Efficacy and Safety of Preoperative Embolisation of Bone Tumours: A Tertiary Centre Experience
ORIGINAL ARTICLE
Hong Kong J Radiol 2024 Sep;27(3):e156-63 | Epub 28 August 2024
Efficacy and Safety of Preoperative Embolisation of Bone Tumours: A Tertiary Centre Experience
FFY Wan, TWY Chin, KC Lai, MK Chan
Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr FFY Wan, Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: wfy471@ha.org.hk
Submitted: 22 May 2023; Accepted: 19 October 2023.
Contributors: All authors designed the study, acquired and analysed the data. FFYW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon Central / Kowloon East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: KC/KE-23-0038/ER-4). The requirement for informed patient consent was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
Preoperative embolisation of bone tumours minimises risk of major intraoperative haemorrhage. Technical success is defined as obliteration of tumour vascularity by ≥70% on post-embolisation angiography. We retrospectively reviewed the technical success, efficacy, and safety of preoperative embolisation of bone tumours in our centre.
Methods
Nineteen patients underwent preoperative embolisation of bone tumours from December 2010 to July 2022. Subsequent surgery was performed 1 day post-embolisation. Patient demographics, tumour histology and location, presence of pathological fracture or spinal cord compression, primary embolic agent used, technical success, intraprocedural blood loss, need for blood transfusion, and major complications related to embolisation or subsequent surgery were assessed.
Results
Most of the bone tumours were metastases (n = 14) with the majority being hypervascular metastases from renal cell carcinoma or thyroid cancer. The primary bone tumours (n = 5) included vertebral haemangioma (n = 2), plasmacytoma (n = 2), and chordoma (n = 1). Pathological fractures were present in 11 patients. Among the 11 tumours in the spine, eight of them were complicated by spinal cord compression before embolisation. Particles were used as the main embolisation agent in all cases, with 89% technical success. There were no major embolisation-related complications. In patients after successful embolisation, the estimated intraprocedural blood loss ranged from 20 to 3,000 mL.
Conclusion
Preoperative embolisation of bone tumours is safe and feasible with high technical success.
Key Words: Bone neoplasms; Hemangioma; Radiology, interventional; Spinal cord compression
中文摘要
骨腫瘤術前栓塞的有效性和安全性:一個三級醫療中心的經驗
尹芳盈、錢永恩、黎國忠、陳文光
引言
骨腫瘤術前栓塞盡量減少了術中大出血的風險。技術成功的定義是栓塞後血管攝影中腫瘤血管消失≥70%。我們對本中心骨腫瘤術前栓塞的技術成功率、有效性和安全性進行回顧性分析。
方法
2010年12月至2022年7月期間,19例患者接受了術前骨腫瘤栓塞治療,他們於栓塞後1天進行手術。我們分析了患者基本數據、腫瘤組織學和位置、是否存在病理性骨折或脊髓壓迫、使用的主要栓塞劑、技術成功率、術中失血、輸血需求以及與栓塞或後續手術相關的主要併發症。
結果
大多數骨腫瘤是轉移瘤(n = 14),其中大多數是腎細胞癌或甲狀腺癌的富血管轉移瘤。原發性骨腫瘤(n = 5)包括椎體血管瘤(n = 2)、漿細胞瘤(n = 2)和脊索瘤(n = 1)。11名患者存在病理性骨折。11個脊椎腫瘤中,有8個在栓塞前併發脊髓受壓。所有病例均使用以顆粒為主的栓塞劑,技術成功率為89%。沒有嚴重的栓塞相關併發症個案。栓塞成功的患者的預計術中失血量為20至3,000 mL。
結論
骨腫瘤術前栓塞是安全且可行的,技術成功率亦高。
INTRODUCTION
The management of bone tumours is complex and
requires a multidisciplinary approach. In general, the
best line of treatment is surgical resection. Bone tumours
with impending or completed pathological fractures
require early surgical intervention to prevent or stabilise
the fractures. Nevertheless, surgery may be technically
difficult due to large or hypervascular tumours, difficult
anatomical locations, or close proximity to adjacent vital
structures such as the spine. In these scenarios, arterial
embolisation plays a pivotal role as a preoperative
methodology to achieve devascularisation of the
tumour, thus minimising intraoperative bleeding and
complications. In this study, we aimed to evaluate the
technical success, efficacy, and safety of preoperative
embolisation of bone tumours in our tertiary
musculoskeletal tumour centre.
METHODS
This retrospective study evaluated 19 patients who
underwent preoperative embolisation of bone tumours
followed by surgery at our centre from December 2010
to July 2022. Patient demographics, tumour histology
and location, presence of pathological fractures or spinal
cord compression, and choice of primary embolic agent
were recorded. The technical success of embolisation,
defined as reduction of tumour arterial blush by ≥70%
on postoperative angiography,[1] [2] as shown in our case (Figure 1), was assessed. In cases of no definite tumoural
staining identified on preoperative angiography, which
was seen in one of our patients, technical success
could not be reliably evaluated. Clinical notes as well
as operative and anaesthetic records were reviewed
for major surgical complications, intraoperative blood
loss, and requirements for transfusion. Categorical data
are presented as percentages, while numerical data are
presented as medians with ranges.
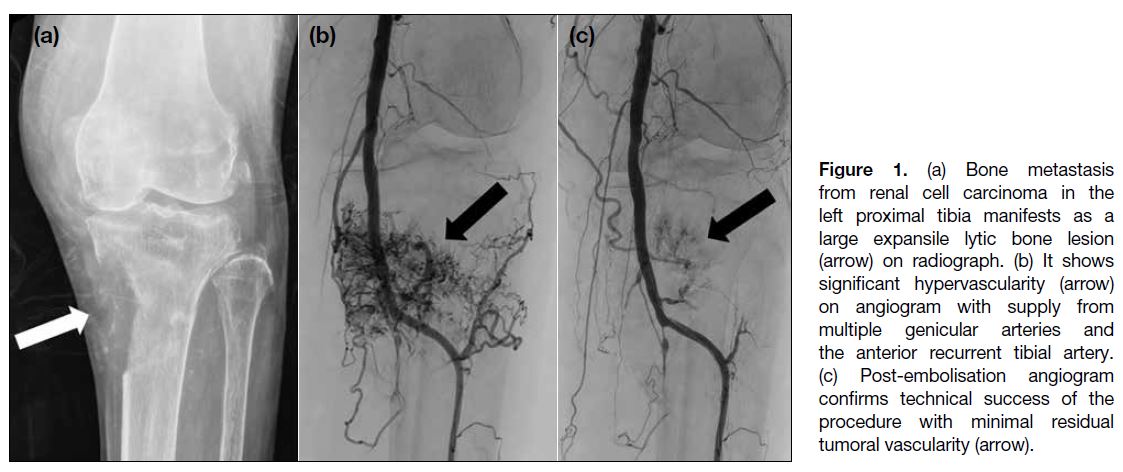
Figure 1. (a) Bone metastasis from renal cell carcinoma in the left proximal tibia manifests as a large expansile lytic bone lesion (arrow) on radiograph. (b) It shows significant hypervascularity (arrow) on angiogram with supply from multiple genicular arteries and the anterior recurrent tibial artery. (c) Post-embolisation angiogram confirms technical success of the procedure with minimal residual tumoral vascularity (arrow).
Techniques
Case selection for preoperative embolisation requires a multidisciplinary team discussion. Factors to consider
include tumour histology, location, size and vascularity,
and the risk of significant intraprocedural haemorrhage.[2]
Tumour histology is confirmed by image-guided core
needle biopsy. The location, size, and vascularity of
the tumour are assessed on imaging. Preprocedural
review of imaging, in particular computed tomography
angiography, is important for identifying blood supply
and drainage, tumour extension into adjacent structures,
and proximity to vital structures potentially sharing the
arterial supply. Before the embolisation procedure, the
results of laboratory tests including clotting profile,
platelet count, haemoglobin level, and creatinine values
are reviewed. Abnormal coagulation should be corrected
since many of the embolic agents require a functioning
intrinsic clotting mechanism.
All patients in our centre had surgery performed 1
day following the embolisation. In view of potential
revascularisation with a prolonged interval between
embolisation and surgery, the timing of embolisation
should be as close as possible to that of the operation,
ideally within 3 days after embolisation.[2] The procedure
was performed by radiologists with 8 to 26 years of
experience in vascular interventional radiology. The
embolisation procedure was done under local anaesthesia
in the angiography suite. Vascular access was obtained
via femoral arterial puncture. A 5-Fr or 6-Fr vascular
sheath and a 4-Fr or 5-Fr pre-shaped catheter were used.
A pre-embolisation angiogram was obtained to assess the
degree of tumour vascularity, identify the major supplying
arteries, and confirm the safety of embolisation. For
instance, careful attention must be paid to ensure there is
no opacification of a spinal pial artery such as the artery
of Adamkiewicz. If embolisation was not contraindicated
for any of these reasons, a microcatheter was introduced
coaxially through the catheter to achieve superselective
catheterisation of tumour feeding arteries and reduce the
chance of non-target embolisation. Micron-sized solid
embolic particles were primarily used in all cases. They
lodged in the tumour vessels proximal to or at capillary
level, thus occluding vessels within the tumour to
achieve distal tumour microvasculature penetration. The
particles were suspended in non-ionic contrast medium to
enable visualisation during the angiographic procedure.
The choice of particle diameter was determined by
vessel size and desired distal embolisation. Injection of
embolic agents must be performed under fluoroscopic
guidance to guard against reflux into non-target vessels.
All embolisation procedures were performed under continuous fluoroscopic guidance. Multiple angiograms
were acquired to evaluate the degree of vessel occlusion.
The endpoint of the procedure was reached when all the
major tumour-supplying vessels were occluded with
near-complete obliteration of tumour blush. Finally, a
post-embolisation angiogram was performed to assess
the technical success of embolisation, which was defined
as catheterisation of the major tumour feeding arteries
with reduction of the tumour blood supply by ≥70%.[1] [2]
RESULTS
There were 10 female and nine male patients who
underwent preoperative embolisation during the study
period (Table). Patient age ranged between 22 and 77
years and the median age was 61 years. The majority of
the tumours were bone metastases (n = 14, 74%) and most
of them were either metastases from renal cell carcinoma
(n = 6, 32%) or thyroid carcinoma (n = 5, 26%). The
rest of the bone tumours (n = 5, 26%) included vertebral
haemangioma (n = 2, 11%), plasmacytoma (n = 2, 11%),
and chordoma (n = 1, 5%). More than half (n = 11, 58%)
of the tumours were located within multiple vertebrae.
The rest were located in the extremities (n = 6, 32%)
or the pelvis (n = 2, 11%). Pathological fractures were
present in 58% of the patients (n = 11). Among the 11
vertebral tumours, cord compression was seen in eight
(73%) of them.
Table. Demographics, clinical, and pathological characteristics of patients (n = 19).
Technical success was achieved in 16 out of 18 (89%)
patients and selected case examples are shown in Figures 2, 3, and 4. Only partial embolisation could be performed
in two patients due to the proximity of the tumour
feeding arteries to the spinal artery in one patient and
the occurrence of chest pain during the procedure in
another patient. Technical success could not be reliably
evaluated in one patient since no definite tumour
staining was evident on pre-embolisation angiogram
for comparison. The primary embolic agents used
included trisacryl gelatin microspheres (Embosphere;
Merit Medical, Warrington [PA], US) [n = 8], polyvinyl
alcohol (PVA) particles (Contour; Boston Scientific,
Marlborough [MA], US) [n = 7], and PVA hydrogel
microspheres (Bead Block; Terumo Medical, Tokyo,
Japan) [n = 4].
Figure 2. (a) Preoperative
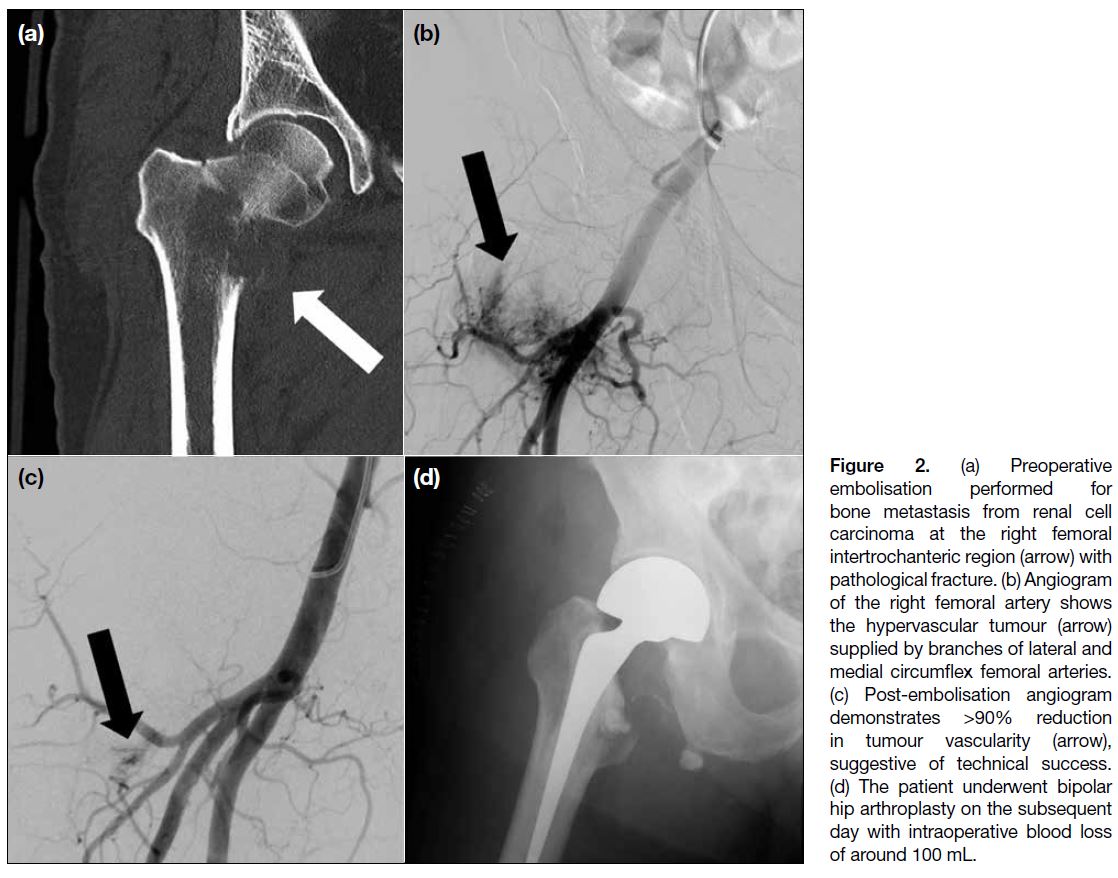
embolisation performed for bone metastasis from renal cell carcinoma at the right femoral intertrochanteric region (arrow) with pathological fracture. (b) Angiogram of the right femoral artery shows the hypervascular tumour (arrow) supplied by branches of lateral and medial circumflex femoral arteries. (c) Post-embolisation angiogram demonstrates >90% reduction in tumour vascularity (arrow), suggestive of technical success. (d) The patient underwent bipolar hip arthroplasty on the subsequent day with intraoperative blood loss of around 100 mL.
Figure 3. Histologically proven spinal metastasis from solitary fibrous tumour undergoing preoperative embolisation. Computed tomography shows the L5 spinal tumour (arrows) with intraspinal (a) and paraspinal (b) extension. Pre-embolisation angiograms confirm the hypervascular tumour (arrows) to be supplied by branches of the left fourth lumbar artery (c) and iliac branch of the left iliolumbar artery (d). Superselective pre-embolisation angiograms by microcatheters advanced into the branches of the left fourth lumbar artery (e) and the branches of the iliac branch of the left iliolumbar artery (f) reveal significant tumour blush (arrows). Post-embolisation angiograms of branches of the left fourth lumbar artery (g) and the iliac branch of the left iliolumbar artery (h) demonstrate absent tumour blush, suggestive of technical success.
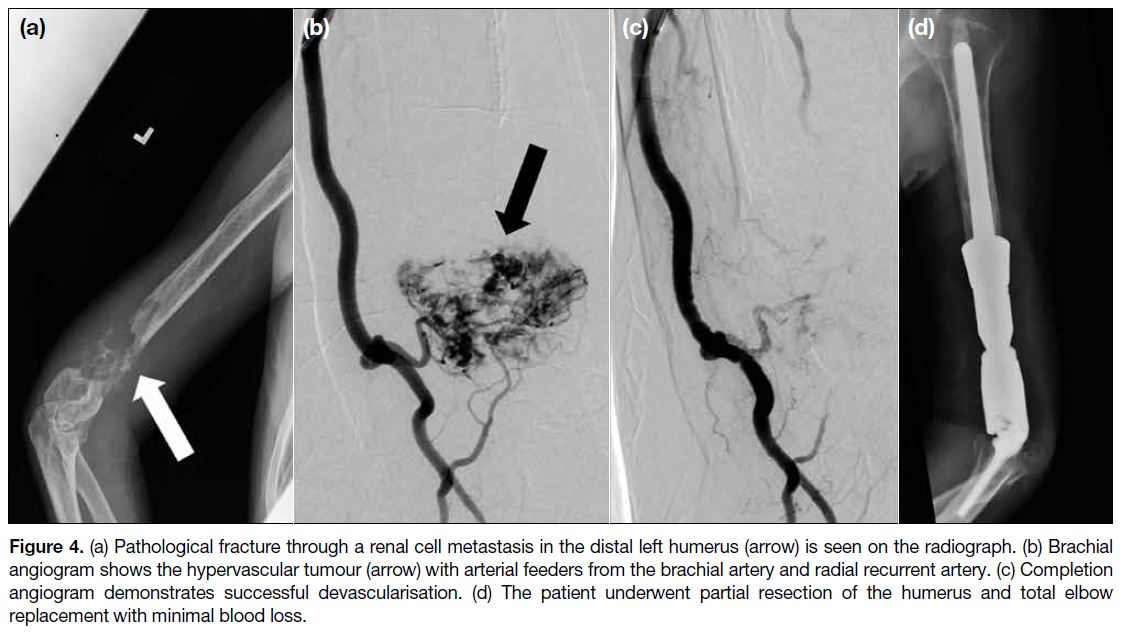
Figure 4. (a) Pathological fracture through a renal cell metastasis in the distal left humerus (arrow) is seen on the radiograph. (b) Brachial
angiogram shows the hypervascular tumour (arrow) with arterial feeders from the brachial artery and radial recurrent artery. (c) Completion
angiogram demonstrates successful devascularisation. (d) The patient underwent partial resection of the humerus and total elbow
replacement with minimal blood loss.
There was no mortality related to embolisation.
Minor complications in the form of post-embolisation
syndrome and pain from ischaemic necrosis of tumours
occurred in six patients (32%) and these were treated
with analgesics and fluid. In patients with embolisation of vertebral tumours, there were no procedure-related
neurological deficits. The median of intraprocedural
blood loss was 700 mL (range, 20-14,000). Two patients
(11%) suffered major haemorrhages requiring massive
intraprocedural blood transfusions. One of them had a
spinal metastasis from renal cell carcinoma with supply
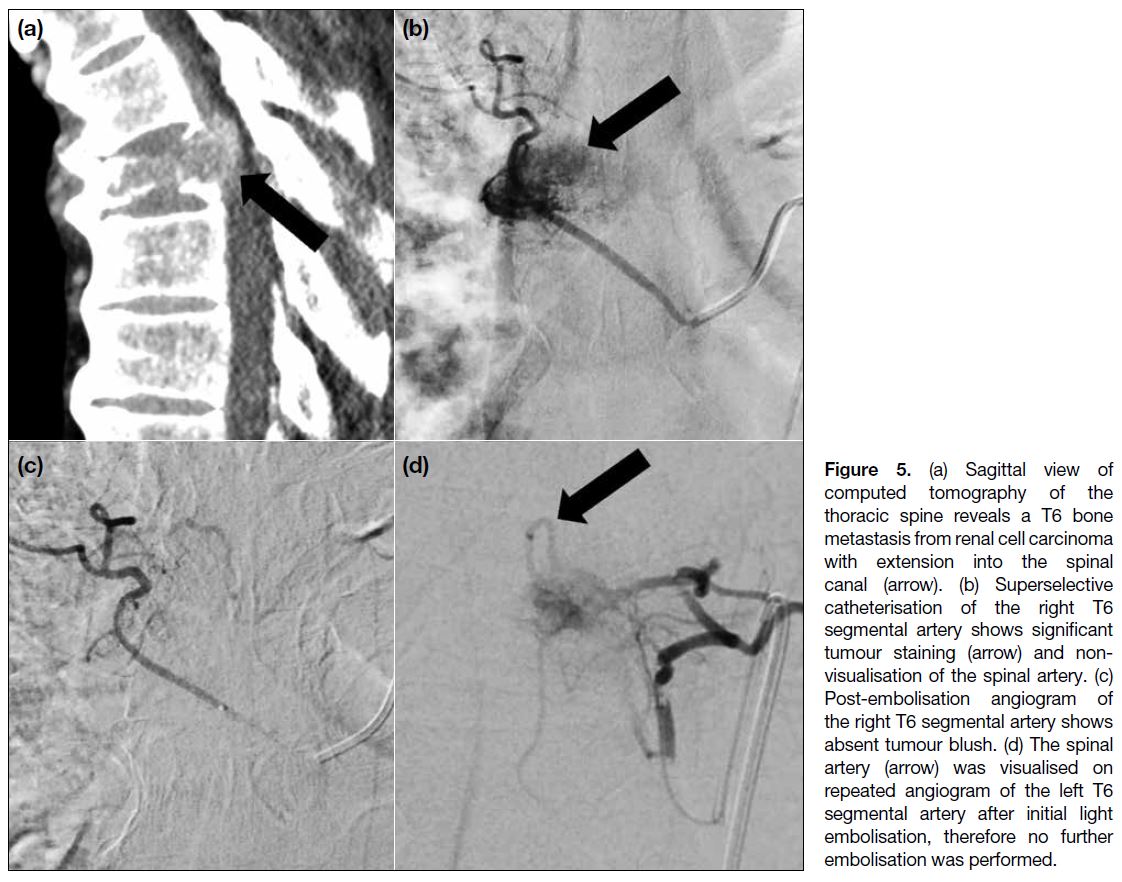
from the bilateral T6 segmental arteries. However,
successful embolisation was only achieved at the right
T6 segmental artery because the spinal artery was
seen in repeated angiograms of the left T6 segmental
artery (Figure 5). To minimise the risk of spinal cord
infarction, the procedure was abandoned after only
light embolisation of the left T6 segmental artery and
the target of technical success could not be achieved.
The patient had significant intraprocedural blood loss
requiring massive transfusion and the transfused blood
volume was around 3 L. Postoperatively, there was
diplegia of the lower limbs, suggestive of spinal cord
injury. Another patient had sacral chordoma with no
definite tumour staining on preoperative angiography.
As a result, technical success of the embolisation
procedure could not be reliably evaluated. Embolisation
was performed pre-emptively in view of the possibility
of massive intraoperative bleeding. Unfortunately, major
intraprocedural haemorrhage was still encountered,
necessitating massive transfusion with transfused blood
volume of around 4 L.
Figure 5. (a) Sagittal view of computed tomography of the thoracic spine reveals a T6 bone metastasis from renal cell carcinoma with extension into the spinal canal (arrow). (b) Superselective catheterisation of the right T6 segmental artery shows significant tumour staining (arrow) and non-visualisation of the spinal artery. (c) Post-embolisation angiogram of the right T6 segmental artery shows absent tumour blush. (d) The spinal artery (arrow) was visualised on repeated angiogram of the left T6 segmental artery after initial light embolisation, therefore no further embolisation was performed.
DISCUSSION
Successful embolisation of bone tumours may potentially decrease intraoperative blood loss and improve
visualisation of the surgical field, thus minimising risks
of major complications and enabling safer and more
complete resection. It is particularly beneficial when
there is a high risk of intraoperative bleeding, spinal
involvement with cord or neural encroachment or in
technically difficult locations with expected prolonged
surgery,[3] such as hypervascular spinal and pelvic bone
metastases. In our case series, the median estimated
intraprocedural blood loss was 700 mL, which was lower
than that reported in other studies.[4] [5]
Apart from its role as an adjuvant therapy to surgery,
embolisation may also be performed as a palliative
treatment for symptomatic relief of bone metastases. It
may be done as a standalone treatment or combined with
ablation or cementoplasty.[6] It has been used successfully
to achieve neurological improvement in patients with
hypervascular vertebral metastases causing acute spinal
cord compression[7] and symptomatic relief in patients
with painful bone metastases from renal cell carcinoma.[8]
There have been studies supporting embolisation as
a primary treatment for benign bone tumours such as
aneurysmal bone cysts and giant cell tumours.[9] [10] [11] It is
particularly beneficial in tumours located in the spine or pelvis, where surgery and radiation are associated
with high rates of morbidity and recurrence. Serial
embolisation of these tumours is usually performed until
there is symptomatic relief or near complete resolution
of tumour vascularity.[10] Radiological response can
also be assessed and it manifests as reduction in
tumour vascularity and increase in ossification. In
patients with vertebral haemangiomas complicated
with spinal cord compression or spinal pain, surgery
or radiotherapy has been the traditional treatment of
choice. However, surgery alone is associated with risk of
significant bleeding from these highly vascular tumours.
Preoperative embolisation has been shown to be a useful
adjunctive therapy to minimise bleeding risk.[12] [13]
Particulate materials, namely PVA particles and
microspheres, are primarily used for embolisation.
PVA is water-soluble synthetic polymer made from
polyvinyl acetate through partial or full hydrolysis to remove the acetate groups, with size ranging from 50
to 1000 μm. It has the ability to penetrate and occlude
the tumour blood supply. It is compressible after drying
and will expand to up to 15 times its compressed size
after rehydration.[14] Most interventional radiologists have
extensive experience in using it and it is relatively easy
to deliver. It is safe without any long-term side-effects.
The conventional preparation (Contour PVA) has
irregular outlines and therefore occludes vessels larger
than its diameter due to aggregation of particles. Some
newer preparations, e.g., Bead Block PVA hydrogel
microspheres, are engineered PVA particles with
relatively uniform size. Their microporous nature also
enables them to be compressible and facilitates delivery
through small catheters. Embosphere microspheres are
trisacryl gelatin microspheres with size ranging from
40 to 1200 μm. Their compressibility allows smooth
passage through microcatheter with a diameter smaller
than its size. They are more uniform in size than PVA and their sizes do not change in liquids. They also have
less tendency to clump after injection. The choice of the
primary particulate embolic agent is mainly determined
by the operator’s experience and preference. There
is currently little published literature comparing the
efficacy of different embolic materials in preoperative
embolisation of bone tumours. A study performed to
assess the intraprocedural blood loss post-embolisation
showed no clinically significant difference between
trisacryl gelatin microspheres and PVA particles.[15]
Liquid embolic agents may induce more tumour necrosis
than particles and be beneficial when definitive treatment
is aimed. Nonetheless, they are technically more difficult
to handle and their use requires an experienced operator.
They are also associated with a higher risk of non-target
embolisation and non-target necrosis compared with
particles.[6] As a general rule, if embolisation is performed
as preoperative or palliative treatment, liquid agents
have little advantage over particulate agents.
Complications of embolisation of bone tumours
include arterial dissection, pain due to ischaemic
necrosis of tumour, non-target embolisation, infection,
haemorrhage, and post-embolisation syndrome.[8] [16]
Post-embolisation syndrome is a common but usually
self-limiting side-effect. Patients present with symptoms
such as pain, fever, and malaise, which could be treated
with analgesics and fluid. Non-target embolisation is
another potential complication. Aside from the use of
microcatheters to reduce its risk, coils may be employed
to embolise and protect the non-target vessels more
proximally, which could not be navigated beyond to get
close to the tumour feeding vessels.[7]
Limitations
There were limitations in our study. First, it was
retrospective in nature and a non-embolisation group
was not available for comparison. With reference to other
studies from the literature, it still offered a reasonable
view of preoperative embolisation as a potentially helpful
procedure in the management of bone tumours. Another
limitation was the heterogeneous study population with
different tumour pathologies and surgeries performed,
but this reflected the diversity of primary and metastatic
bone tumours that could be considered for preoperative
embolisation. Ideally, a prospective randomised
controlled trial with a larger study population would be
optimal for determining the exact value of preoperative
embolisation compared with non-embolisation. Other
factors that could affect intraprocedural blood loss,
including patient factors, and the surgery performed, should also be taken into account.
CONCLUSION
Preoperative embolisation is safe, technically feasible, and potentially useful in the treatment of bone tumours,
although a high risk of intraoperative bleeding should be
taken into consideration.
REFERENCES
1. Kwon JH, Shin JH, Kim JH, Gwon DI, Yoon HK, Ko GY, et al. Preoperative transcatheter arterial embolization of hypervascular metastatic tumors of long bones. Acta Radiol. 2010;51:396-401. Crossref
2. Geraets SE, Bos PK, van der Stok J. Preoperative embolization in surgical treatment of long bone metastasis: a systematic literature review. EFORT Open Rev. 2020;5:17-25. Crossref
3. Barton PP, Waneck RE, Karnel FJ, Ritschl P, Kramer J, Lechner GL. Embolization of bone metastases. J Vasc Interv Radiol. 1996;7:81-8. Crossref
4. Owen RJ. Embolization of musculoskeletal bone tumors. Semin Intervent Radiol. 2010;27:111-23. Crossref
5. Manke C, Bretschneider T, Lenhart M, Strotzer M, Neumann C, Gmeinwieser J, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001;22:997-1003.
6. Kickuth R, Waldherr C, Hoppe H, Bonel HM, Ludwig K, Beck M, et al. Interventional management of hypervascular osseous metastasis: role of embolotherapy before orthopedic tumor resection
and bone stabilization. AJR Am J Roentgenol. 2008;191:W240-7. Crossref
7. Gottfried ON, Schmidt MH, Stevens EA. Embolization of sacral
tumors. Neurosurg Focus. 2003;15:E4. Crossref
8. Munk PL, Legiehn GM. Musculoskeletal interventional radiology:
applications to oncology. Semin Roentgenol. 2007;42:164-74. Crossref
9. Smit JW, Vielvoye GJ, Goslings BM. Embolization for vertebral
metastases of follicular thyroid carcinoma. J Clin Endocrinol Metab.
2000;85:989-94. Crossref
10. Forauer AR, Kent E, Cwikiel W, Esper P, Redman B. Selective
palliative transcatheter embolization of bony metastases from renal
cell carcinoma. Acta Oncol. 2007;46:1012-8. Crossref
11. Lin PP, Guzel VB, Moura MF, Wallace S, Benjamin RS, Weber KL,
et al. Long-term follow-up of patients with giant cell tumor of
the sacrum treated with selective arterial embolization. Cancer.
2002;95:1317-25. Crossref
12. Luther N, Bilsky MH, Härtl R. Giant cell tumor of the spine.
Neurosurg Clin N Am. 2008;19:49-55. Crossref
13. Rossi G, Rimondi E, Bartalena T, Gerardi A, Alberghini M,
Staals EL, et al. Selective arterial embolization of 36 aneurysmal
bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal
Radiol. 2010;39:161-7. Crossref
14. Bandiera S, Gasbarrini A, De Iure F, Cappuccio M, Picci P,
Boriani S. Symptomatic vertebral hemangioma: the treatment of
23 cases and a review of the literature [in English, Italian]. Chir
Organi Mov. 2002;87:1-15.
15. Acosta FL Jr, Dowd CF, Chin C, Tihan T, Ames CP, Weinstein PR. Current treatment strategies and outcomes in the management of symptomatic vertebral hemangiomas. Neurosurgery. 2006;58:287-95. Crossref
16. Sheth RA, Sabir S, Krishnamurthy S, Avery RK, Zhang YS,
Khademhosseini A, et al. Endovascular embolization by
transcatheter delivery of particles: past, present, and future. J Funct
Biomater. 2017;8:12. Crossref