Moderately Hypofractionated Versus Conventionally Fractionated Volumetric Modulated Arc Therapy for Definitive Treatment of Localised Prostate Cancer
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2024 Jun;27(2):e89-99 | Epub 20 May 2024
Moderately Hypofractionated Versus Conventionally Fractionated Volumetric Modulated Arc Therapy for Definitive Treatment of Localised Prostate Cancer
TC Liu, PY Wu, KYC Zheng, HM Hung, K Chan
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr TC Liu, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: ltc265@ha.org.hk
Submitted: 18 July 2023; Accepted: 27 November 2023.
Contributors: TCL, PYW and KC designed the study. TCL and HMH acquired the data. TCL, PYW, KYCZ and KC analysed the data. TCL
drafted the manuscript. TCL, PYW and KC critically revised the manuscript for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: HKECREC 2022-068). The requirement of patient consent was waived by the Committee due to the retrospective nature of the research.
Declaration: This research was presented orally at the 10th Joint Scientific Meeting of The Royal College of Radiologists and Hong Kong College of Radiologists (HKCR) and 31st Annual Scientific Meeting of HKCR (18-19 November 2023, Hong Kong).
Supplementary Material: The supplementary material was provided by the authors and some information may not have been peer reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by the Hong Kong College of Radiologists. The Hong Kong College of Radiologists disclaims all liability and responsibility arising from any reliance placed on the content.
Abstract
Introduction
This retrospective study compares the treatment outcome of a reduced dose with moderate hypofractionation (60 Gray in 20 fractions [60 Gy/20 fr]) with conventionally fractionated (76 Gy/38 fr) volumetric modulated arc therapy for definitive treatment of localised prostate cancer in a public hospital in Hong Kong.
Methods
All patients with low- or intermediate-risk prostate cancer (defined according to the National Comprehensive Cancer Network Guidelines) who received definitive radiotherapy from 1 January 2017 to 30 June 2022 were included.
Results
A total of 105 patients were identified (58 receiving moderate hypofractionation and 47 receiving conventional fractionation). The median follow-up period was 38.3 months. Grade 2 acute gastrointestinal (GI) toxicity was more common with moderate hypofractionation than with conventional fractionation (15.5% vs. 2.1%, 95% confidence interval = 1.03-69.33; p = 0.02). In the moderate hypofractionation cohort, the planning target volume (PTV) in patients who experienced grade ≥2 acute genitourinary (GU) toxicity was significantly higher than those who did not (p = 0.03). None of the patients developed grade ≥3 acute GI toxicity. The incidence of grade 3 acute or late GU and late GI toxicities was rare with both fractionation schedules.
Conclusion
This study shows that moderately hypofractionated radiotherapy is a safe, effective and feasible alternative to conventionally fractionated radiotherapy for low- and intermediate-risk prostate cancer in the Chinese community. Patients should be counselled on the potential increase in low-grade acute GI toxicity with moderate hypofractionation, which is usually self-limited and is not associated with increases in long-term toxicity. Close monitoring for acute GU toxicity in patients with larger PTVs is warranted.
Key Words: Neoplasms; Prostate; Radiotherapy
中文摘要
中等強度大分割及傳統大分割體積調控弧型放射治療作為局部前列腺癌根治性治療方案的比較
廖芷霑、吳宇光、鄭裕誠、孔慶明、陳娟
引言
本回顧性研究旨在比較在香港一所公立醫院進行的已減劑量的中等強度大分割(60 Gy分20次)及傳統大分割(76 Gy分38次)體積調控弧型放射治療作為局部前列腺癌根治性治療方案。
方法
本研究納入在2017年1月1日至2022年6月30日期間接受根治性放射治療的所有低風險及中風險前列腺癌患者(根據美國國家綜合癌症網絡指引定義)。
結果
我們共找到105名患者(58名接受中等強度大分割,47名接受傳統大分割)。隨訪時間中位數為38.3個月。第2級急性腸胃道毒性於中等強度大分割中較常見,在傳統大分割則較少見(15.5%與2.1%,95%置信區間 = 1.03-69.33;p = 0.02)。在中等強度大分割隊列中,有第2級或以上急性泌尿生殖系統毒性的患者的治療計劃靶體積顯著高於沒有相關毒性的患者(p = 0.03)。沒有患者有第3級或以上急性腸胃道毒性。第3級急性或晚期泌尿生殖系統毒性及晚期腸胃道毒性在兩個分割治療計劃中均屬罕見。
結論
本研究顯示對於低風險及中風險前列腺癌華裔患者而言,中等強度大分割放射治療是傳統大分割放射治療的安全、有效且可行的替代方案。患者應獲告知中等強度大分割的低級急性腸胃道毒性有可能增加,而該增加通常具自限性,並與長期毒性增加無關。醫護人員應密切監察治療計劃靶體積較高的患者的急性泌尿生殖系統毒性。
INTRODUCTION
Globally, prostate cancer ranks second in cancer
incidence and fifth in cancer mortality among males;
it has become the most frequently diagnosed cancer in
>100 countries.[1] In Hong Kong, prostate cancer ranks fourth in cancer incidence.[2]
For low- or intermediate-risk prostate cancer, external
beam radiotherapy (EBRT) and radical prostatectomy are
associated with lower incidence of disease progression
and metastasis compared to active surveillance. There
is no difference in 10-year overall survival or disease-free
survival between the two treatments.[3] Radiotherapy has the benefit of sparing patients from surgical and
anaesthetic risks, which is especially relevant for patients
of advanced age or with medical co-morbidities.
Dose escalation in definitive radiotherapy for prostate
cancer increases tumour biological effective dose which
leads to improvement in relapse-free survival.[4] [5] On the
other hand, the location of the prostate near organs at risk
(OARs) such as the rectum and bladder leads to inevitably
heightened gastrointestinal (GI) and genitourinary (GU) toxicities.[4] [5] [6] [7] [8] Recent advances in planning techniques and
image guidance have led to improvement in treatment
precision. Volumetric modulated arc therapy (VMAT)
allows better dose conformation than traditional
conformal EBRT, thus minimising dose to surrounding
OARs. Image guidance strategies such as cone beam
computed tomography (CT), in contrast to traditional
two-dimensional kilovoltage imaging, allows more
accurate definition and verification of targets and pelvic
organs, thereby allowing tighter margins and smaller
treatment volumes.
In the past decade, moderately hypofractionated EBRT
(generally fractional doses of 2.4 Gray [Gy] to 3.4 Gy[9])
has been increasingly adopted to overcome the limitations
of dose escalation in conventional radiotherapy by
exploiting the low alpha/beta ratio of prostate cancer.
The alpha/beta ratio is inversely correlated to the effect of
change in fractional size in normal or malignant tissues.
Most cancers have an alpha/beta ratio of approximately
10, whereas OARs typically have an alpha/beta ratio of
about 3. Prostate cancer, in contrast to other cancers,
has a lower alpha/beta ratio of approximately 1.5.[10] [11] Hypofractionation takes advantage of the low alpha/beta
ratio of prostate cancer relative to surrounding OARs
to enhance biologically equivalent tumour doses while
minimising toxicity to normal tissues.[10] [12] [13] [14] [15] [16]
Multiple studies have shown the comparable efficacy
of moderate hypofractionation to conventional
fractionation schedules.[17] [18] [19] [20] [21] [22] The guidelines published
by ASTRO/ASCO/AUA (the American Society for
Radiation Oncology, the American Society of Clinical
Oncology, and the American Urological Association) in
2018 recommended moderately hypofractionated EBRT
over conventional schedules, especially when nodal
irradiation was not required.[9] One of the most widely
adopted regimens in clinical practice is 60 Gy in 20 daily
fractions (60 Gy/20 fr).
The most relevant toxicities in prostate cancer
radiotherapy include GU and GI toxicities as well as sexual
dysfunction due to the target’s proximity to the bladder,
rectum, small bowel, and penile bulb. Most prospective
clinical trials had demonstrated slightly increased acute
GI toxicity in moderate hypofractionation compared to
conventional schedules. Some trials had shown increases
in late GU and GI side-effects (mostly of low grade),
while others showed no significant differences. Overall,
in all trials, there was no significant safety concern with
moderately hypofractionated EBRT.[18] [19] [20] [23] [24] [25]
The patient population of most large-scale prospective
clinical trials has consisted mainly of Caucasians.
So far, there are relatively scarce data reporting on
the clinical utility, safety, and efficacy of moderately
hypofractionated EBRT in Chinese populations.[26] [27]
Furthermore, most randomised trials did not necessitate
the use of modern radiotherapy techniques such as
VMAT,[17] [18] [22] nor the mode or intensity of image
verification.[17] [18] [19] [22]
Moderately hypofractionated VMAT for definitive
treatment of low- and intermediate-risk prostate cancer
was introduced in the Department of Clinical Oncology
of Pamela Youde Nethersole Eastern Hospital in Hong
Kong in January 2017. Since then, both moderate
hypofractionation and conventional fractionation can
be used for low- and intermediate-risk prostate cancer
graded according to the National Comprehensive
Cancer Network (NCCN) Guidelines at the clinician’s
discretion, although the former has been more commonly
prescribed in recent years. In this study, we report
our 6-year institutional experience in both treatment strategies, which provides real-world data on the toxicity
and early treatment outcomes using modern EBRT
technique in the local Chinese community under a public
hospital setting. This is particularly relevant considering
that moderate hypofractionation for prostate cancer has
not been universally adopted in Hong Kong.
METHODS
Patients
This study included 105 consecutive patients with NCCN
low- or intermediate-risk localised prostate cancer who
received moderately hypofractionated or conventionally
fractionated VMAT as definitive treatment from 1
January 2017 to 30 June 2022 at the Department of
Clinical Oncology of Pamela Youde Nethersole Eastern
Hospital. Patients had histologically confirmed prostate
carcinoma, with clinical tumour (T) stage ≥2 disease
by clinical and multiparametric magnetic resonance
imaging staging, as well as a pretreatment prostate-specific
antigen (PSA) level of ≤20 ng/mL and a Gleason
score of ≤7. A bone scan or 68Gallium–prostate-specific
membrane antigen–HBED-CC positron emission
tomography–CT was used for staging when clinically
indicated.
Treatment Method
Moderate Hypofractionation
For moderate hypofractionation, EBRT consisted of
inversely planned VMAT of 60 Gy/20 fr, administering
5 fractions per week. Patients were simulated and treated
in the supine position on a flat tabletop in a customised
vacuum bag (or alpha cradle). They were instructed to
maintain a comfortably full bladder and empty rectum
(using micro-enema). Non-contrast CT images of the
pelvis with slice thickness of 3 mm were acquired
and fused with diagnostic multiparametric magnetic
resonance imaging images for radiotherapy planning.
Perirectal spacer was not employed in all cases.
The clinical target volume (CTV) was the whole prostate
and proximal 1 cm of seminal vesicles for low- and
favourable intermediate-risk disease, while the seminal
vesicles were included in their entirety for unfavourable
intermediate-risk disease. The planning target volume
(PTV) was an 8-mm circumferential expansion from
the CTV, except 5 mm posteriorly (towards the rectum).
Online verification with cone beam CT was performed
before each treatment fraction, complemented by the
6 degrees-of-freedom treatment couch for corrections.
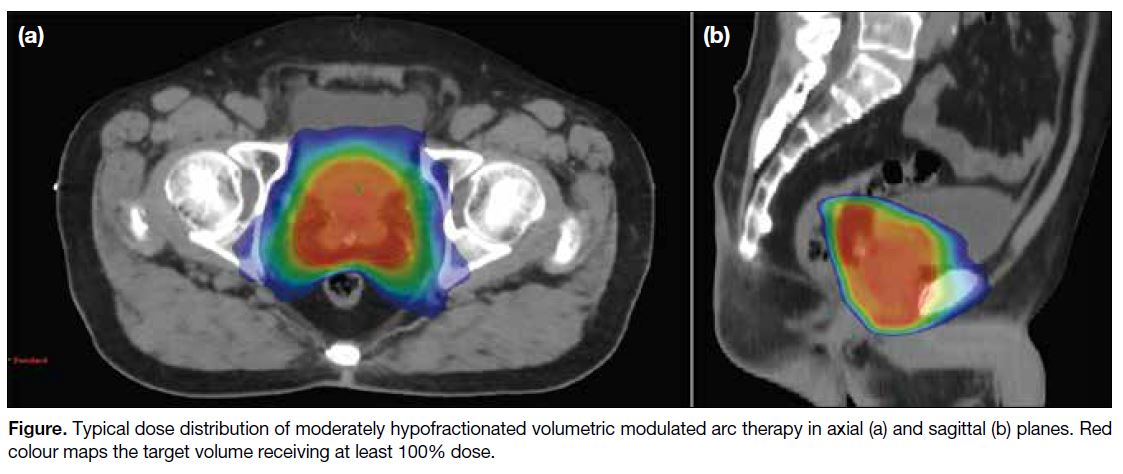
Typical dose distribution of moderately hypofractionated
VMAT is illustrated in the Figure.
Figure. Typical dose distribution of moderately hypofractionated volumetric modulated arc therapy in axial (a) and sagittal (b) planes. Red colour maps the target volume receiving at least 100% dose.
Conventional Fractionation
For conventional fractionation, EBRT consisted of
inversely planned VMAT of 76 Gy/38 fr, administering
5 fractions per week. Patients were simulated and
treated in the same setup as that used for moderate
hypofractionation.
The CTV was the same as that of moderate
hypofractionation, i.e., the whole prostate and proximal
1 cm of seminal vesicles for low- and favourable
intermediate-risk disease, while the seminal vesicles were
included in their entirety for unfavourable intermediate-risk
disease. The PTV was a 10-mm circumferential
expansion from the CTV, except 5 mm posteriorly
(towards the rectum). Online verification consisted
of daily on-board orthogonal kilovoltage imaging,
and then with cone beam CT before the first three
treatment fractions weekly. Planning objectives and dose
constraints to OARs followed the standard institutional
protocol (online supplementary Table). Normal organ
and target dosimetric priorities were rectum and bladder
and PTV coverage.
Neoadjuvant-concurrent luteinising hormone-releasing
hormone analogue for 6 months was permitted for
intermediate-risk patients in both treatment groups with
adverse risk features. If given, this was initiated 3 months
prior to radiotherapy.
Follow-up
Clinical assessment was performed at least twice weekly
during, at the end of, and 2 weeks after radiotherapy treatment, followed by every 3 to 6 months in the first 5
years, and annually thereafter. Post-treatment PSA level
was checked at least half-yearly after radiotherapy.
Assessment
The acute and late GI and GU toxicities arising from
radiotherapy were scored according to the National
Cancer Institute’s CTCAE (Common Terminology
Criteria for Adverse Events) version 5.0.[28] Acute
treatment toxicities in this study were defined as events
occurring within 18 weeks from the start of radiotherapy.
Late toxicities were those appearing >18 weeks from the
start of radiotherapy.
Biochemical failure was defined by the Phoenix criteria
(rise of PSA level of ≥2 ng/mL above the nadir PSA
level).[29] Clinical recurrence was defined by any clinical
or radiological evidence of disease recurrence at local,
regional, or distant sites.
Endpoints and Statistical Analyses
The primary endpoint was the development of
radiotherapy-related toxicity. Time-to-event was defined
from the first fraction of radiotherapy to the appearance
of treatment toxicity at follow-up. The data cut-off was
30 June 2023. Patients were censored at death or at data
cut-off, whichever occurred first.
The influence of clinicopathological characteristics and radiotherapy-related parameters on radiotherapy-related
toxicities were analysed. For acute toxicities, continuous
variables were analysed by logistic regression; categorical variables were analysed by Pearson’s Chi
squared test and Fisher’s exact test. For late toxicities,
continuous variables were analysed by univariate Cox
regression; categorical variables were analysed by log-rank test.
A p value of < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted
using SPSS (Windows version 26.0; IBM Corp, Armonk
[NY], United States).
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist was implemented in the preparation of the manuscript.
RESULTS
Patient Characteristics
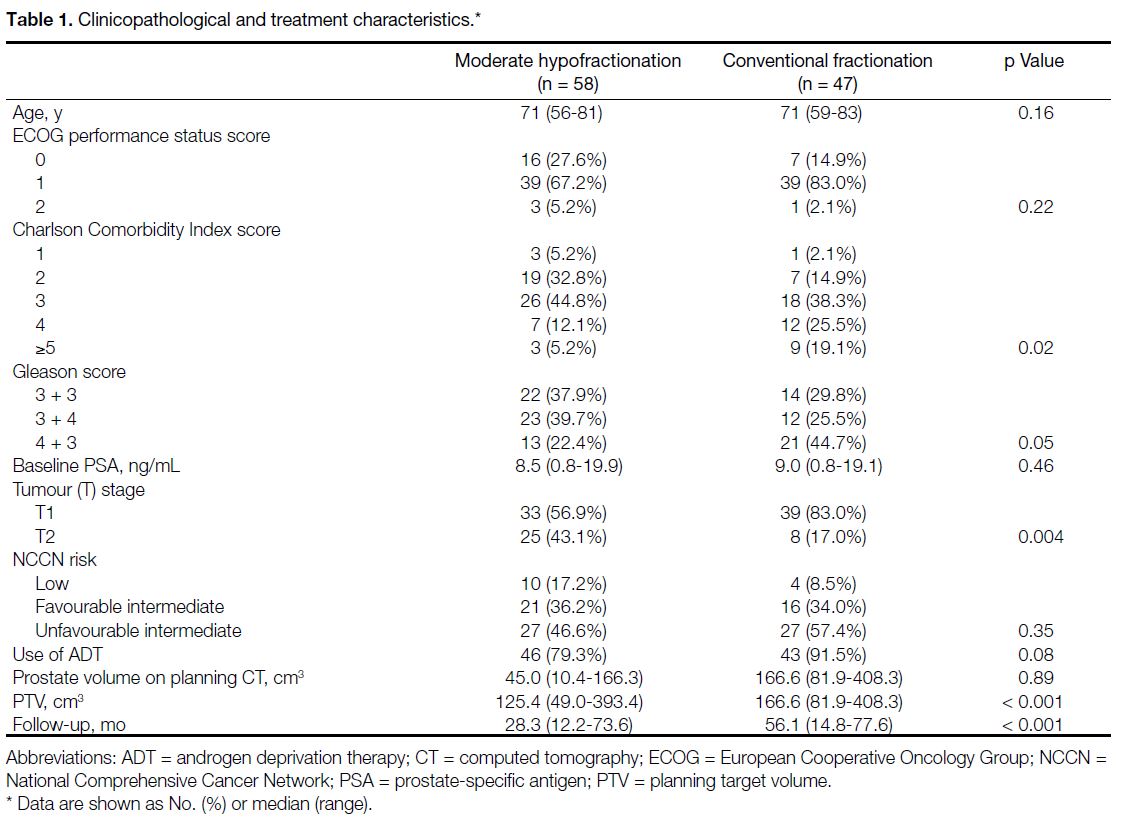
Of the 105 patients identified, 58 had undergone moderate hypofractionation and 47 had undergone conventional
fractionation. Table 1 shows the clinicopathological and
treatment characteristics of both groups.
Table 1. Clinicopathological and treatment characteristics.
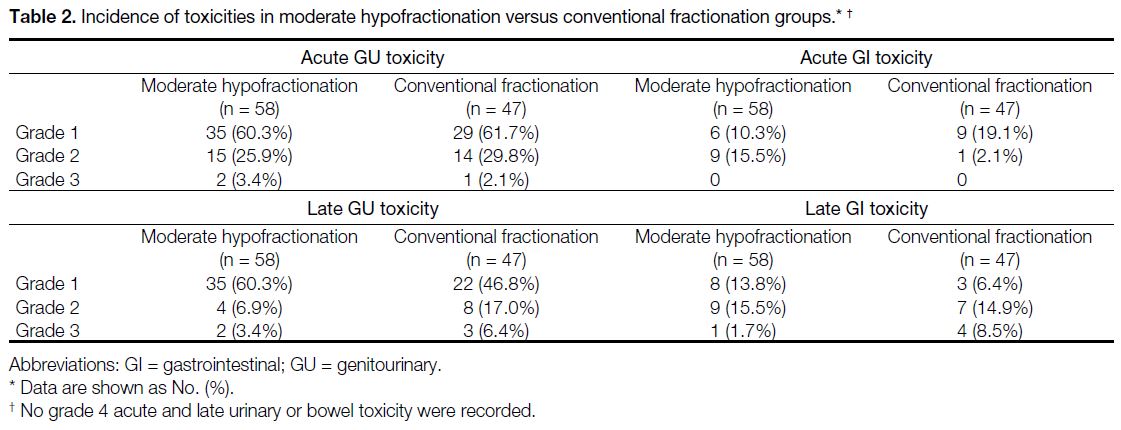
Clinical Manifestations of Acute Genitourinary Toxicity
Among patients with any grade acute GU toxicities,
the most frequently reported symptoms were urinary
frequency (65.4% in the moderate hypofractionation
group and 52.3% in the conventional fractionation
group), followed by nocturia (13.5% in the moderate
hypofractionation group and 25.0% in the conventional
fractionation group) and dysuria (7.7% in the moderate
hypofractionation group and 9.1% in the conventional
fractionation group). Two patients in the moderate
hypofractionation group had grade 3 acute GU toxicity,
one presented as haematuria and another one as acute
urinary retention. One patient in the conventional
fractionation group had grade 3 acute GU toxicity, which
presented as acute urinary retention (Table 2).
Table 2. Incidence of toxicities in moderate hypofractionation versus conventional fractionation groups.
Clinical Manifestations of Acute
Gastrointestinal Toxicity
Among patients with any grade acute GI toxicities, the
most reported symptoms were diarrhoea (75.0% in the moderate hypofractionation group and 80.0% in the
conventional fractionation group), followed by rectal
bleeding (16.7% in the moderate hypofractionation group
and 10.0% in the conventional fractionation group).
Clinical Manifestations of Late
Genitourinary Toxicity
Among patients with any grade late GU toxicities,
the most reported symptoms in the moderate
hypofractionation group were nocturia (43.9%),
followed by urinary frequency (29.3%) and incontinence
(9.8%); the most reported symptoms in the conventional
fractionation group were nocturia (51.5%), followed
by urinary frequency (24.2%), haematuria (15.2%),
and incontinence (3.0%). Two patients in the moderate
hypofractionation group had grade 3 late GU toxicity,
both present as haemorrhagic cystitis. Three patients in
the conventional fractionation group had grade 3 late GU
toxicity, two presented as haemorrhagic cystitis and one
presented as urethral stricture (Table 2).
The median time to develop grade ≥2 late GU toxicity
was 32.6 months for the moderate hypofractionation
group and 26.4 months for the conventional fractionation
group. There was no statistically significant difference
between the two groups in time for occurrence of grade
≥2 late GU toxicity (p = 0.48).
Clinical Manifestations of Late
Gastrointestinal Toxicity
Among patients with any grade late GI toxicities, the
most reported late GI toxicity was predominantly
proctitis (88.9% in the moderate hypofractionation group
and 100% in the conventional fractionation group). No faecal incontinence was reported. The incidence of grade
≥2 late GI toxicity was 17.2% in the hypofractionation
group and 23.4% in the conventional fractionation group.
One patient in the moderate hypofractionation group and
four patients in the conventional fractionation group had
grade 3 GI toxicity, all presented as proctitis (Table 2).
The median time to grade ≥2 late GI toxicity was 20.1
months for the moderate hypofractionation group and
22.4 months for the conventional fractionation group.
There was no statistically significant difference between
the two groups in time for occurrence of grade ≥2 late GI
toxicity (p = 0.48).
Erectile Dysfunction
The incidence of any grade erectile dysfunction was
6.9% in the moderate hypofractionation group and
10.6% in the conventional fractionation group. The
median time to develop erectile dysfunction was 13.1
months in the moderate hypofractionation group and
6.5 months in the conventional fractionation group.
There was no statistically significant difference between
the two groups in the probability of developing erectile
dysfunction (p = 0.17).
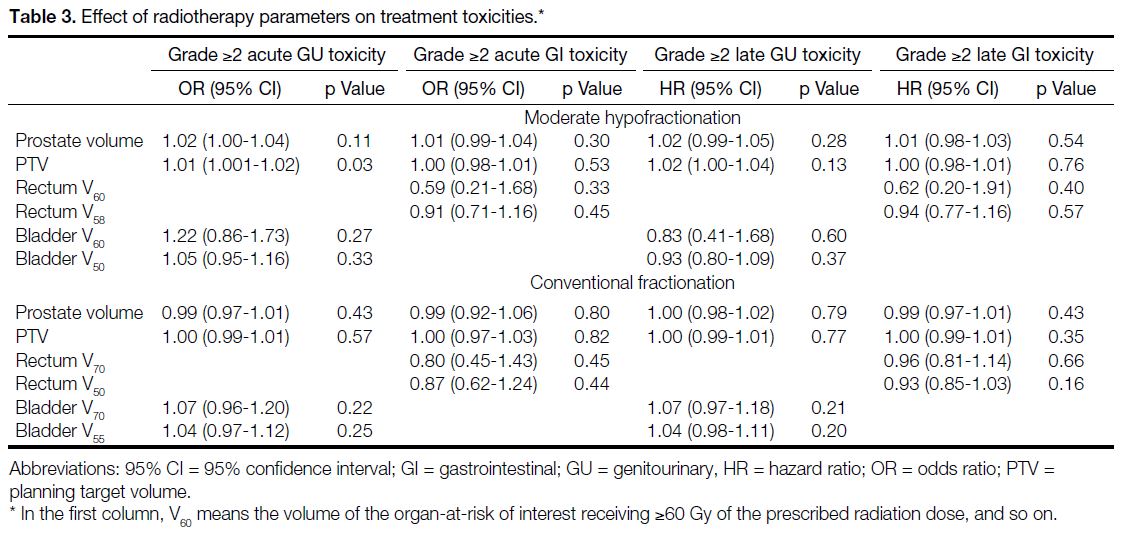
Effect of Radiotherapy Parameters on Radiotherapy Toxicities
Table 3 shows the effect of radiotherapy parameters
on radiotherapy toxicities. The PTV in patients who
experienced grade ≥2 acute GU toxicity is significantly
higher those who did not (p = 0.03). No significant clinical
or treatment parameters were found to be predictive
of other toxicity endpoints for the two fractionation
schedules.
Table 3. Effect of radiotherapy parameters on treatment toxicities.
Biochemical Failure and Clinical Recurrence
There was one biochemical failure with clinical
recurrence in the conventional fractionation arm and
none in the moderate hypofractionation arm.
DISCUSSION
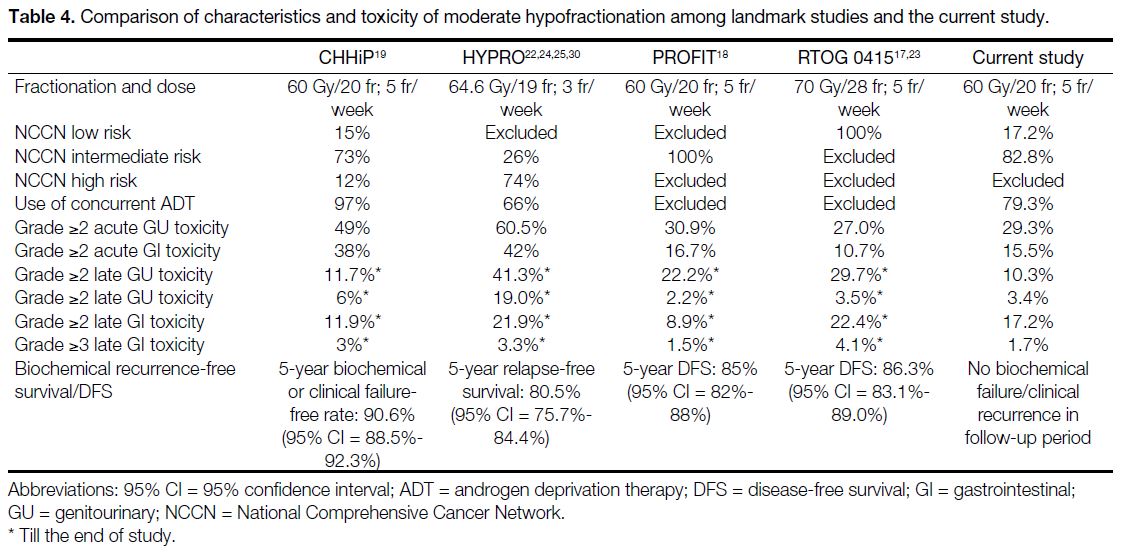
Landmark trials including CHHiP,[19] HYPRO
(HYpofractionated irradiation for PROstate
cancer),[22] [24] [25] [30] PROFIT (Prostate Fractionated
Irradiation Trial),[18] and RTOG 0415[17] [23] have shown that moderately hypofractionated radiotherapy to prostate is
as effective as conventional fractionation. However, the
data on toxicities were less consistent (Table 4).
Table 4. Comparison of characteristics and toxicity of moderate hypofractionation among landmark studies and the current study.
Our real-world data showed that the increased acute GI
toxicity from moderately hypofractionated radiotherapy
was limited to grade 2 and did not lead to increase in
long-term toxicity. As recommended by the ASTRO/ASCO/AUA 2018 guideline, patients should be counselled on the small increased risk of acute GI toxicity with moderate hypofractionation.[9] Both the
CHHiP[19] and PROFIT[18] trials showed lower rates of late
GI toxicities with moderately hypofractionated EBRT.
In contrast, the late GI toxicity rate was higher with
moderate hypofractionation in the RTOG 0415 trial.[17]
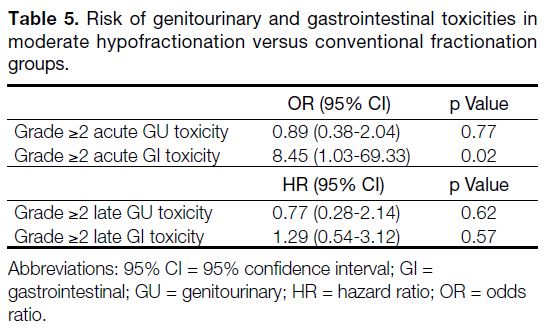
In our study, no difference was detected between the
fractionation schedules for grade ≥2 late GI toxicity (p = 0.57) [Table 5]. For the incidence of grade 3 late GU and
GI toxicities in the moderate hypofractionation cohorts,
the rates were low in the current study (Table 2).
Table 5. Risk of genitourinary and gastrointestinal toxicities in
moderate hypofractionation versus conventional fractionation
groups
In general, the incidence of radiotherapy-related toxicities
in the current study was lower than that reported in the
landmark trials on moderate hypofractionation. There
may be several reasons. First, the lower late toxicities may
be attributed to shorter follow-up time. The landmark
trials had a median follow-up of at least 60 months (62.4
months in CHHiP,[19] 60 months in HYPRO,[22] 72 months
in PROFIT,[18] and 70 months in RTOG 0415[17] [23]), while
the median follow-up time in the current study was 38.3
months (range, 12.2-77.6). We expect more mature
late toxicity data with longer follow-up. The long-term
radiotherapy-related toxicities may not be fully reflected;
nevertheless, it is worth noting that in the landmark
prospective trials, the majority of grade ≥2 late toxicity
occurred within the first 2 years of follow-up. Second,
we adopted inversely planned VMAT with stringent
planning aims and dose constraints for OARs, together
with daily cone beam CT verification for moderate
hypofractionation. Most of the landmark prospective
trials did not mandate the use of intensity-modulated
radiotherapy or VMAT,[17] [18] [22] nor did they require the
technique or intensity of image verification.[17] [18] [19] [22] In our
study, modern dose planning with VMAT technique and
intensive image guidance allowed tight PTV margins
and more precise treatment delivery, which could be a
contributing factor to the lower incidence of treatment-related
toxicities. Third, due to the retrospective nature
of this study that reflects on real-world clinical practice, meticulous and frequent documentation of toxicity was
difficult. Our reporting on toxicities was limited by
inter-clinician variation in toxicity charting (especially
for low-grade events), and lack of formal reporting of
patient-reported outcome. In addition, there may be
cultural variations in toxicity reporting by patients,
especially sexual dysfunction, which is often considered
a sensitive topic and often underrepresented in the local
Chinese population.
The PTV in patients who experienced grade ≥2 acute
GU toxicity is significantly higher those who did not (p = 0.03). Interestingly, bladder V60 and V50, which reflect
the bladder volume receiving high doses (≥60 Gy and
≥50 Gy, respectively), were not significant predictors
of acute GU toxicity (Table 3). This may be due to
interfractional variation in bladder filling during the
course of radiotherapy, which may result in variation
between planned and actual bladder doses. Nevertheless,
in real life practice, it would be helpful to offer close
monitoring of acute GU toxicities for patients with larger
PTVs.
There was only one biochemical failure in the
conventional fractionation arm and none in the moderate
hypofractionation arm. The relatively lower incidence
of biochemical or clinical failure in our local cohort
compared to other landmark trials may be explained
by shorter follow-up duration, differences in patient
selection, and use of androgen deprivation therapy
(ADT). As discussed above, the landmark trials had a
median follow-up of at least 60 months, in contrast to
38.3 months in the current study, making it difficult
to conclude on long-term disease control based on the
current results. Furthermore, in this study, all patients
were classified into low- or intermediate-risk categories
according to the NCCN Guidelines. The presence of
high-risk patients in CHHiP[19] and HYPRO[22] trials may
be a reason for the higher biochemical or clinical failure
rate in these trials. In PROFIT[18] and RTOG 0415 trials,[17]
the use of ADT was not allowed. On the other hand,
79.3% patients in the hypofractionation cohort received
ADT in the current study, which likely contributed to
better biochemical control (Table 4).
We have observed a gradual but significant shift in
practice from conventional fractionation to moderate
hypofractionation over the years. Among patients
treated between 2017 and 2019, adoption of moderate
hypofractionation was 24.1%. The rate rose to 80.9%
in 2020 to 2022. This reflects the evolution in treatment paradigms with increasing clinical data and local
experience to support moderate hypofractionation.
Limitations
Other limitations of our current study include
imbalance in baseline characteristics including Charlson
Comorbidity Index score, tumour stage, PTV, and
median follow-up time, which may be confounders on
toxicity outcomes. This was due to the intrinsic nature
of a retrospective study. The difference in follow-up
duration between the two patient cohorts reflects real-world
gradual adoption of moderate hypofractionation
and growth in local experience in this technique. Despite
the above limitations, it is worth noting that the study
cohort represents local real-world data of all consecutive
patients treated in the same institution over 5 years,
using contemporary radiotherapy planning and intensive
image guidance, for which similar reports in Chinese
patients are scarce.
Future Directions
Further dose escalation with an intraprostatic boost may improve disease control. The FLAME trial (Fluoxetine
for motor recovery after acute ischaemic stroke)
demonstrated superior biochemical disease-free survival
with a focal 95-Gy boost to macroscopic tumour whilst
toxicities and quality of life were not compromised.[31]
The ongoing multicentre phase III PIVOTALboost trial
may offer phase III data on dose escalation to the prostate
(using brachytherapy or EBRT) on top of moderately
hypofractionated EBRT in high-intermediate to high-risk patients.[32]
In recent years, there has been growing interest in
ultra-hypofractionated EBRT for definitive treatment
of prostate cancer (using ≥5 Gy per fraction) to further
exploit the biological advantage of its low alpha/beta
ratio. The HYPO trial showed comparable 5-year failure-free
survival and late toxicities but increased acute GU
and GI toxicities for ultra-hypofractionation compared to
conventional schedules.[33] The 2-year toxicity data of the
PACE-B trial[34] [35] and early toxicity data of HEAT (The
Helicobacter Eradication Aspirin Trial)[36] had not shown
significant safety concerns with ultra-hypofractionated
radiotherapy. The long-term data of these studies are
eagerly awaited. Considering the encouraging results
so far, the ASTRO/ASCO/AUA 2018 guidelines
conditionally recommended that ultra-hypofractionated
radiotherapy may be offered for low- and intermediate-risk
prostate cancer but strongly encouraged treatment
of intermediate-risk patients in a clinical trial or multi-institutional multiinstitutional
registry.[9] It is noteworthy that stereotactic
ablative radiotherapy demands high precision in setup,
planning, dosimetry, verification, and quality assurance.
The availability of biodegradable spacers placed between
the rectum and prostate has been reported to reduce the
volume of rectum irradiated and thus further mitigates GI
toxicity. Both hydrogel and hyaluronic acid spacers have
been demonstrated in phase III clinical trials to improve
rectal sparing and reduce GI toxicity.[37] [38] As moderately
hypofractionated EBRT has been reported to result
in more acute GI toxicity compared to conventional
schedules, use of perirectal spacers may play a role in
improving the therapeutic window in suitable patients.
The effect of high dose volumes (i.e., the volume
receiving high dose) of rectum and bladder on
radiotherapy-related toxicities was reported in this
study. These OAR parameters were chosen due to
the more established dose-response relationship with
radiotherapy-related toxicities and reflected our local
OAR constraints. Low dose volumes to OARs are
potential predictors of low-grade toxicities, and it will
be a meaningful future research direction to explore the
dose-response relationship between low dose volumes to
OARs (e.g., V20) and radiotherapy-related toxicities.
CONCLUSION
A major advantage of moderate hypofractionation is
the reduction of ≥40% treatment visits, translating to
improved patient convenience, alleviation of clinical
manpower pressures, and demands on healthcare
resources. With the demanding workload in the healthcare
system, moderately hypofractionated radiotherapy is
considered a cost-effective treatment strategy.[39]
Local institutional outcomes suggested that image-guided
moderately hypofractionated radiotherapy
using VMAT technique is a safe, effective, and feasible
alternative to conventionally fractionated radiotherapy
for low- and intermediate-risk prostate cancer in the
Chinese community in a public hospital setting. Patients
should be counselled on the potential increase in acute
GI toxicity that is likely to be low-grade. It is encouraged
to take note of the PTV during radiotherapy planning and
to offer close monitoring for acute toxicities.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I,
Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49. Crossref
2. Hospital Authority, Hong Kong SAR Government. Overview of Hong Kong Cancer Statistics of 2020. 2022. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202020.pdf. Accessed 1 Jun 2023.
3. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery,
or radiotherapy for localized prostate cancer. N Engl J Med.
2016;375:1415-24. Crossref
4. Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW,
Bahary JP, et al. Effect of standard vs dose-escalated radiation
therapy for patients with intermediate-risk prostate cancer: the
NRG Oncology RTOG 0126 randomized clinical trial. JAMA
Oncol. 2018;4:e180039. Crossref
5. Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA,
Graham JD, et al. Escalated-dose versus control-dose conformal
radiotherapy for prostate cancer: long-term results from the MRC
RT01 randomised controlled trial. Lancet Oncol. 2014;15:464-73. Crossref
6. Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D,
Cowan RA, et al. Escalated-dose versus standard-dose conformal
radiotherapy in prostate cancer: first results from the MRC RT01
randomised controlled trial. Lancet Oncol. 2007;8:475-87. Crossref
7. Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot
A, Dielwart MF, et al. Dose-response in radiotherapy for localized
prostate cancer: results of the Dutch multicenter randomized phase
III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol.
2006;24:1990-6. Crossref
8. Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097-105. Crossref
9. Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol. 2018;36:JCO1801097. Crossref
10. Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021-31. Crossref
11. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095-101. Crossref
12. Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors
show high sensitivity to fractionation (low alpha/beta ratio), similar
to late-responding normal tissue. Int J Radiat Oncol Biol Phys.
2002;52:6-13. Crossref
13. Khoo VS, Dearnaley DP. Question of dose, fractionation and
technique: ingredients for testing hypofractionation in prostate
cancer—the CHHiP trial. Clin Oncol (R Coll Radiol). 2008;20:12-4. Crossref
14. Proust-Lima C, Taylor JM, Sécher S, Sandler H, Kestin L,
Pickles T, et al. Confirmation of a low α/β ratio for prostate
cancer treated by external beam radiation therapy alone using a
post-treatment repeated-measures model for PSA dynamics. Int J
Radiat Oncol Biol Phys. 2011;79:195-201. Crossref
15. Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys. 2013;85:89-94. Crossref
16. Zaorsky NG, Palmer JD, Hurwitz MD, Keith SW, Dicker AP,
Den RB. What is the ideal radiotherapy dose to treat prostate
cancer? A meta-analysis of biologically equivalent dose escalation.
Radiother Oncol. 2015;115:295-300. Crossref
17. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325-32. Crossref
18. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PW, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884-90. Crossref
19. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D,
et al. Conventional versus hypofractionated high-dose intensity-modulated
radiotherapy for prostate cancer: 5-year outcomes of the
randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol.
2016;17:1047-60. Crossref
20. Hickey BE, James ML, Daly T, Soh FY, Jeffery M. Hypofractionation for clinically localized prostate cancer. Cochrane Database Syst Rev. 2019;9:CD011462. Crossref
21. Avkshtol V, Ruth KJ, Ross EA, Hallman MA, Greenberg RE,
Price RA Jr, et al. Ten-year update of a randomized, prospective
trial of conventional fractionated versus moderate hypofractionated
radiation therapy for localized prostate cancer. J Clin Oncol.
2020;38:1676-84. Crossref
22. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E,
Krol S, et al. Hypofractionated versus conventionally fractionated
radiotherapy for patients with localised prostate cancer (HYPRO):
final efficacy results from a randomised, multicentre, open-label,
phase 3 trial. Lancet Oncol. 2016;17:1061-9. Crossref
23. Bruner DW, Pugh SL, Lee WR, Hall WA, Dignam JJ, Low D, et al.
Quality of life in patients with low-risk prostate cancer treated
with hypofractionated vs conventional radiotherapy: a phase 3
randomized clinical trial. JAMA Oncol. 2019;5:664-70. Crossref
24. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H,
et al. Hypofractionated versus conventionally fractionated
radiotherapy for patients with prostate cancer (HYPRO): late
toxicity results from a randomised, non-inferiority, phase 3 trial.
Lancet Oncol. 2016;17:464-74. Crossref
25. Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP,
et al. Hypofractionated versus conventionally fractionated
radiotherapy for patients with prostate cancer (HYPRO): acute
toxicity results from a randomised non-inferiority phase 3 trial.
Lancet Oncol. 2015;16:274-83. Crossref
26. Zhong QZ, Xia X, Gao H, Xu YG, Zhao T, Wu QH, et al.
Hypofractionated versus conventionally fractionated image-guided
volumetric-modulated arc radiotherapy for localized prostate
cancer: a phase II randomized trial from China. Aging (Albany
NY). 2021;13:6936-44. Crossref
27. Yao L, Shou J, Wang S, Song Y, Fang H, Lu N, et al. Long-term
outcomes of moderately hypofractionated radiotherapy (67.5 Gy
in 25 fractions) for prostate cancer confined to the pelvis: a single
center retrospective analysis. Radiat Oncol. 2020;15:231. Crossref
28. United States Department of Health and Human Services. Common
Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
2017. Available from: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed 1 Jun 2023.
29. Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU,
Sokol GH, et al. Defining biochemical failure following
radiotherapy with or without hormonal therapy in men with
clinically localized prostate cancer: recommendations of the RTOGASTRO
Phoenix Consensus Conference. Int J Radiat Oncol Biol
Phys. 2006;65:965-74. Crossref
30. Wortel RC, Pos FJ, Heemsbergen WD, Incrocci L. Sexual
function after hypofractionated versus conventionally fractionated
radiotherapy for prostate cancer: results from the randomized phase
III HYPRO trial. J Sex Med. 2016;13:1695-703. Crossref
31. Kerkmeijer LG, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal boost to the intraprostatic tumor in
external beam radiotherapy for patients with localized prostate
cancer: results from the FLAME randomized phase III trial. J Clin
Oncol. 2021;39:787-96. Crossref
32. Syndikus I, Cruickshank C, Staffurth J, Tree A, Henry A,
Naismith O, et al. PIVOTALboost: a phase III randomised
controlled trial of prostate and pelvis versus prostate alone
radiotherapy with or without prostate boost (CRUK/16/018). Clin
Transl Radiat Oncol. 2020;25:22-8. Crossref
33. Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C,
Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus
conventionally fractionated radiotherapy for prostate cancer: 5-year
outcomes of the HYPO-RT-PC randomised, non-inferiority, phase
3 trial. Lancet. 2019;394:385-95. Crossref
34. Brand DH, Tree AC, Ostler P, van der Voet H, Loblaw A, Chu W, et al.
Intensity-modulated fractionated radiotherapy versus stereotactic
body radiotherapy for prostate cancer (PACE-B): acute toxicity
findings from an international, randomised, open-label, phase 3,
non-inferiority trial. Lancet Oncol. 2019;20:1531-43. Crossref
35. Tree AC, Ostler P, van der Voet H, Chu W, Loblaw A, Ford D, et al. Intensity-modulated radiotherapy versus stereotactic body
radiotherapy for prostate cancer (PACE-B): 2-year toxicity results
from an open-label, randomised, phase 3, non-inferiority trial.
Lancet Oncol. 2022;23:1308-20. Crossref
36. Abramowitz MC, Kwon D, Freeman DE, Dogan N, Eade T,
Punnen S, et al. Early toxicity and patient reported outcomes from
a radiation hypofractionation randomized trial of extended vs
accelerated therapy for prostate cancer (HEAT). Int J Radiat Oncol
Biol Phys. 2018;102:e98-9. Crossref
37. Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R,
et al. Continued benefit to rectal separation for prostate radiation
therapy: final results of a phase III trial. Int J Radiat Oncol Biol
Phys. 2017;97:976-85. Crossref
38. Mariados NF, Orio PF 3rd, Schiffman Z, Van TJ, Engelman A,
Nurani R, et al. Hyaluronic acid spacer for hypofractionated
prostate radiation therapy: a randomized clinical trial. JAMA
Oncol. 2023;9:511-8. Crossref
39. Kraus RD, Weil CR, Abdel-Wahab M. Benefits of adopting hypofractionated radiotherapy as a standard of care in low-and middle-income countries. JCO Glob Oncol. 2022;8:e2200215. Crossref