Diabetic Ketoacidosis after Pembrolizumab Treatment in a Patient with Thymic Carcinoma and No Known Diabetes Mellitus: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Sep;27(3):e171-5 | Epub 19 September 2024
Diabetic Ketoacidosis after Pembrolizumab Treatment in a Patient with Thymic Carcinoma and No Known Diabetes Mellitus: A Case Report
HCY Wong1, HF Hung2, CH Kwok1
1 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
2 Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr HCY Wong, Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China. Email: henrywong3011@gmail.com
Submitted: 2 May 2023; Accepted: 5 October 2023.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong [Ref No.: KW/EX-22-065(175-04)]. The requirement for patient consent was waived by the Committee as the patient had passed away at the time of writing.
Supplementary Material: The supplementary material was provided by the authors and some information may not have been peer reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by the Hong Kong College of Radiologists. The Hong Kong College of Radiologists disclaims all liability and responsibility arising from any reliance placed on the content.
CASE PRESENTATION
A 64-year-old Chinese man presented in December
2020 with a 3-month history of neck pain. Contrast-enhanced
magnetic resonance imaging of the cervical
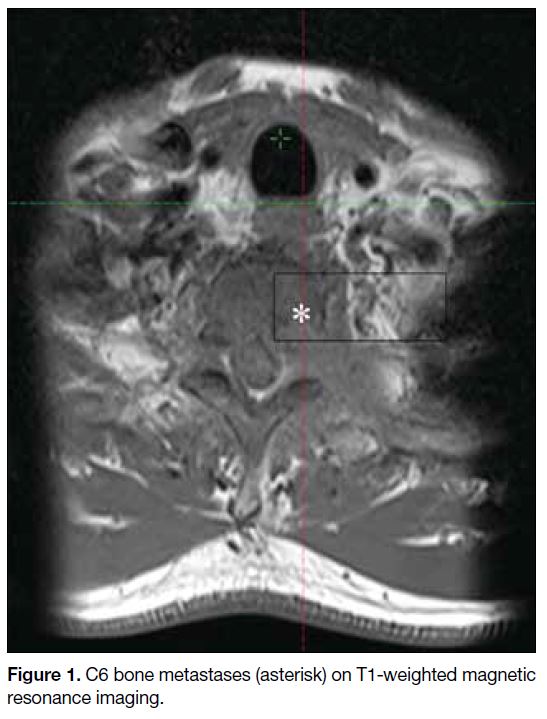
spine revealed a large mass at the C6 vertebra (Figure 1). Computed tomography (CT)–guided biopsy of
the mass revealed poorly differentiated carcinoma,
with immunohistochemistry tests positive for p40,
cytokeratin, CD5, PAX8 and c-kit, and negative for
thyroid transcription factor 1, CDX2, leukocyte common
antigen, S100 protein, desmin, synaptophysin and CD56.
These results were suggestive of thymic squamous cell
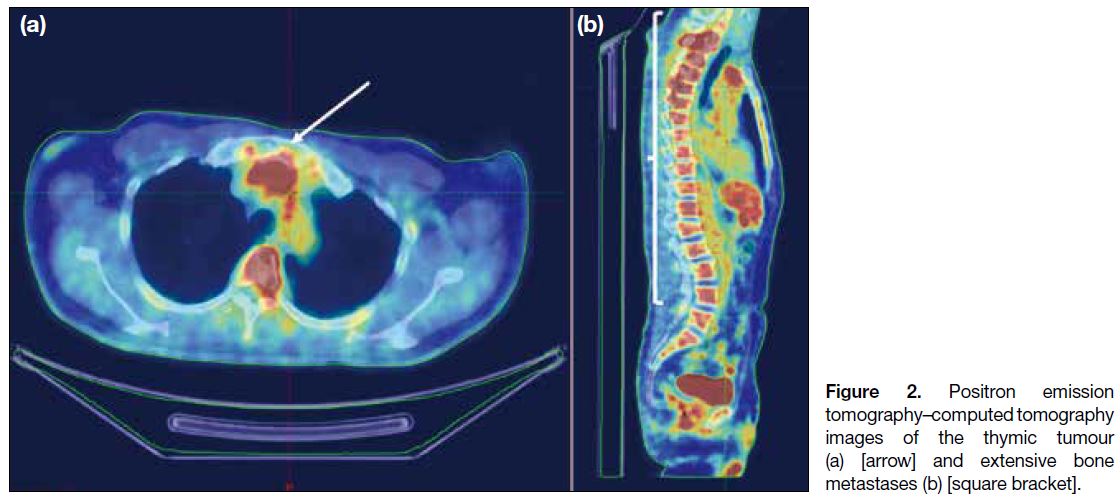
carcinoma. Positron emission tomography–CT showed
a hypermetabolic thymic mass and multiple bone
metastases, confirming the diagnosis of metastatic thymic
carcinoma (Figure 2). No metastases were observed in the pancreas or adrenal glands. He had a past medical
history of hypertension well controlled on amlodipine 5
mg daily. His cell counts, organ function, fasting glucose
level and lipid profile were normal 1 month before the
diagnosis of malignancy. He had no family history of
diabetes mellitus.
Figure 1. C6 bone metastases (asterisk) on T1-weighted magnetic resonance imaging.
Figure 2. Positron emission
tomography–computed tomography images of the thymic tumour (a) [arrow] and extensive bone metastases (b) [square bracket].
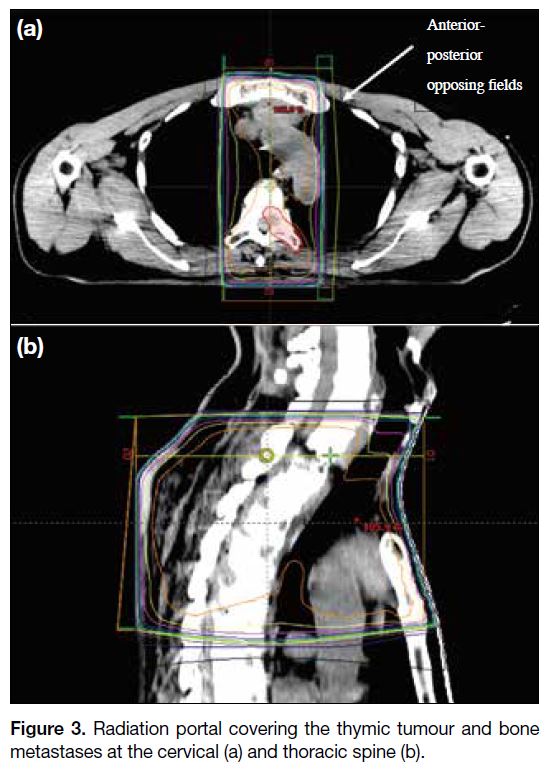
The patient received palliative radiotherapy to the
painful cervical and thoracic spine bone metastases at
a dose of 22.5 Gy in five daily fractions over 1 week
with anterior-posterior opposing fields. The thymic
tumour was covered in the radiation portal (Figure 3).
Subsequently, he was started on palliative chemotherapy
with etoposide and cisplatin (etoposide 100 mg/m2 and
cisplatin 30 mg/m2 daily from day 1 to day 3 every
3 weeks) in January 2021. Regular zoledronic acid every 4 weeks was also given. He developed grade 4 neutropenia requiring granulocyte colony-stimulating factor support, treatment deferrals, and dose reduction.
After six cycles of etoposide and cisplatin, CT showed
mixed response with stable bone metastases but
enlarging thymic tumour. As the patient was
asymptomatic, he opted for a drug holiday.
Figure 3. Radiation portal covering the thymic tumour and bone metastases at the cervical (a) and thoracic spine (b).
Six months later, the patient complained of increasing
lower back pain, and CT confirmed disease progression.
He was started on pembrolizumab 200 mg every 3
weeks in November 2021. His fasting glucose level
before treatment was 5.8 mmol/L. Baseline morning
cortisol level was low at 36 nmol/L (normal: 133-537)
while thyroid function was normal (thyroid-stimulating
hormone level: 3.67 mIU/L [normal: 0.27-4.20],
thyroxine level: 16.9 pmol/L [normal: 12.0-22.0]). He
was given hydrocortisone replacement of 10 mg twice
daily before starting immunotherapy. No significant
side-effects were observed during the first three cycles.
The patient was admitted to the hospital for coma in
January 2022, 3 days after the fourth cycle. Blood
results showed severe hyperglycaemia (blood glucose
level: 55.7 mmol/L) and metabolic acidosis (pH
value: 7.22, bicarbonate level: 9.6 mmol/L). Multistix
urine test revealed large amounts of ketones. Coupled
with an elevated beta-hydroxybutyrate level, the
clinical diagnosis of diabetic ketoacidosis (DKA) was
suggested. He was treated with insulin infusion and
fluid resuscitation. Subsequent investigations after
stabilisation showed glycated haemoglobin level of
10.8% and low C-peptide (0.06 nmol/L; normal: 0.30-2.40) and insulin (1.6 mIU/L; normal 2.6-24.9) levels.
Anti-GAD65 and anti-IA2 antibodies were negative.
Insulin infusion was weaned off and switched to
subcutaneous insulin glargine.
The oncology team decided to stop pembrolizumab as
the severe hyperglycaemia and DKA could be related to the treatment. The plan was to consider second-line
chemotherapy if there was progressive disease.
A CT performed 2 months after the presentation of
hyperglycaemia showed stable disease.
The patient’s diabetic control was brittle and he required three admissions within 2 months for insulin titration.
The first admission was due to hyperglycaemia,
whereas the latter two were for hypoglycaemia. In
the third admission, he had persistent hypotension
requiring escalation of hydrocortisone replacement
for stabilisation. He ran a progressive downhill course
with deconditioning and was readmitted for Klebsiella
pneumoniae chest infection. He succumbed in May
2022, 4 months after the presentation of DKA and 17
months after the diagnosis of thymic carcinoma. Details
about the patient’s timeline of events are illustrated in
online supplementary Figure 1.
DISCUSSION
This patient developed life-threatening DKA following pembrolizumab treatment. Since the patient’s baseline
fasting glucose level was normal and type 1 diabetes
mellitus (T1DM) was considered unlikely for the
patient’s age, his condition was most probably related to
pembrolizumab.
In the past decade, immune checkpoint inhibitors
(ICIs) have revolutionised the field of oncology.
Pembrolizumab, a programmed cell death protein 1
(PD-1) inhibitor, has been studied in thymic carcinoma
and shown promising efficacy in this entity with a poor
prognosis.[1] [2] Despite important clinical benefits, ICIs are
known for their immune-related adverse events (irAEs).
These can target virtually any organ system and their
severity can range from mild to life threatening. ICI-associated
autoimmune diabetes mellitus (CIADM)
is a rare complication of therapy, with an incidence of
0.2% to 1.4%.[3] With increasing clinician awareness of
CIADM, its incidence is likely to increase.
The pathophysiology of CIADM involves the
development of autoreactive T cells to pancreatic beta
cells in response to a previous environmental trigger
in genetically predisposed individuals. These T cells
are generally controlled by immune checkpoints but
pathology may result when activated by anti–PD-1/programmed death-ligand 1 (PD-L1) therapy.[2] [3]
The presentation of CIADM is variable, ranging from
asymptomatic hyperglycaemia to severe diabetic
complications. This patient’s presentation with DKA is
the most common presentation of CIADM. In a pooled
analysis of 200 case reports, 67.5% of CIADM patients
presented with DKA.[4] The onset of CIADM varies with
a median of 6 to 9 weeks but can occur as early as 1 week
and as late as after the end of ICI treatment.[5]
The diagnosis of CIADM is characterised by two
hallmark features of hyperglycaemia and low C-peptide
level. When C-peptide level is normal, alternative
causes of hyperglycaemia during ICI therapy should be
considered, including exacerbation of type 2 diabetes
mellitus, steroid-induced hyperglycaemia, autoimmune
pancreatitis, and lipodystrophy.[3] Compared with T1DM
where autoantibodies are present in >90% of cases,
autoantibody positivity is lower in CIADM, ranging
from 0% to 71%.[3] Therefore, negative values for this patient did not exclude CIADM.
Due to the rarity of CIADM, evaluating its risk factors based on clinical characteristics and biomarkers is challenging. A recent systematic review identified that
close to 60% of CIADM patients had susceptibility
haplotypes for T1DM, and patients with positive T1DM
antibodies had an earlier onset of CIADM.[4] Although
this provides important information about the disease
nature and clinical course of CIADM, it does not
help clinicians assess which patients need enhanced
surveillance. Suazo-Zepeda et al[6] demonstrated that high
PD-L1 expression is associated with the development
of immune-related adverse reactions in patients with
non–small cell lung cancer. Whether this correlation
is also observed for CIADM and patients with thymic
carcinoma is uncertain. Unfortunately, our patient had
passed away at the time of writing this case report, and
it was not possible to retrieve his archival specimen for
PD-L1 testing.
The mainstay of treatment for CIADM is insulin. In
contrast to other irAEs, treatment with glucocorticoids
or immunosuppressants is not effective in these patients
due to the almost complete destruction of beta cells.[3] [5]
Steroids will likely negatively influence diabetes
control in these patients and are not advised. In view of
the irreversible damage to beta cells, similar to that in
T1DM, a multi-dose basal-bolus regimen or continuous
insulin pump is recommended to achieve glycaemic
targets.[3] Our patient was prescribed long-acting insulin
glargine only and discharged before C-peptide result
was available, possibly one of the reasons for his labile
glycaemic control.
Close surveillance for irAEs is essential while using
ICIs. The 2021 American Society of Clinical Oncology
guideline suggests testing of baseline fasting glucose
level and monitoring of random glucose level before
each dose of ICI.[7] Although CIADM is rare, regular
monitoring to facilitate early endocrine team referral
and insulin treatment to prevent life-threatening diabetic
complications should be advocated. This patient had an
elevated glycated haemoglobin level at presentation with
DKA, suggesting he may have been hyperglycaemic
during the preceding months. If regular surveillance of
glucose level was performed, CIADM could have been
diagnosed at an earlier stage. The suggested workflow
for monitoring and treatment of the condition is depicted
in online supplementary Figure 2.
In general, treatment of severe irAEs requires permanent discontinuation of the checkpoint inhibitor. Nonetheless
similar to other immune-related endocrinopathies where
the damage is irreversible, restarting treatment may be considered with close monitoring of diabetic control once glucose levels stabilise.[7]
In the two prospective phase II studies of the role of
pembrolizumab in patients with thymic carcinoma,
around 15% of patients developed grade >3 irAEs,[1] [2]
much higher than the pooled incidence of <2% in a
systematic review and meta-analysis of clinical trials
evaluating anti-PD1 and anti–PD-L1 checkpoint
inhibitors.[8] Notably, the types of high-grade irAEs
in these patients were rarely seen in other tumour
histologies. Of the 66 thymic carcinoma patients in the
two studies, three developed myasthenia gravis (4.5%),
two developed myocarditis (3.0%), one developed
myositis (1.5%), and one developed myoclonus (1.5%).[1] [2]
CIADM was observed in one patient (1.5%).[1] [2] The
higher incidence and unusual clinical presentations of
irAEs in patients with thymic carcinoma warrant further
study and validation in larger patient cohorts.
Another reason this patient developed CIADM is that
he may have had an underlying autoimmune condition.
This patient had a low baseline cortisol level before
treatment with pembrolizumab. It is possible that
he had undiagnosed autoimmune adrenalitis since
thymic carcinomas are associated with autoimmune
paraneoplastic syndromes, albeit at lower rates
compared with thymomas.[9] In retrospect, further workup
with blood tests for adrenocorticotropic hormone level
and antiadrenal antibodies should have been performed.
Patients with preexisting autoimmune conditions are
known to have higher risks for irAEs and have flare-ups
during immunotherapy.[10] This may also explain the need to escalate our patient’s hydrocortisone dose after commencing pembrolizumab.
This case highlights the need for a heightened degree of suspicion amongst physicians for CIADM when treating
patients with immunotherapy, especially those with
thymic carcinoma, malignancies prone to paraneoplastic
syndromes, or a past history of autoimmune diseases.
Blood testing for C-peptide in patients who present
with hyperglycaemia following immunotherapy aids the
diagnosis of CIADM.
REFERENCES
1. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic
carcinoma: a single-arm, single-centre, phase 2 study. Lancet
Oncol. 2018;19:347-55. Crossref
2. Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. 2019;37:2162-70. Crossref
3. Wu L, Tsang VH, Sasson SC, Menzies AM, Carlino MS, Brown DA, et al. Unravelling checkpoint inhibitor associated
autoimmune diabetes: from bench to bedside. Front Endocrinol
(Lausanne). 2021;12:764138. Crossref
4. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord. 2021;22:337-49. Crossref
5. Clotman K, Janssens K, Specenier P, Weets I, De Block CE. Programmed cell death-1 inhibitor–induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:3144-54. Crossref
6. Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann TJ, de Bock GH,
Sidorenkov G. Risk factors for adverse events induced by immune
checkpoint inhibitors in patients with non–small-cell lung
cancer: a systematic review and meta-analysis. Cancer Immunol Immunother. 2021;70:3069-80. Crossref
7. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline update. J Clin Oncol. 2021;39:4073-126. Crossref
8. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al.
Comprehensive meta-analysis of key immune-related adverse
events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients.
Cancer Immunol Res. 2017;5:312-8. Crossref
9. Padda SK, Yao X, Antonicelli A, Riess JW, Shang Y, Shrager JB,
et al. Paraneoplastic syndromes and thymic malignancies: an
examination of the international thymic malignancy interest group
retrospective database. J Thorac Oncol. 2018;13:436-46. Crossref
10. Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol.
2019;71:2100-11. Crossref