of interventional radiology in
management.
RADIOLOGICAL FEATURES OF THE CASES
Three patients with a radiological diagnosis of OWRS were included (Table 1). [1] [2] [3] They underwent contrastenhanced
computed tomography (CECT) and magnetic
resonance imaging (MRI) examinations followed by
vascular interventions at our hospital from February
2019 to September 2020.
Table 1. Curaçao clinical criteria for diagnosis of hereditary
haemorrhagic telangiectasia.[1] [2] [3]
Computed Tomography and Magnetic Resonance Imaging Acquisition
CECT examinations of the abdomen and the pelvis
were acquired using a Revolution EVO Gen 2 128-slice
unit (GE HealthCare, Beijing, China). CT images were
obtained with parameters of 120 kV and current in auto
mode. Axial thin sections were acquired from the dome
of the diaphragm to the symphysis pubis and the data
were processed into multiplanar reconstruction (MPR)
images with 5-mm slice thickness and three-dimensional
images. The reconstruction interval was 0.625 mm.
Approximately 80 mL of non-ionic contrast material
(iohexol 350 mg iodine/mL; GE HealthCare, Milwaukee
[WI], US) was administered with a power injector at a rate of 3.5 mL/s. Image data were acquired 18 to 20 seconds
(arterial phase) and 60 seconds (portal venous phase)
post contrast injection. For CT pulmonary angiography,
60 mL of non-ionic contrast was administrated
intravenously at the rate of 5 mL/s followed by 30 mL
of a normal saline chaser. Axial thin sections acquired
from the sternal notch to the xiphisternum were used to
reconstruct three-dimensional and MPR images.
For MRI of the brain, the sequences were axial T1-weighted, T2-weighted, diffusion-weighted imaging,
sagittal T1-weighted, coronal fast fluid-attenuated
inversion recovery, two-dimensional time-of-flight, and
three-dimensional time-of-flight acquired on a Signa
HDxt 1.5T scanner (GE HealthCare, Milwaukee [WI],
US). The CT and MRI images were sent to a picture
archiving and communication system and interpreted at
workstations. Interventional procedures were performed
using a biplane system (Allura Biplane FD20/20; Philips,
Best, the Netherlands).
Case 1
A 43-year-old female, who had three pregnancies and
three live births previously, presented with abnormal
uterine bleeding for 1 month. On pelvic ultrasound, an
anterior wall uterine fibroid and a right ovarian cyst was
noted. A CT of the abdomen and pelvis showed a right
paraovarian cyst and a heterogeneous lesion within the
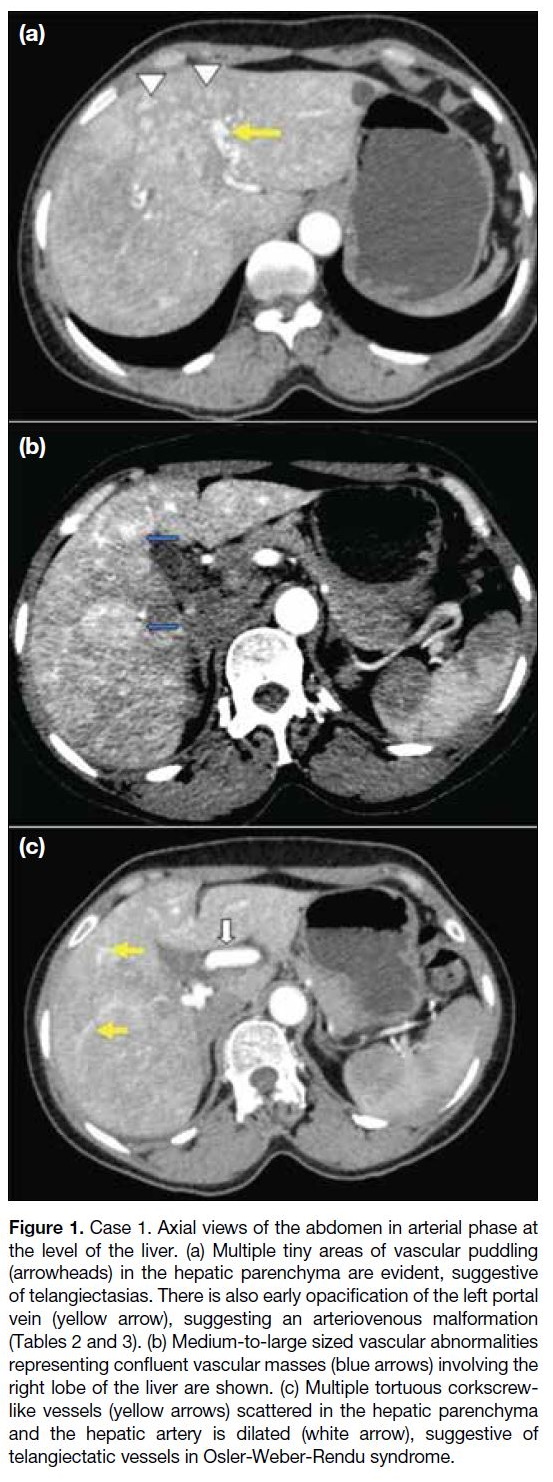
right lobe of the liver. CECT of the abdomen showed
extensive telangiectasias[4] (Figure 1a, 1b, and Tables 2 and 3) and confluent masses[5] [6] within both lobes of the
liver, with the right one larger than the left one. Early
opacification of the left portal vein was noted in the
arterial phase, suggestive of AVMs[5] (arteriovenous
shunts) [Figure 1c]. The extra- and intrahepatic arteries
were dilated, with the common hepatic artery measuring
around 10 mm in diameter.[5] [6] On clinical examination,
telangiectasias were noted in the fingertips and oral
cavity of the patient.
Figure 1. Case 1. Axial views of the abdomen in arterial phase at
the level of the liver. (a) Multiple tiny areas of vascular puddling
(arrowheads) in the hepatic parenchyma are evident, suggestive
of telangiectasias. There is also early opacification of the left portal
vein (yellow arrow), suggesting an arteriovenous malformation
(Tables 2 and 3). (b) Medium-to-large sized vascular abnormalities
representing confluent vascular masses (blue arrows) involving the
right lobe of the liver are shown. (c) Multiple tortuous corkscrew-like
vessels (yellow arrows) scattered in the hepatic parenchyma
and the hepatic artery is dilated (white arrow), suggestive of
telangiectatic vessels in Osler-Weber-Rendu syndrome.
Table 2. Salient features of arteriovenous malformations within different organs with presenting signs/symptoms and treatment options.[4] [8] [11]
The diagnosis of OWRS was made (Table 1). The
patient needed intervention in the liver AVMs to prevent
development of portal hypertension as a complication.
However, due to logistical reasons and the patient’s
financial difficulties, follow-up imaging was suggested.
Case 2
A 2-year-old boy presented with a sudden onset episode
of generalised seizures. His father had a history of
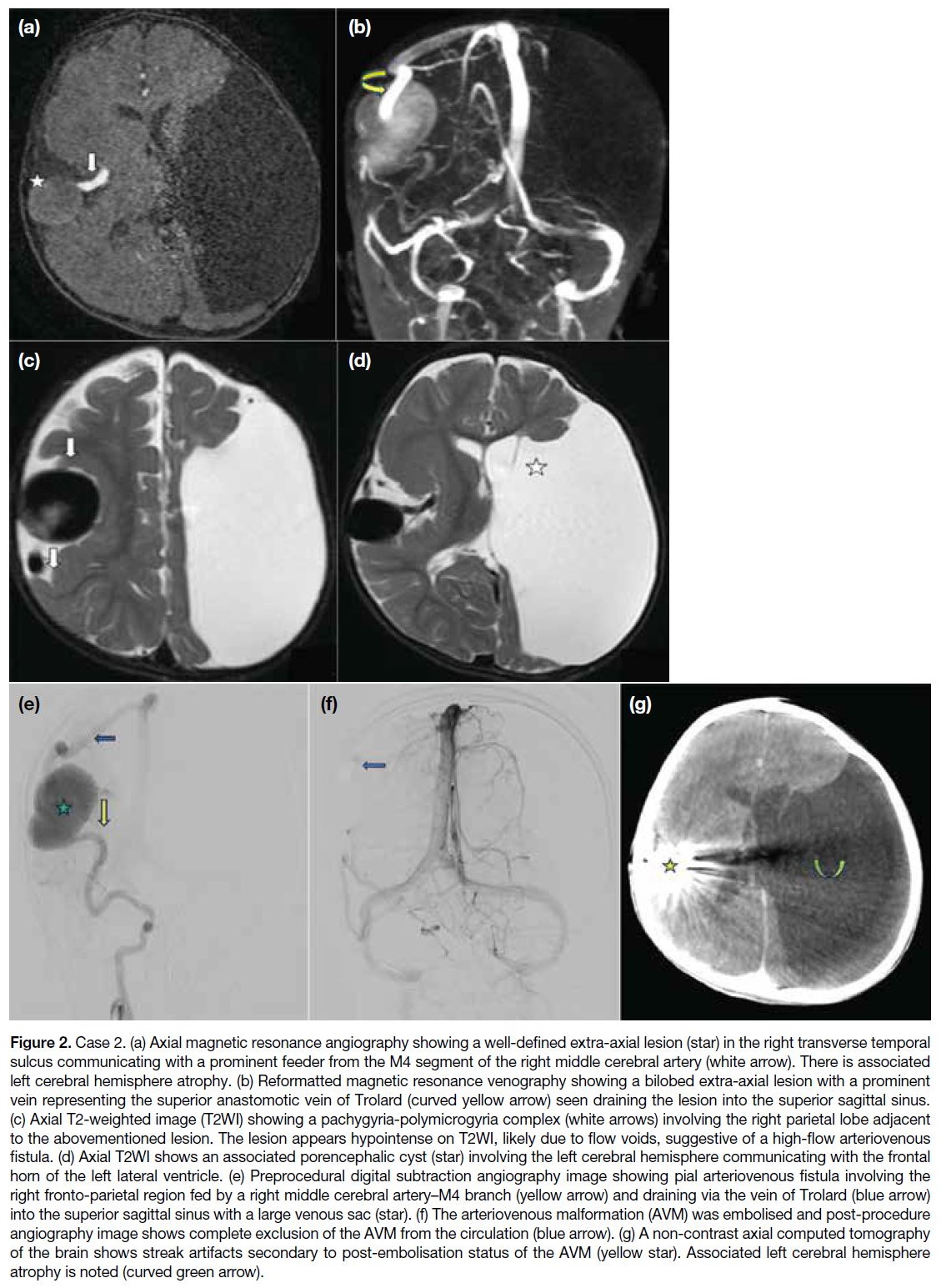
brain AVMs. On brain MRI, a bilobed extra-axial lesion was seen in the right transverse temporal sulcus
with blooming on gradient echo sequences and flow
void on other sequences. A prominent feeder from the
M4 segment of the right middle cerebral artery and a
prominent vein from the superior anastomotic vein of
Trolard were seen draining the lesion into the superior sagittal sinus, suggestive of AVM (Figure 2a and 2b).[1] [7]
Figure 2. Case 2. (a) Axial magnetic resonance angiography showing a well-defined extra-axial lesion (star) in the right transverse temporal
sulcus communicating with a prominent feeder from the M4 segment of the right middle cerebral artery (white arrow). There is associated
left cerebral hemisphere atrophy. (b) Reformatted magnetic resonance venography showing a bilobed extra-axial lesion with a prominent
vein representing the superior anastomotic vein of Trolard (curved yellow arrow) seen draining the lesion into the superior sagittal sinus.
(c) Axial T2-weighted image (T2WI) showing a pachygyria-polymicrogyria complex (white arrows) involving the right parietal lobe adjacent
to the abovementioned lesion. The lesion appears hypointense on T2WI, likely due to flow voids, suggestive of a high-flow arteriovenous
fistula. (d) Axial T2WI shows an associated porencephalic cyst (star) involving the left cerebral hemisphere communicating with the frontal
horn of the left lateral ventricle. (e) Preprocedural digital subtraction angiography image showing pial arteriovenous fistula involving the
right fronto-parietal region fed by a right middle cerebral artery–M4 branch (yellow arrow) and draining via the vein of Trolard (blue arrow)
into the superior sagittal sinus with a large venous sac (star). (f) The arteriovenous malformation (AVM) was embolised and post-procedure
angiography image shows complete exclusion of the AVM from the circulation (blue arrow). (g) A non-contrast axial computed tomography
of the brain shows streak artifacts secondary to post-embolisation status of the AVM (yellow star). Associated left cerebral hemisphere
atrophy is noted (curved green arrow).
A pachygyria-polymicrogyria complex involved the
right parietal lobe adjacent to the lesion (Figure 2c and 2d).[7] A large left-sided porencephalic cyst[7]
communicating with the ipsilateral lateral ventricle
exerted mass effect in the form of a midline shift of
7 mm to the right. There was associated left cerebral hemiatrophy (Figure 2c and 2d). The diagnosis of
OWRS was made (Table 1). In view of the high-flow
AVM, embolisation was advised. Endovascular glue
embolisation of the right parietal pial arteriovenous
fistula was performed by administering iodixanol
contrast (GE HealthCare, Milwaukee [WI], US) using a
microcatheter (Excelsior XT-17; Stryker, Fremont [CA],
US), microwire combination (Transcend; Meditech,
Watertown [MA], US) and a HyperGlide balloon (eV3
Endovascular Inc, Irvine [CA], US) to inject 66% glue
[n-butyl-2-cyanoacrylate liquid embolic system (Trufill;
Cordis Neurovascular, Miami Lakes [FL], US)] as an
embolisation agent mixed with ethiodised oil in various
dilution ratios depending on the application to control
polymerisation rate. Post-procedure angiography and
brain CT were performed, which showed complete exclusion of the AVM from the circulation (Figure 2e to 2g). The patient was started on antiepileptic drugs and
did not have any other seizure episode during the course
in the hospital.
Case 3
A 43-year-old male presented with one episode of
haemoptysis (around 10 mL of blood) and two episodes
of generalised tonic-clonic seizures. Multiple scattered non-haemorrhagic lacunar infarcts were noted in the right
occipital lobe and both fronto-parietal regions. Chest
radiography showed multiple homogenous mass lesions
in the right lower zone and left upper and lower zones
(Figure 3a). Subsequent CT pulmonary angiography
revealed multiple AVMs involving the anterior basal
segment of the right lower lobe, the apicoposterior
segment of the left upper lobe, and the lateral basal
segment of the left lower lobe. Multiple tiny AVMs were
identified in the right lower lobe.[8] [9] The feeding artery
diameter of a few of the AVMs was >3 mm[10] (Figure 3b). The diagnosis of OWRS was made (Table 1). In view of feeding artery diameter size being >3 mm, the patient was advised to undergo curative embolisation to
prevent pulmonary as well as cerebral complications.
The preprocedural angiogram (Figure 3c and 3d)
showed pulmonary AVMs supplied from the basal
segmental arteries. Endovascular glue embolisation of
the pulmonary AVMs was performed by administering
iohexol contrast using a microcatheter and/or microwire
and coils with 50% glue as an embolisation agent mixed
with ethiodised oil in various dilution ratios. Post-embolisation angiography (Figure 3e) showed complete exclusion of the AVMs from the circulation.
Figure 3. Case 3. (a) Chest radiograph showing multiple homogenous mass lesions (stars) seen in the right lower zone and left upper and
lower zones. (b) Reformatted coronal maximum intensity projection image showing multiple arteriovenous malformations (AVMs) involving
the antero-basal segment of the right lower lobe, apicoposterior segment of the left upper lobe, and the lateral basal segment of the left
lobe (red arrows). Multiple tiny AVMs are seen in the right lower lobes (yellow arrowheads). (c) Pre-procedure digital subtraction angiography
image of the right lung showing pulmonary AVM (star) with supply from the basal segmental arteries (blue arrow). (d) The AVM nidus (star)
was progressively embolised using micro coils with 60% glue and subsequent opacification was seen. Blue arrow indicates vascular supply
to the nidus from the basal segmental arteries. (e) Post-procedure angiogram shows complete exclusion of AVM from circulation (blue arrow).
DISCUSSION
Aetiologically, OWRS has been classified into different
types based on the genetic mutations found in these
patients. HHT type 1, found in nearly 61% of cases,[8]
shows a mutation in the endoglin gene located on
chromosome 9 and is found on the inner cell membrane
of the endothelial cells lining the blood vessels. Patients
with these mutations are generally predisposed to cerebral
and pulmonary AVMs.[8] HHT type 2, found in nearly
37% of cases, shows a mutation in the activin A receptor-like
type 1 (ACVRL-1) gene or the activin receptor-like
kinase-1 (ALK-1) gene located on chromosome 12.[8] [11]
These patients have been shown to have liver AVMs.[4]
Mutations in the mothers against decapentaplegic
homolog 4 (SMAD4) gene,[9] which encodes protein for
signal transmission from the transforming growth factor
beta receptor, has been implicated in 2% of cases of HHT
with juvenile gastrointestinal polyposis.[9] Patients having
bone morphogenetic protein-9 (BMPR-9) and RSA-1
gene mutations have also shown phenotypic overlap
with telangiectasia.[9]
Clinical Diagnosis, Signs, and Symptoms
The clinical diagnosis of OWRS is made using the four
Curaçao clinical criteria,[1] [2] [3] namely: (1) recurrent
epistaxis; (2) telangiectasias involving sites including the lips, oral cavity, nose, and fingers; (3) visceral lesions
with the gastrointestinal tract, liver, pulmonary, cerebral
or spinal involvement; and (4) family history of HHT in
a first-degree relative (Table 1).
Recurrent epistaxis is the most common symptom
which can begin in childhood or adolescensce.[8] Low-pressure
packing techniques can be used to manage
such episodes. Telangiectasias affected individuals can present post puberty or in adulthood. It happens
when capillaries fail to develop between arterioles
and venules, commonly involving the face, lips,
tongue, palm, and fingers (periungual and nail bed).[8]
Telangiectasias can also develop in the gastrointestinal
tract, presenting most commonly in the fourth
decade of life with stomach and duodenum being
the most common sites.[6] [8] AVMs, which are direct
communications between blood vessels having a calibre greater than telangiectatic vessels, are also seen in the patients.[9] [11]
Brain Involvement
Distal emboli containing blood clots or bacteria from
pulmonary AVMs may result in abscess formation and
ischaemic stroke (Table 2).[4] [8] [11]
The brain abscesses are generally multiple and recurrent, and involve the superficial layers of the cerebral lobes,
most commonly occurring in the parietal lobe. A higher
incidence is seen between the third and the fifth decades
of life, corresponding to increased pulmonary AVMs.[4] [11]
Imaging Features of Cerebral Arteriovenous
Malformations
Cerebral AVMs are seen as serpiginous areas of flow
void with invasion into the brain parenchyma on
MRI. The feeding artery and the draining veins can
be identified on different sections (Figure 2a and 2b).
Some patients may have high signal intensity on T1-weighted imaging within the basal nuclei that can be a
result of the metabolic disorder caused by hepatic artery-portal
venous shunting. In equivocal lesions, cerebral
angiography is performed, which may show high flow
pial AVFs occurring due to the lack of an intervening capillary bed (Figure 2a and 2b).[1]
Cortical Development Malformation
Cortical developmental malformation is another feature
which can be seen in the paediatric population. It involves
two main entities: polymicrogyria and heterotopia.[7]
Patients with polymicrogyria can present with
developmental delay, cognitive abnormalities, and
epilepsy (about 78% of cases).[4] Epilepsy shows
earlier onset in patients having higher degrees of
polymicrogyria.[7] A favourable prognosis is present in
patients with unilateral and localised polymicrogyria.
The imaging features of polymicrogyria on MRI are
smaller gyri with thin, shallow sulci separating them
(Figure 2c and 2d). The cortex appears thickened, with
an irregular surface and abnormal vasculature in close
proximity. It is most commonly seen in the perisylvian
region, followed by parietal, parietotemporal, and frontal
regions.[7]
Heterotopia is an abnormal location of normal neuronal
cells due to abnormal migration. The most commonly
seen variant in OWRS is the periventricular nodular
type. Bilateral occurrence is more common in the frontal
lobes.[7]
Brain and pulmonary AVMs seem to have a higher
incidence in patients with cortical developmental
malformations.[7] [8]
Lung Involvement
Pulmonary AVMs are the most striking features of
lung involvement, seen in nearly 50% of HHT cases
(Table 2).[8] The anatomical structure of AVMs can be
simple, with one feeding artery and one draining vein, or
complex with ≥ 2 arterial branches and draining veins.[12]
Chest radiography shows well-defined nodules within
the lung (Figure 3a). Cardiomegaly and prominent
pulmonary arteries can also be seen (Table 2).
Chest CT with MPR and maximum intensity projections
show one or multiple serpiginous masses/nodules with
≥1 enlarged feeding artery (diameter of ≥3 mm) and
draining vein (Table 2 and Figure 3b).[10] [12] Contrast-enhanced
magnetic resonance angiography can show
all pulmonary AVMs with feeding arteries having a
diameter >3 mm.[10]
Liver Involvement
Ultrasound imaging can show an increase in common
hepatic artery calibre (>7 mm) and intrahepatic
hypervascularity.[6] Doppler imaging shows pulsatile
portal flow in cases of arterioportal shunting and pulsatile
hepatic venous flow in cases of arteriovenous shunting.[6]
Focal nodular hyperplasia and hepatic AVMs are
more commonly seen in patients with ALK-1 gene
mutation. Increased sinusoidal blood flow leads to portal
hypertension, pseudocirrhosis of the liver, and hepatic
encephalopathy in later stages.[4] [6]
Liver Telangiectasias
CECT of the abdomen with MPR and maximum intensity
projections can show telangiectasias in proximity to
large vessels[5] and also in the subcapsular regions. These
are seen as focal hyperattenuating rounded nodular
lesions in the arterial and late arterial phases, which
become isodense with the hepatic parenchyma in the hepatic phase (Figure 1a, 1b, and Tables 2 and 3).[4] [5] [6] MRI
shows high signal intensity on T2-weighted imaging and
appears hypointense on T1-weighted imaging.
Large Confluent Masses
On CECT of the abdomen, large confluent masses within
the liver (>10 mm) may be seen enhancing in enhancing
arterial phase with persistent enhancement in hepatic phase.
Hereditary Haemorrhagic Telangiectasia
and Cirrhosis
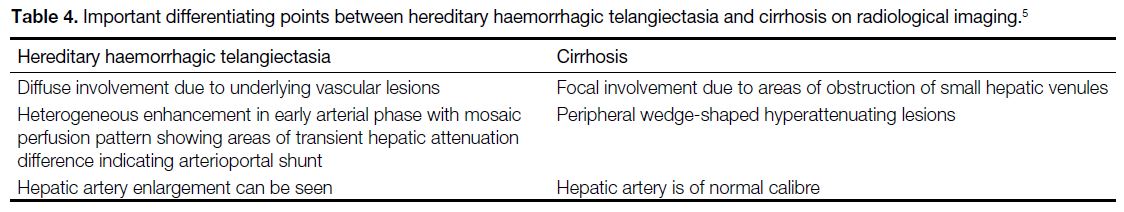
The differentiating features between HHT and cirrhosis
on imaging for hepatic perfusion abnormalities are listed
in Table 4.[5]
Table 4. Important differentiating points between hereditary haemorrhagic telangiectasia and cirrhosis on radiological imaging.[5]
Pancreatic Arteriovenous Malformations
Pancreatic AVMs are the most common extrahepatic
AVMs. CT imaging shows focal lesions (diameter: 5-10
mm) with increased vascularity. Arteriovenous shunting
to the splenic vein or superior mesenteric vein may be
noted.[5] [6]
Gastrointestinal Involvement
The most common manifestation is telangiectasias,
both in the small bowel (around 60%) and the stomach
(around 30%). Patients with HHT have an increased
incidence of small bowel polyps as compared to the
general population.[6] [8] Gastrointestinal haemorrhage is a
common presentation, usually seen in the fourth to the
fifth decades[6] (Table 2). On colonoscopy, 31% to 32% of
OWRS patients show colonic AVMs associated with the
HHT1 genotype having the endoglin mutation.[4] SMAD4
mutations in OWRS patients result in juvenile polyposis,
which is difficult to differentiate from the juvenile
polyposis caused by BMPR1A mutations in the general
population. Rarely, OWRS cases show intramural
haematomas on endoscopic evaluation.[6] [8] [9]
Ocular Manifestations
The ocular signs and symptoms include conjunctival
telangiectasias or AVMs, bloody tears, conjunctival post-haemorrhagic
granulomatous lesions, and the recently
described association of choriocapillaris atrophy with
HHT. However, retinal involvement prevalence is only
around 1%.[3] [9]
Management
Embolisation of pulmonary and cerebral AVMs is
important to avoid serious complications. Pulmonary
AVMs having a feeding artery diameter >3 mm are
ideal candidates for embolisation to decrease the risk
of pulmonary haemorrhages and paradoxical emboli
to the brain.[10] Patients should be advised about the
side-effects related to embolotherapy for pulmonary
AVMs, such as transient post-procedural chest pain
and self-limiting pleurisy. Brain AVMs need treatment
to prevent the occurrence of stroke and abscess.
Microsurgical resection, stereotactic radiation surgery,
and endovascular embolisation can be performed.
Embolisation can be pre-microsurgical, pre-radiation
surgery, curative, or palliative depending on the patient.
Preprocedural CT angiography and antibiotic prophylaxis
for the risk of bacteraemia is advised. Digital subtraction
angiography is used for the procedure.[13] In our patients,
since the AVMs were relatively small with few feeding
pedicles and without perinidal angiogenesis, curative
embolisation was performed (Table 2).
Follow-up is an important step in management of these
patients. A baseline post-embolisation scan is performed
at 6 months and then repeated at 12 months to ensure sac
involution, followed by follow-up intervals of 2 years
to detect growth of untreated pulmonary AVMs and
reperfusion of treated AVMs.[14] Chest CT or contrast-enhanced
MRI are used for follow-up post-coiling to
look for reperfusion of occluded pulmonary AVMs.[15]
The risk of reperfusion increases with larger feeding
artery diameter, using less number of coils, oversized
coils, or more proximal placement of the coil within the
feeding artery. In such cases, it is increasingly difficult
to treat these reperfused AVMs, resulting in higher
recurrence rates.[15]
CONCLUSION
AVMs associated with OWRS are ticking time
bombs which can result in catastrophic events such as
pulmonary haemorrhages, brain abscess, stroke, chronic
gastrointestinal bleeds, high-output cardiac failure,
paraparesis, and, rarely, paraplegia. Since affected individuals are generally asymptomatic, the lesions are
often discovered incidentally. Radiologists must always
be on the lookout for such classical findings to not only
aid the diagnosis but also lower the risk of complications.
REFERENCES
1. Geibprasert S, Pongpech S, Jiarakongmun P, Shroff MM, Armstrong DC, Krings T. Radiologic assessment of brain
arteriovenous malformations: what clinicians need to know.
Radiographics. 2010;30:483-501. Crossref
2. Ha M, Kim YJ, Kwon KA, Hahm KB, Kim MJ, Kim DK, et al. Gastric angiodysplasia in a hereditary hemorrhagic telangiectasia type 2 patient. World J Gastroenterol. 2012;18:1840-4. Crossref
3. Rinaldi M, Buscarini E, Danesino C, Chiosi F, De Benedictis A, Porcellini A, et al. Ocular manifestations in hereditary hemorrhagic
telangiectasia (Rendu-Osler-Weber disease): a case-series.
Ophthalmic Genet. 2011;32:12-7. Crossref
4. Singh A, Suri V, Jain S, Varma S. Rare manifestations in a case of Osler-Weber-Rendu disease. BMJ Case Rep.
2015;2015:bcr2014207852. Crossref
5. Siddiki H, Doherty MG, Fletcher JG, Stanson AW, Vrtiska TJ,
Hough DM, et al. Abdominal findings in hereditary hemorrhagic
telangiectasia: pictorial essay on 2D and 3D findings with isotropic
multiphase CT. Radiographics. 2008;28:171-84. Crossref
6. Jackson SB, Villano NP, Benhammou JN, Lewis M, Pisegna JR, Padua D. Gastrointestinal manifestations of hereditary hemorrhagic telangiectasia (HHT): a systematic review of the literature. Dig Dis Sci. 2017;62:2623-30. Crossref
7. Palagallo GJ, McWilliams SR, Sekarski LA, Sharma A, Goyal MS, White AJ. The prevalence of malformations of cortical development
in a pediatric hereditary hemorrhagic telangiectasia population.
AJNR Am J Neuroradiol. 2017;38:383-6. Crossref
8. Macri A, Wilson AM, Shafaat O, Sharma S. Osler-Weber-Rendu disease. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482361/. Accessed 8 Nov 2022.
9. National Organization for Rare Disorders. Hereditary hemorrhagic
telangiectasia. 2021. Available from: https://rarediseases.org/rare-diseases/hereditary-hemorrhagic-telangiectasia/. Accessed 8 Nov
2022.
10. Majumdar S, McWilliams JP. Approach to pulmonary arteriovenous malformations: a comprehensive update. J Clin Med. 2020;9:1927. Crossref
11. Maher CO, Piepgras DG, Brown RD Jr, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32:877-82. Crossref
12. Lee HN, Hyun D. Pulmonary arteriovenous malformation and its vascular mimickers. Korean J Radiol. 2022;23:202-17. Crossref
13. Vollherbst DF, Chapot R, Bendszus M, Möhlenbruch MA. Glue, Onyx, Squid or PHIL? Liquid embolic agents for the embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas. Clin Neuroradiol. 2022;32:25-38. Crossref
14. Hong J, Lee SY, Cha JG, Lim JK, Park J, Lee J, et al. Pulmonary arteriovenous malformation (PAVM) embolization: prediction of angiographically-confirmed recanalization according to PAVM Diameter changes on CT. CVIR Endovasc. 2021;4:16. Crossref
15. Maruno M, Kiyosue H, Hongo N, Matsumoto S, Mori H. Where is the origin of the last normal branch from feeding artery of pulmonary arteriovenous malformations? Cardiovasc Intervent Radiol. 2018;41:1849-56. Crossref