Analysis of Discordant Histologically Benign Breast Lesions and Predictive Factors Associated with True Discordance on Imaging
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2024 Jun;27(2):e80-8 | Epub 17 May 2024
Analysis of Discordant Histologically Benign Breast Lesions and Predictive Factors Associated with True Discordance on Imaging
FFY Wan, KM Chu, TWY Chin, L Xu, WL Wong, JLF Chiu
Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr Dr FFY Wan, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: wfy471@ha.org.hk
Submitted: 2 January 2023; Accepted: 21 June 2023.
Contributors: All authors designed the study and acquired and analysed the data. FFYW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon Central Cluster Research Ethics Committee/ Kowloon East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: KC/KE-22-0069/ER-1). The requirement for informed consent from the patients was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
Discordant benign breast lesions are suspicious for malignancy on imaging but show benign histology on initial biopsy. These lesions require further histological workup. This study sought to determine the frequency of discordant benign lesions and the rate of true discordance among them, and to identify predictive factors associated with true discordance.
Methods
Clinical, radiological, and pathological data on all discordant benign breast lesions biopsied between
2012 and 2021 were retrieved from the departmental database of a Hong Kong hospital. Rate of discordant benign
lesions, true and false discordance rates, and proportion of high-risk and benign lesions among false discordance
were calculated. If the discordant benign lesion was found to be malignant in repeat percutaneous biopsy or excisional
biopsy, it was true discordance. If a lesion’s benignity was confirmed with excisional biopsy, it was false discordance.
Univariate analysis was performed followed by multivariable logistic regression analysis to identify independent
predictors associated with true discordance.
Results
A total of 3080 breast biopsies were performed during the study period, of which 64 lesions (2.1%) were discordant benign lesions. Among 55 lesions with available additional workup results, 17 lesions (30.9%) were true discordant and 38 (69.1%) were false discordant. Nine (23.7%) of the false discordant lesions were high-risk lesions on final pathology. Older age (p = 0.019), presence of symptoms (p = 0.046), BI-RADS category 5 (p = 0.028), presence of microcalcifications with suspicious morphology (p = 0.047), and presence of architectural distortion (p = 0.04) were identified as independent predictors of true discordance.
Conclusion
The high true discordance rate confirmed the importance of further histological workup in discordant
benign breast lesions.
Key Words: Biopsy; Breast; Histology; Neoplasms
中文摘要
不一致組織學良性乳房病變及與影像學真正不一致相關的預測因子分析
尹芳盈、朱嘉敏、錢永恩、徐璐、黃慧琳、趙朗峰
引言
不一致良性乳房病變在影像學上疑似惡性腫瘤,但在初次活檢時顯示良性組織學,這些病變需要進一步組織學檢查。本研究旨在確定不一致良性病變的發病率以及它們真正不一致的比率,並確定與真正不一致相關的預測因子。
方法
我們從香港一家醫院的部門資料庫中檢索於2012至2021年間活檢的所有不一致良性乳房病變的臨床、放射學和病理數據,並計算不一致良性病變率、真假不一致率及假性不一致中的高風險病例和良性病變比例。如果重複經皮活檢或切除活檢發現不一致的良性病變為惡性,則為真正不一致;如果切除活檢證實病變為良性,則屬假性不一致。本研究先進行單變量分析,然後進行多變量邏輯迴歸分析,以確定與真正不一致相關的獨立預測因子。
結果
研究期間共進行了3080例乳房活檢,其中64例(2.1%)病灶為不一致良性乳房病變。在55例有額外檢查結果的病灶中,17例(30.9%)為真正不一致,38例(69.1%)為假性不一致。9例(23.7%)假性不一致病變在最終病理學上屬高風險病變。年齡較大(p = 0.019)、存在症狀(p = 0.046)、BI-RADS(乳房影像報告和數據系統)類別5(p = 0.028)、存在形態可疑的微鈣化(p = 0.047)以及存在結構扭曲(p = 0.04)為真正不一致的獨立預測因子。
結論
真正不一致比率高證實了對不一致良性乳房病變進行進一步組織學檢查的重要性。
INTRODUCTION
Image-guided core needle biopsy is the current standard
for initial workup and diagnosis of most BI-RADS
(Breast Imaging Reporting and Data System) category
4 and 5 breast lesions detected on mammography. With
technological advancements in both imaging techniques
and core biopsy devices, the false-negative rates of
image-guided core needle biopsy have been reported to
be down to 2.5%,[1] with most cases identified because of
radiological-pathological discordance. Such discordance
happens when the pathology results do not match the
imaging features, indicating that the lesion may not
have been sampled adequately and creating the need for
further histological workup.
Discordant benign lesions are lesions radiologically
suspicious for malignancy (BI-RADS category 4 or 5)
with a histological result that does not account for the
radiological suspicion.[2] Up to 64% of discordant benign
lesions from image-guided core needle biopsy turned
out to be malignant in subsequent excisional biopsy.[3]
If there is any concern regarding a discordant benign
breast lesion, further investigation by repeating image-guided
core needle biopsy or performing excisional
biopsy is then be considered. If a true discordant benign lesion is recognised promptly, a missed malignancy
can be identified, thus avoiding delay in diagnosis and
treatment.
This retrospective analysis aimed to determine the
frequency of discordant benign lesions and the
proportion of true discordance among them. Potential
predictive factors associated with true discordance were
identified to assist radiologists in better evaluating for
discordance.
METHODS
Data Collection
All cases with discordant benign breast lesions from 2012 to 2021 were retrieved from the departmental database
of Department of Radiology and Imaging of Queen
Elizabeth Hospital, Hong Kong. Data including patients’
clinical details, radiological features, pathological
findings, and imaging methods for biopsy guidance were
described.
Discordant benign lesions were defined as lesions
showing radiological findings suspicious for malignancy
with no evidence of malignancy on initial pathological
examination. In our institution, excisional biopsy was the standard of care for patients with discordant benign
pathological findings after two image-guided core
needle biopsies. If a lesion was found to be malignant
with repeat percutaneous biopsy or excisional biopsy,
it was considered to represent true discordance. If a lesion’s benignity was confirmed with final excisional
biopsy, it was considered to represent false discordance
(or concordance). The rates of discordant benign lesions,
true discordance (Figure 1), and false discordance
(Figure 2) were calculated.
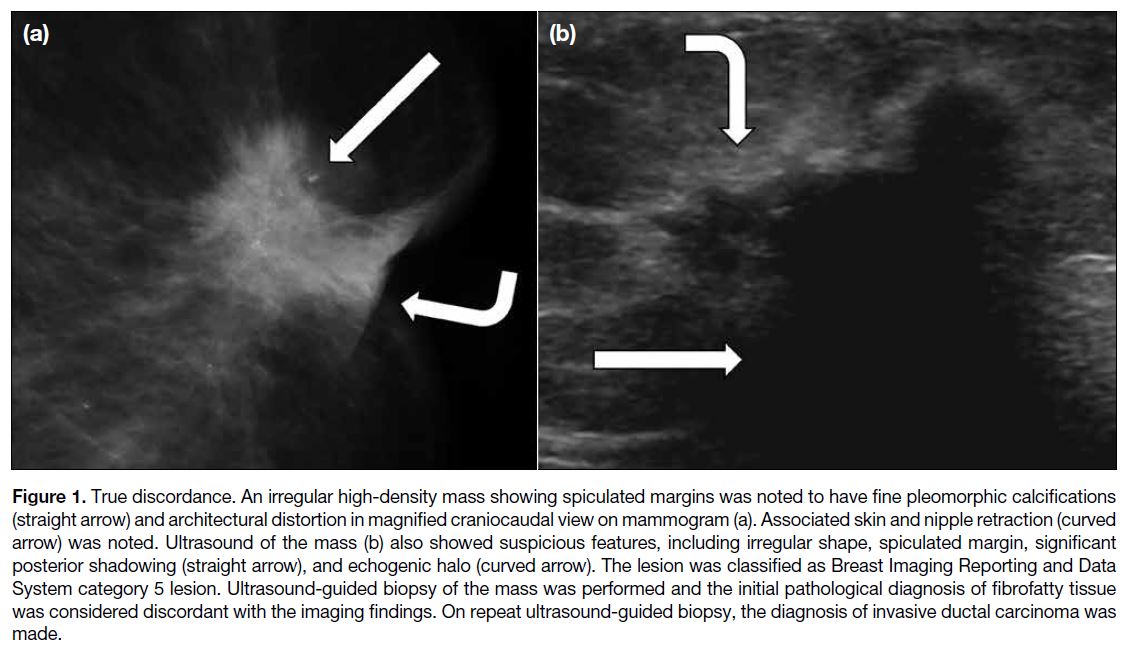
Figure 1. True discordance. An irregular high-density mass showing spiculated margins was noted to have fine pleomorphic calcifications
(straight arrow) and architectural distortion in magnified craniocaudal view on mammogram (a). Associated skin and nipple retraction (curved
arrow) was noted. Ultrasound of the mass (b) also showed suspicious features, including irregular shape, spiculated margin, significant
posterior shadowing (straight arrow), and echogenic halo (curved arrow). The lesion was classified as Breast Imaging Reporting and Data
System category 5 lesion. Ultrasound-guided biopsy of the mass was performed and the initial pathological diagnosis of fibrofatty tissue
was considered discordant with the imaging findings. On repeat ultrasound-guided biopsy, the diagnosis of invasive ductal carcinoma was
made.
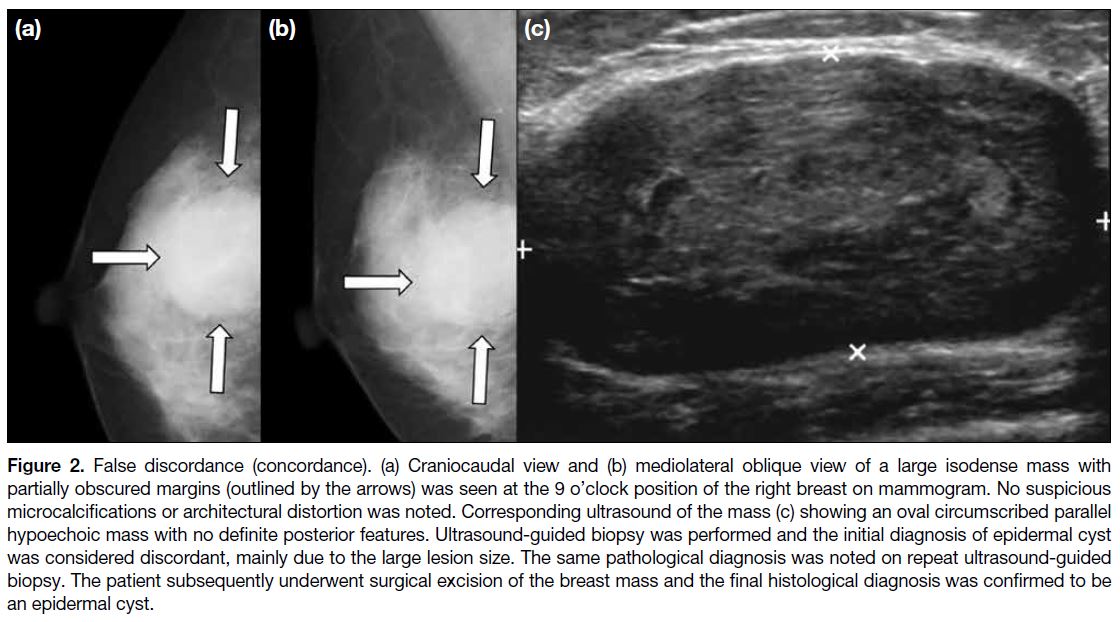
Figure 2. False discordance (concordance). (a) Craniocaudal view and (b) mediolateral oblique view of a large isodense mass with
partially obscured margins (outlined by the arrows) was seen at the 9 o’clock position of the right breast on mammogram. No suspicious
microcalcifications or architectural distortion was noted. Corresponding ultrasound of the mass (c) showing an oval circumscribed parallel
hypoechoic mass with no definite posterior features. Ultrasound-guided biopsy was performed and the initial diagnosis of epidermal cyst
was considered discordant, mainly due to the large lesion size. The same pathological diagnosis was noted on repeat ultrasound-guided
biopsy. The patient subsequently underwent surgical excision of the breast mass and the final histological diagnosis was confirmed to be
an epidermal cyst.
Patients’ clinical details including age, presence of
signs and symptoms (e.g., palpable breast mass or
pathological nipple discharge), synchronous breast
malignancy, past medical history or family history
of breast cancer, and history of previous ipsilateral
breast procedure were collected. Radiological features
including lesion size (measured on ultrasound except
for lesions only visualised on mammogram), BI-RADS
category, presence of suspicious microcalcifications or
architectural distortion on mammogram, presence of
other radiologically suspicious lesions in the ipsilateral
or contralateral breast, and presence of axillary
lymphadenopathy on ultrasound or mammogram were
reviewed. The imaging method for biopsy guidance
was also noted, and all the above features were
evaluated for their potential association with true or
false discordance.
Diagnostic Imaging Workup
All patients had had mammography and ultrasound
of both breasts performed as the initial diagnostic
workup, with interpretation and reporting performed
by breast radiologists (years of experience: mean, 8.3
years; median, 7; range, 1-30). Assessment for any
suspicious radiological features was made in accordance
with the American College of Radiology BI-RADS
Atlas.[4] The major findings to be evaluated included
mass, microcalcifications, architectural distortion,
and axillary lymphadenopathy. Suspicious features of
masses include irregular shape, non-parallel orientation,
non-circumscribed margins, and posterior shadowing
on ultrasound. Morphology was useful in predicting
the likelihood of malignancy for microcalcifications
and other suspicious calcification morphologies
including amorphous, coarse heterogeneous, fine
pleomorphic, fine linear, and fine linear-branching
microcalcifications. Linear or segmental distribution of
the microcalcifications also elevated the suspicion for
malignancy since they suggest deposits within the ductal
system. Examples of other associated suspicious features
were duct changes and skin changes. Suspicious lymph
nodes usually displayed cortical thickening and hilar
compression or displacement. The BI-RADS category
indicates the likelihood of malignancy and guides the
next step of management. Any lesions of BI-RADS
category 4 or 5 require tissue diagnosis. BI-RADS
category 4 lesions present with imaging findings that
do not possess the classic appearance of malignancy
but are sufficiently suspicious to indicate biopsy. BI-RADS
category 5 lesions carry a very high probability
of malignancy for which any non-malignant biopsy results are automatically considered discordant, leading
to repeat percutaneous biopsy or excisional biopsy. Any
radiologic-pathologic discordances were established in
multidisciplinary meetings.
Biopsy Technique
The choice of imaging modality for biopsy guidance was
based on factors including lesion visibility, operator’s
and patient’s preference, and availability of equipment.
All sonographic-guided biopsies were performed with
an automated biopsy gun (Bard; Magnum, Covington
[GA], US) and a 14-gauge core needle with a 22-mm
throw. A minimum of three core samples were obtained.
All stereotactic-guided biopsies were performed with a
directional vacuum-assisted device (Eviva Breast Biopsy
System; Hologic, Marlborough [MA], US) and a 9-gauge
core needle, and approximately 12 tissue samples were
acquired. Biopsies were performed by breast radiologists.
If microcalcifications or architectural distortion were
deemed to be well-visualised and targeted on ultrasound,
sonographic guidance was considered for biopsy. After
biopsy of calcifications, specimen radiographs were
acquired to verify sampling of the target calcifications.
A marker clip was placed at the biopsy site, and its
location on post-biopsy mammographic images was
confirmed. Correlation with initial diagnostic imaging
was performed to confirm biopsy of the targeted lesion.
Statistical Analysis
The distribution of the numerical variables was first
assessed for normality by using the Shapiro–Wilk test. If
the data are not normally distributed, they are expressed
as medians with interquartile ranges and analysed using
Mann-Whitney U test.
Categorical variables were reported as counts and
proportions. If there were <20% of cells with an
expected frequency of <5 in a contingency table, the
analysis of differences in characteristics between groups
was performed using the Chi squared test. If there were
≥20% of cells with an expected frequency of ≤5 in a
contingency table, the analysis between groups was
assessed using Fisher’s exact test for a 2 × 2 contingency
table and Fisher-Freeman-Halton exact test for a
contingency table larger than 2 × 2.
The variables with p < 0.1 in the univariate analysis were included in the multivariable logistic regression analysis
to assess their abilities as independent predictors. The
variables with p < 0.05 were considered statistically
significant.
Statistical analyses were performed using SPSS
(Windows version 28.0; IBM Corp, Armonk [NY], US).
RESULTS
A total of 3080 breast biopsies were performed from
2012 to 2021, of which 64 lesions (2.1%) were discordant
benign lesions. Among the 55 lesions with available
additional workup results, 17 lesions (30.9%) were
true discordant and 38 (69.1%) were false discordant
(concordant). Overall, there were 14 lesions (25.5%)
graded BI-RADS category 5, 13 lesions (23.6%) graded
BI-RADS category 4C, and 28 lesions (50.9%) graded
BI-RADS category 4B. There were no BI-RADS
category 4A lesions. Repeat biopsy of the lesions was
performed under either sonographic (n = 50, 90.9%) or
stereotactic guidance (n = 5, 9.1%).
In univariate analysis, older age (p = 0.002), larger
lesion size (p < 0.001), presence of symptoms (p = 0.076), BI-RADS category 5 (p < 0.001), presence of microcalcifications with suspicious morphology (p = 0.062), and presence of architectural distortion (p = 0.091) were predictors of true discordance. Synchronous
breast malignancy (p = 0.149), past medical history
(p = 1) or family history of breast cancer (p = 1), history
of previous ipsilateral breast procedure (p = 0.309),
presence of other radiologically suspicious lesions in
the ipsilateral or contralateral breast (p = 0.36), presence
of axillary lymphadenopathy (p = 0.435), and imaging
guidance methods (p = 0.31) were non-significant
variables (Tables 1 and 2).
Table 1. Association of patient demographics with true and false discordance.
Table 2. Association of radiological features with true and false discordance.
In multivariable logistic regression analysis, older age (p = 0.019), presence of symptoms (p = 0.046), BI-RADS
category 5 (p = 0.028), presence of microcalcifications
with suspicious morphology (p = 0.047), and presence
of architectural distortion (p = 0.04) were independent
predictors of true discordance, while lesion size
(p = 0.196) failed to remain a statistically significant
independent predictor (Tables 1 and 2).
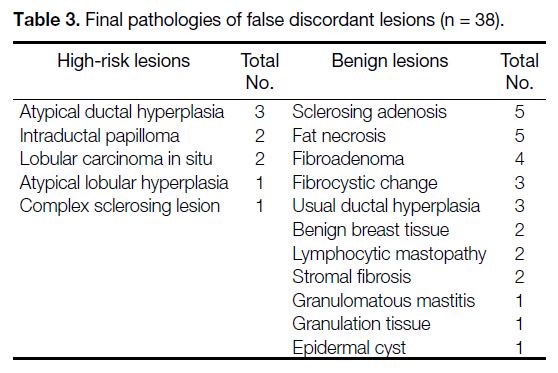
A total of 9 out of 38 (23.7%) false discordant lesions
were high-risk on final pathology. The high-risk lesions
included atypical ductal hyperplasia as the commonest
pathology, followed by intraductal papilloma and lobular
carcinoma in situ. The common final pathologies in
concordant cases were sclerosing adenosis, fat necrosis,
and fibroadenoma (Table 3).
Table 3. Final pathologies of false discordant lesions (n = 38).
DISCUSSION
The reported percentages of imaging-pathology
discordant lesions among biopsied breast lesions ranged
from 2.2% to 5.8%.[5] [6] [7] The prevalence of discordant
benign breast lesions in our institution was relatively low
(2.1%). This might be explained by our quality control
methods. During ultrasound-guided biopsy, satisfactory
needle position was confirmed by obtaining post-fire
images in orthogonal planes. For breast lesions with
suspicious microcalcifications, specimen radiographs
were performed to confirm the presence of target
microcalcifications. Moreover, adequate sampling was
achieved by obtaining at least three cores with minimal
fragmentations.
In our institution, excisional biopsy is the standard of
care for patients with discordant benign pathological
findings after two image-guided core needle biopsies.
Recently, vacuum-assisted breast biopsy has emerged
as a potentially less invasive alternative to excisional
biopsy for discordant benign lesions, with an upgrade
rate ranging from 4.6% to 22.7%.[8] Because of the high
sensitivity of contrast-enhanced magnetic resonance
imaging (MRI) for detection of breast malignancies,
it has been suggested to be of value in patients with
discordant benign breast lesions to avoid further
excisional biopsy. In a recent retrospective analysis,
incorporating MRI into the algorithm for management of discordant benign breast lesions was shown to
obviate the need for excisional biopsy in nearly 70% of
patients with BI-RADS category 4 findings (excluding
clusters of microcalcifications which are suspicious of
underlying ductal carcinoma in situ).[9] A previous study
also supported the use of MRI in determining the need
for biopsy of BI-RADS category 4 lesions.[10] MRI is a
potential tool for further workup of discordant benign
breast lesions in the BI-RADS 4 category, especially in
patients reluctant to undergo invasive excisional biopsy.
However, using the criterion of non-enhancement to
justify non-surgical management warrants further studies
with larger populations since false-negative results could
still occur with MRI.[10] Of note, the use of MRI in this
setting has not been studied for BI-RADS category 5
lesions, likely due to the assumption that BI-RADS 5
category from mammography and ultrasound studies is
unlikely to be overridden by the absence of suspicious
malignant findings on MRI. Surgical resection still
remains the gold standard for management of discordant
benign lesions of BI-RADS category 5.
To our knowledge, this is the first study to evaluate
for the potential predictive factors associated with
true discordance. Although determining radiological-pathological
concordance is crucial, no standard or
guideline is currently available to assist in decision
making. For this reason, evaluating for concordance still
remains a subjective decision which could certainly vary
among radiologists. Identification of predictors for true
discordance may therefore be useful in decision making,
especially in equivocal cases.
From our study, older age was a significant predictor
of true discordance. The incidence of breast cancer is
strongly related to older age, with the highest incidence
rates in older women. For example, in Hong Kong from
2000 to 2020, more than half of the new cases of invasive
breast cancers were in people aged ≥55 years.[11] The
increase in incidence with age largely reflects cell DNA
damage accumulating over time, which can be related to
biological processes or exposure to risk factors. Hence, it
is worth considering the age of the patient when assessing
concordance of breast lesions. On the contrary, personal
history and family history of breast cancers were not
significant predictors for true discordance in this study.
However, a definite conclusion could not be arrived at
due to the small number of patients having a personal or
family history of breast cancer in this study.
Imaging findings in patients with postprocedural changes may pose challenges in imaging interpretation
and assessment of concordance, especially in view
of the differential diagnosis of recurrent lesions.[1] Five
discordant benign breast lesions in our study had a
history of previous ipsilateral breast intervention.
Subsequent histological workup confirmed all to be
non-malignant with four of them being fat necrosis. Fat
necrosis is an inflammatory condition commonly seen
after breast surgery, radiation, infection or trauma. It is
known to be a mimicker of malignancy both clinically
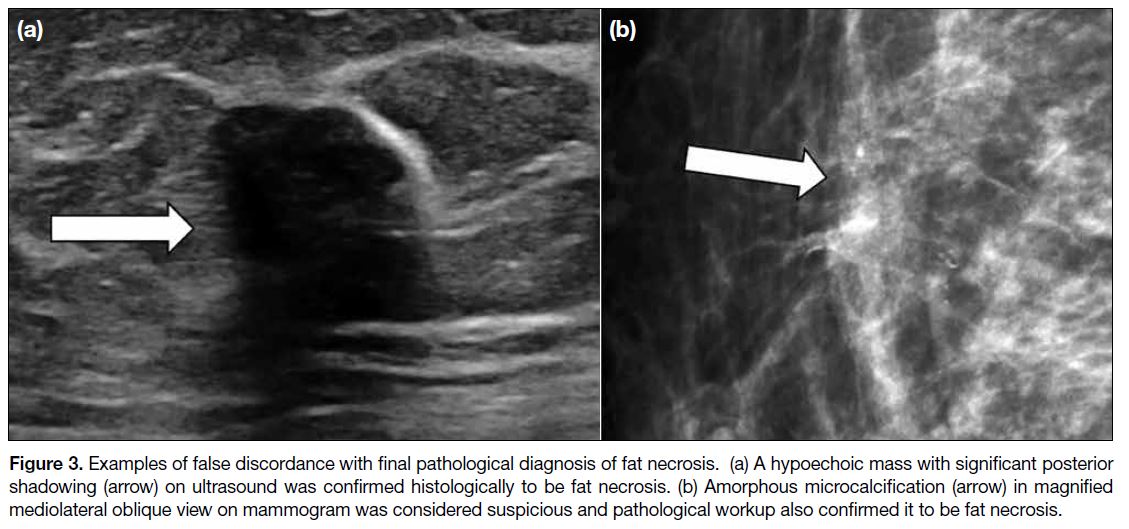
and radiologically. Knowledge about the spectrum of
suspicious radiological features of fat necrosis (Figure 3) as well as careful review of the biopsy technique
and confirmation of biopsy adequacy are useful in the
assessment for concordance in these patients.
Figure 3. Examples of false discordance with final pathological diagnosis of fat necrosis. (a) A hypoechoic mass with significant posterior shadowing (arrow) on ultrasound was confirmed histologically to be fat necrosis. (b) Amorphous microcalcification (arrow) in magnified mediolateral oblique view on mammogram was considered suspicious and pathological workup also confirmed it to be fat necrosis.
BI-RADS is the internationally accepted standard for
reporting breast imaging and BI-RADS category 5
lesions are highly suggestive of malignancy. With >95%
probability of malignancy, BI-RADS category 5 was
proven in our study to be a reliable factor in identifying
true discordance. This also showed that our radiologists
can successfully stratify lesions using BI-RADS risk
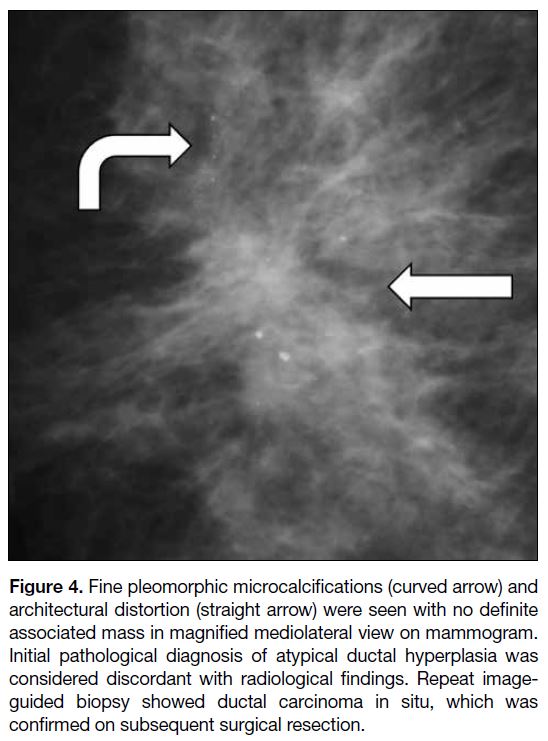
assessment categories. In addition, microcalcifications
of suspicious morphology and architectural distortion
(Figure 4) are suspicious imaging findings included in
the BI-RADS lexicon. Among the discordant benign
breast lesions in this analysis, the presence of either
finding was shown to be associated with a statistically
significant higher malignancy rate.
Figure 4. Fine pleomorphic microcalcifications (curved arrow) and architectural distortion (straight arrow) were seen with no definite associated mass in magnified mediolateral view on mammogram. Initial pathological diagnosis of atypical ductal hyperplasia was considered discordant with radiological findings. Repeat image-guided biopsy showed ductal carcinoma in situ, which was
confirmed on subsequent surgical resection.
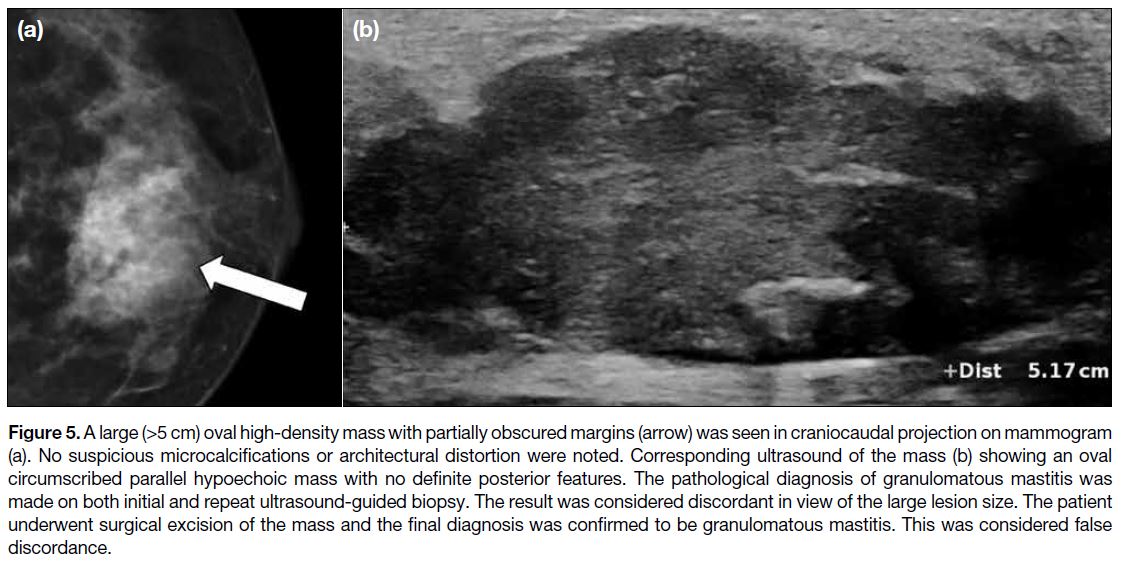
Lesion size was not a criterion for determination of the BI-RADS category and was confirmed in this study to be
a non-significant predictor of true discordance. Although large breast masses (>5 cm) are understandably
worrying for both patients and doctors, not all of them
are malignant. Some benign breast lesions can present
as large breast masses (Figure 5). According to the study
by Sickles,[12] no statistically significant difference in the
likelihood of cancer was found in relation to lesion size
in non-palpable breast masses.
Figure 5. A large (>5 cm) oval high-density mass with partially obscured margins (arrow) was seen in craniocaudal projection on mammogram
(a). No suspicious microcalcifications or architectural distortion were noted. Corresponding ultrasound of the mass (b) showing an oval
circumscribed parallel hypoechoic mass with no definite posterior features. The pathological diagnosis of granulomatous mastitis was
made on both initial and repeat ultrasound-guided biopsy. The result was considered discordant in view of the large lesion size. The patient
underwent surgical excision of the mass and the final diagnosis was confirmed to be granulomatous mastitis. This was considered false
discordance.
The imaging method for biopsy guidance was not found
to be a significant factor in predicting true discordance.
In our department, ultrasound-guided core needle
biopsies were often performed with 14-gauge biopsy
needles, while stereotactic-guided biopsies were usually
done with 9-gauge vacuum-assisted biopsy devices.
Since the size of the biopsy needles used between these
two methods was also different, this acted as a potential
confounder limiting proper comparison. However, it
was worth noting that all five discordant benign lesions
biopsied with stereotactic-guided vacuum-assisted
biopsy were false discordant. This observation could
possibly be explained by the higher biopsy adequacy
obtained using a larger-bore biopsy needle with a
vacuum-assisted device.
Among the false discordant lesions in our study, nearly
one-fourth were high-risk lesions. This category refers
to non-malignant lesions with increased lifetime risk
of developing breast cancer, including atypical ductal
hyperplasia, lobular neoplasia, papillary lesion, and radial scar.[13] Controversy exists regarding the appropriate
management of these lesions, which is primarily related
to the need for subsequent surgical excision. These
patients should be managed by a multidisciplinary team
with personalised management recommendations based
on clinical, imaging, and pathological correlations.[14]
For example, a pathological diagnosis of atypical
ductal hyperplasia in a small lesion that was nearly
entirely removed by vacuum-assisted biopsy may not
require subsequent surgical resection.[15] On the other
hand, if the same pathological diagnosis of atypical
ductal hyperplasia was obtained with imaging showing
extensive suspicious findings, there was a high possibility
of co-existing higher-grade lesions and further surgical
excision would be justified. Therefore, a single-standard
approach does not exist for high-risk breast lesions and
individualised management should be offered.
Limitations
Our study was limited by its small sample size and
retrospective approach. The strength of association
between the independent predictors and true discordance
(i.e., odds ratio) therefore cannot be reliably assessed and
reported.
CONCLUSION
The high true discordance rate of this study emphasised
the importance of careful radiological-pathological
correlation. Radiologists performing breast biopsy should be aware of the possibility of false-negative
diagnoses and be familiar with how to determine
radiological-pathological concordance as well as the
appropriate subsequent management. Future studies with
larger populations are necessary to develop a predictive
model for true discordance.
REFERENCES
1. Park VY, Kim EK, Moon HJ, Yoon JH, Kim MJ. Evaluating imaging-pathology concordance and discordance after ultrasound-guided breast biopsy. Ultrasonography. 2018;37:107-20. Crossref
2. Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics. 2007;27:79-94. Crossref
3. Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002;40:483-500, vi. Crossref
4. American College of Radiology. Breast Imaging Reporting & Data System (BI-RADS®) Atlas 5th Edition. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads. Accessed 26 Apr 2024.
5. Soyder A, Taşkin F, Ozbas S. Imaging-histological discordance after sonographically guided percutaneous breast core biopsy. Breast Care (Basel). 2015;10:33-7. Crossref
6. Sohn YM, Yoon JH, Kim EK, Moon HJ, Kim MJ. Percutaneous
ultrasound-guided vacuum-assisted removal versus surgery
for breast lesions showing imaging-histology discordance
after ultrasound-guided core-needle biopsy. Korean J Radiol.
2014;15:697-703. Crossref
7. Son EJ, Kim EK, Youk JH, Kim MJ, Kwak JY, Choi SH. Imaging-histologic
discordance after sonographically guided percutaneous
breast biopsy: a prospective observational study. Ultrasound Med
Biol. 2011;37:1771-8. Crossref
8. Jörg I, Wieler J, Elfgen C, Bolten K, Hutzli C, Talimi J, et al.
Discrepancies between radiological and histological findings in
preoperative core needle (CNB) and vacuum-assisted (VAB) breast
biopsies. J Cancer Res Clin Oncol. 2021;147:749-54. Crossref
9. Sanders LM, El-Madany M, Persing A, Mehta A. Use of contrast-enhanced MRI in management of discordant core biopsy results. AJR Am J Roentgenol. 2019;212:1157-65. Crossref
10. Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 lesions detected with screening mammography and screening US: utility of MR imaging.
Radiology. 2015;274:343-51. Crossref
11. Hong Kong Cancer Registry, Hospital Authority, Hong Kong. Hong Kong Cancer Statistics 2000-2020. Available from: https://www3.ha.org.hk/cancereg/". Accessed 1 Dec 2022.
12. Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994;192:439-42. Crossref
13. Parikh J, Tickman R. Image-guided tissue sampling: where radiology meets pathology. Breast J. 2005;11:403-9. Crossref
14. Krishnamurthy S, Bevers T, Kuerer H, Yang WT. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol. 2012;198:W132-40. Crossref
15. Krishnamurthy S, Bevers T, Kuerer HM, Smith B, Yang WT. Paradigm shifts in breast care delivery: impact of imaging in a multidisciplinary environment. AJR Am J Roentgenol.
2017;208:248-55. Crossref