Radiologic-Pathologic Review of Non-Epithelial Malignancies and Metastases in the Breast: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024 Jun;27(2):e125-34 | Epub 13 June 2024
Radiologic-Pathologic Review of Non-Epithelial Malignancies and Metastases in the Breast: A Pictorial Essay
RYS Mak1, AHC Wong1, CKM Mo1, KH Chin1, JSC Wong2, AYT Lai1, WWC Wong1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
2 Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr RYS Mak, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: mys877@ha.org.hk
Submitted: 25 March 2023; Accepted: 29 September 2023.
Contributors: RYSM, JSCW, AYTL and WWCW designed the study. RYSM, AHCW, CKMM, KHC and AYTL acquired the data. RYSM,
AHCW, JSCW and AYTL drafted the manuscript. CKMM, KHC, AYTL and WWCW critically revised the manuscript for important intellectual
content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No: HKECREC-2022-035). The requirement for patient consent was waived by the Committee due to the retrospective nature of the study.
Acknowledgement: The authors thank Dr KC Leung from the Department of Clinical Pathology of Pamela Youde Nethersole Eastern Hospital for critically revising the manuscript.
Declaration: Part of the manuscript was previously presented as a poster in the 30th Annual Scientific Meeting of the Hong Kong College of Radiologists (12-13 November 2022, virtual).
INTRODUCTION
Most malignant tumours in the breast are primary
epithelial malignancies. Non-epithelial malignancies
and metastases from other organs or tissues are rare.
Many such malignancies have variable and non-specific
radiological features and may resemble epithelial breast
carcinomas or even benign breast lesions. Nevertheless,
familiarity with their common imaging appearance is
crucial for facilitating timely diagnosis and determining
radiopathological concordance. This pictorial essay
reviews the radiological appearance of important non-epithelial
malignancies and metastases in the breast with
histopathological correlation.
METASTASES TO THE BREAST
Metastases to the breasts from non-mammary primary
tumours account for 0.5% to 2.0% of all breast malignancies.[1] The most common sources are melanoma;
non-Hodgkin lymphoma; sarcoma; and carcinoma of
the lung, stomach, ovaries, and kidney.[1] Clinically,
metastases to the breast are generally not associated with
chest wall fixation, peau d’orange appearance, Paget’s
disease of bone, skin retraction, nipple retraction, or
discharge.[2] The lesions tend to be superficially located in the upper outer quadrant.[3]
The most common mammographic manifestations
of metastases to the breast are one or more
circumscribed upper outer quadrant masses (Figure 1)
without spiculation or calcifications.[4] Another
reported manifestation is a diffuse pattern resembling
inflammatory breast carcinoma in cases of lymphatic
metastases, most commonly from contralateral breast
cancer, gastric, and ovarian carcinoma.[2] [5]
Figure 1. An 81-year-old woman with known carcinoma of the lung was found to have synchronous primary right breast invasive ductal
carcinoma and a right axillary metastatic lesion from lung adenocarcinoma. (a) Mammogram showing an oval isodense mass in the upper
outer quadrant of the right breast at anterior depth (arrow). (b) Ultrasound reveals a corresponding irregular hypoechoic mass in the
upper outer quadrant of the right breast. (c) Another oval hypoechoic mass with non-parallel (taller-than-wide) orientation is seen in the right
axillary subcutaneous region. (d-f) Low-power views (×4) of the right breast mass biopsy in haematoxylin and eosin (H&E) staining (d), p63
staining (e) and GATA binding protein 3 (GATA-3) staining (f) showing invasive ductal carcinoma (yellow brackets) featuring infiltrative nests
and tubules. On immunohistochemistry (IHC), the tumour cells are diffusely positive for GATA-3, supportive of primary breast malignancy.
A small component of ductal carcinoma in situ (yellow arrows) with preservation of the myoepithelial layer is highlighted by p63 staining
(blue arrowheads in [e]). (g-i) Low-power views (×4) of the right axillary mass biopsy in H&E staining (g), thyroid transcription factor-1
(TTF-1) staining (h) and GATA-3 staining (i) showing fibroadipose tissue infiltrated by irregular glands, with some extracellular mucin pools
in proximity (curved arrows in [g]). The morphology and IHC profile were similar to those of a specimen of prior lung carcinoma in the
same patient (not shown). The tumour cells are diffusely positive for TTF-1 and negative for GATA-3. Overall findings are consistent with
metastatic adenocarcinoma with mucinous features of a lung primary. Image courtesy of Dr KC Leung, Department of Clinical Pathology,
Pamela Youde Nethersole Eastern Hospital, Hong Kong (d-i).
Sonographic findings are similarly non-specific; they
can appear as solid masses that are circumscribed or
ill-defined and hypo- or hyperechoic, with posterior
shadowing or enhancement (Figure 1).[4] [6]
Metastatic tumours can be recognised pathologically
by their unusual histological patterns, lack of an
in situ component, predominant periductal and/or
perilobular distribution, and extensive lymphovascular
involvement.[7] In addition, clinical history, comparison
of the histology of mammary and extramammary
tumours (if any), and use of immunohistochemical
markers that are organ- or tumour-specific provide
indispensable information for delineating the origin of
the primary tumour (Figure 1).[7]
SARCOMA
Breast sarcomas are a group of aggressive tumours of
mesenchymal origin accounting for <1% of all breast
malignancies.[8] They can be primary, or secondary
to previous radiotherapy for breast or intrathoracic
malignancies.[8] Primary sarcomas occur predominantly
in women, with the highest incidence in patients aged
between 45 and 50 years, except for angiosarcomas,
which tend to occur in younger women with a reported
mean age of <40 years.[9] [10] The most common subtypes of
breast sarcomas are angiosarcomas, fibrosarcomas, and
undifferentiated pleomorphic sarcomas.[9] Angiosarcomas have a worse prognosis than other types of breast sarcomas (Figures 2 and 3).[11]
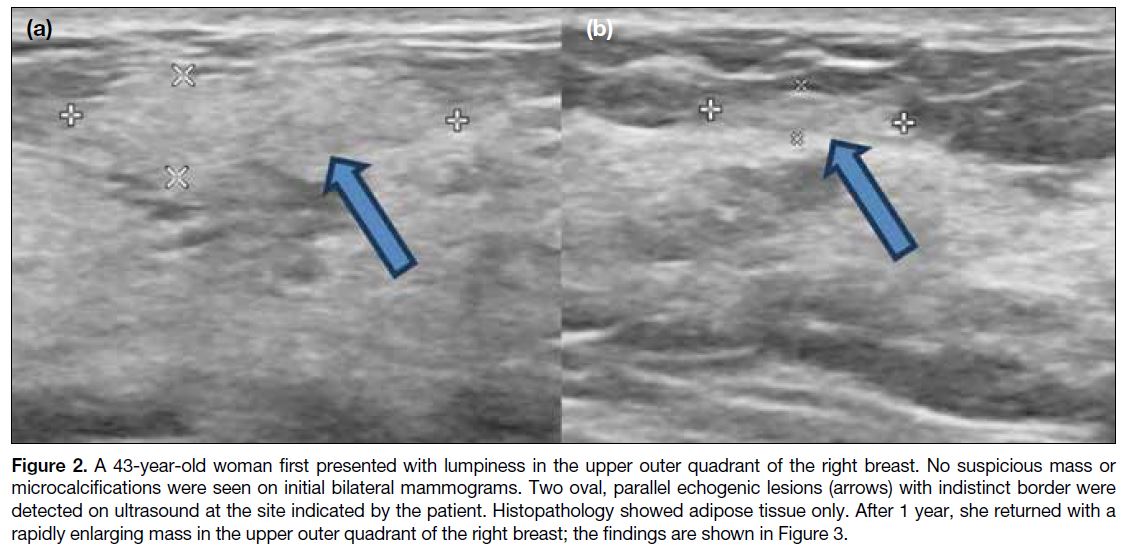
Figure 2. A 43-year-old woman first presented with lumpiness in the upper outer quadrant of the right breast. No suspicious mass or
microcalcifications were seen on initial bilateral mammograms. Two oval, parallel echogenic lesions (arrows) with indistinct border were
detected on ultrasound at the site indicated by the patient. Histopathology showed adipose tissue only. After 1 year, she returned with a
rapidly enlarging mass in the upper outer quadrant of the right breast; the findings are shown in Figure 3.
Figure 3. The same patient described in Figure 2 returned complaining of a rapidly enlarging right breast mass at 1 year after initial
assessment. (a) The current bilateral mammograms in mediolateral oblique view shows a new iso- to hyperdense mass with obscured border
occupying most of the upper outer quadrant of the right breast (thick arrow). (b, c) A large irregular heterogeneous mass (arrowheads)
is seen in the upper outer quadrant of the right breast on ultrasound. Its periphery is mainly hyperechoic and blends in with the normal
breast tissue. Minimal peripheral vascularity is noted (curved arrows in [c]). Staging contrast-enhanced computed tomography (d, e) and
18F-fluorodeoxyglucose positron emission tomography/computed tomography (f) show a corresponding avidly enhancing hypermetabolic
right breast mass (thin arrows) with no distant metastases. The histopathological diagnosis was angiosarcoma.
On mammography, the most common finding is a solitary, oval, high-density mass with either indistinct
or circumscribed margins and no calcifications
(Figure 3).[10] However, coarse osteoid calcifications
may occasionally be found in breast sarcomas with
osteosarcoma features.[11] [12]
On ultrasound, breast sarcomas typically appear as
oval masses with indistinct margins, hypoechoic or
complex echotexture, posterior acoustic shadowing,
and internal vascularity (Figure 3).[10] Diffuse abnormal
mixed hyper-and hypoechogenicity without a discrete
mass has been reported in up to 38% of patients with
breast angiosarcoma.[13] Hypervascularity on colour
Doppler imaging is a typical feature of angiosarcoma; up
to 54% of masses are hyperechoic or mixed hyper- and
hypoechoic, which may also reflect the vascular nature
of angiosarcoma.[13]
On magnetic resonance imaging, breast sarcoma typically
appears as an oval irregular mass that is hypointense on
T1-weighted images and hyperintense on T2-weighted
images, with heterogeneous initial rapid enhancement,
and washout curves (i.e., a relatively rapid uptake with
reduction in enhancement towards the latter part of the
study) or plateau curves (i.e., initial uptake followed by
the plateau phase towards the latter part of the study) on
dynamic imaging.[10]
LYMPHOMA
Breast lymphoma is a haematological neoplasm
that originates in the lymphoid tissue of the breast
and may be primary or secondary. Together, they represent approximately 0.04% to 0.7% of all breast
malignancies.[14] Primary breast lymphoma accounts for
0.85% to 2.2% of all extranodal malignant lymphomas,
while secondary breast lymphoma is the most common
malignancy with secondary involvement of the breast.[15] [16]
Breast lymphoma most commonly presents as a painless,
enlarging, palpable mass.[15] Multiple masses are found in
<10% of patients and bilateral involvement is found in
approximately 10% of patients, although these findings
are more commonly identified in secondary breast
lymphoma cases.[15]
On mammography, breast lymphoma manifests as a
solitary, non-calcified, oval or lobulated mass. It may
have circumscribed or indistinct margins (Figure 4).
Infiltrative patterns such as global asymmetry and
trabecula and skin thickening are uncommon findings.[12]
Skin thickening and lymphedema are reported in only up
to 8% of patients (Figure 5).[17] Calcifications, architectural
distortion, and spiculations are rare.[12]
Figure 4. Primary diffuse large B-cell lymphoma presenting as a palpable left breast lump in an 88-year-old woman. (a) Cropped magnified
views of left mammogram focusing on the upper outer quadrant showing an oval, circumscribed, equal density mass (thin arrows). (b) A
corresponding oval circumscribed, mixed echogenicity mass is seen in the left breast at 1 o’clock position on ultrasound (thick arrow).
(c) The left breast mass is hypermetabolic on 18F-fluorodeoxyglucose positron emission tomography/computed tomography with no
extramammary hypermetabolic foci (arrowhead). (d) High-power view (×400) of the breast mass biopsy with haematoxylin and eosin stain
showing fibroadipose tissue infiltrated by sheets of medium- to large-sized neoplastic lymphoid cells. They possess irregular nuclear
outlines, occasional nucleoli, and frequent apoptotic figures. On immunostaining (×40), the neoplastic lymphoid cells are diffusely positive
for the B-cell marker CD20 (e) and negative for the T-cell marker CD3 (which highlights T lymphocytes in the background) [f]. Image courtesy
of Dr Tiffany HT Chan and Dr HL Li, Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong (d-f).
Figure 5. A 74-year-old woman presented with a right axillary mass. (a) Diffuse skin thickening and oedema (open arrow) of the right
breast and axillary region were seen on bilateral mammograms in mediolateral oblique view. An enlarged right axillary lymph node (curved
arrow) was also noted. (b) Ultrasound of the right axilla confirms the presence of enlarged irregular hypoechoic lymph nodes with loss of
fatty hila (blue arrow). (c) The right axillary mass (arrowhead) demonstrates hypermetabolism on 18F-fluorodeoxyglucose positron emission tomography/computed tomography, with multiple other sites of nodal involvement. (d) Histology sections with haematoxylin and eosin
(H&E) stain in low-power view (×40) reveals the viable areas of the tumour as hypercellular. (e) High-power view (H&E staining, ×400) of the viable area showing sheets of medium- to large-sized neoplastic lymphoid cells with mitotic and apoptotic figures (thin arrow). (f) High-power
view (H&E staining, ×400) of the necrotic area showing ghost outlines of neoplastic cells (thick arrows). On immunostaining (×200),
the neoplastic lymphoid cells are diffusely positive for CD20 (g) and negative for CD3 (h). Overall features are consistent with diffuse large
B-cell lymphoma. Image courtesy of Dr CK Cheung and Dr F Hioe, Department of Clinical Pathology, Pamela Youde Nethersole Eastern
Hospital, Hong Kong (d-h).
Sonographic features of breast lymphoma are non-specific,
appearing as an oval or irregular mass of hyper-, hypo-, or mixed echogenicity, with circumscribed to
indistinct margins (Figure 4).[12] [15] Posterior acoustic
enhancement, echogenic rims, and onion-peel–like rims
are common findings (Figure 6).[14] Masses typically appear hypervascular on Doppler ultrasound.[12] [17] Axillary lymphadenopathy has been reported in up to
32% of patients (Figures 5 and 7).[17]
Figure 6. A 67-year-old woman with known history of stage IV follicular lymphoma with total metabolic response after chemotherapy
presented with multiple new right breast lumps. (a) Bilateral mammograms showed three equal-density oval and circumscribed masses in
the right breast without associated microcalcifications (curved arrows). (b-e) Multiple oval and circumscribed heterogeneous hypoechoic
lesions were seen in the right breast, some with echogenic rims (thick arrows in [b] and [d]) and posterior enhancement (arrowhead in
[d]). Internal vascularity is also visible in some of these lesions. (b, c) The first mass. Vascularity of this mass using Doppler ultrasound is
shown in (c). (d) The second mass. The third mass with vascularity using Doppler ultrasound is shown in (e). (f, g) 18F-fluorodeoxyglucose
positron emission tomography/computed tomography images demonstrating extensive hypermetabolic disease involving the right
breast (g) and multiple organs and systems (f) [black and white thin arrows]. (h) High-power view (×400) of the breast masses biopsy
with haematoxylin and eosin stain showing diffuse sheets of neoplastic lymphoid infiltrate comprising medium- to large-sized cells
with irregular nuclear membranes, hyperchromatic nuclei, and occasional nucleoli. Mitotic figures and apoptotic bodies are noted. On
immunostaining (×200), the neoplastic lymphoid cells were diffusely positive for CD20 (i) and negative for CD3 (j). Overall features are
consistent with diffuse large B-cell lymphoma. Image courtesy of Dr Elaine Lee, Department of Diagnostic Radiology, The University of
Hong Kong (f, g); Dr CK Cheung and Dr MW Ma, Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong
Kong (h-j).
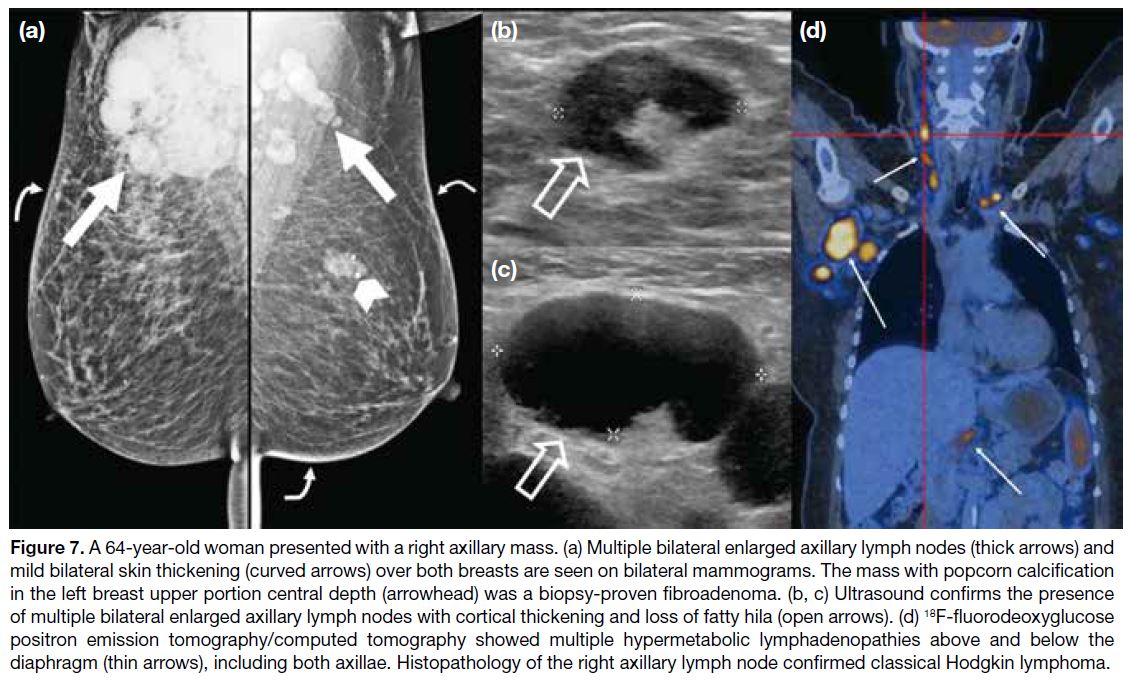
Figure 7. A 64-year-old woman presented with a right axillary mass. (a) Multiple bilateral enlarged axillary lymph nodes (thick arrows) and
mild bilateral skin thickening (curved arrows) over both breasts are seen on bilateral mammograms. The mass with popcorn calcification
in the left breast upper portion central depth (arrowhead) was a biopsy-proven fibroadenoma. (b, c) Ultrasound confirms the presence
of multiple bilateral enlarged axillary lymph nodes with cortical thickening and loss of fatty hila (open arrows). (d) 18F-fluorodeoxyglucose
positron emission tomography/computed tomography showed multiple hypermetabolic lymphadenopathies above and below the
diaphragm (thin arrows), including both axillae. Histopathology of the right axillary lymph node confirmed classical Hodgkin lymphoma.
The overall preponderance of breast lymphomas are
diffuse large B-cell lymphomas,[18] [19] which typically
show 18F-fluorodeoxyglucose (18F-FDG) avidity on
18F-FDG positron emission tomography/computed
tomography (18F-FDG PET/CT).[20] 18F-FDG PET/CT is
recommended for staging and response assessment of
18F-FDG–avid lymphomas[21]; its role in the management
of breast lymphoma has also been suggested.[22]
No specific imaging features can reliably distinguish
between primary and secondary breast lymphoma. In
patients with known extramammary lymphoma, multiple
masses or an inflammatory-like appearance such as
trabecular and skin thickening without a mass are more
likely to suggest secondary breast lymphoma.[15] [17]
PLASMACYTOMA
Plasmacytomas are tumours characterised by neoplastic monoclonal plasma cells in the bone or soft tissue
(extramedullary). Extramedullary breast plasmacytomas
are extremely rare and most commonly occur as a
secondary event in patients with known multiple
myeloma.[23] Clinically, breast plasmacytomas present as
palpable lumps.
A retrospective study involving 53 cases of breast
plasmacytoma examined its radiological features.[23] On
mammography, they had a non-specific appearance,
with dense, round, or oval masses with well- or ill-defined
margins (Figure 8[24]), or as diffuse infiltration.
On ultrasound, they can appear hypoechoic with well-defined
margins but, less commonly, may display mixed
hypo- to hyperechogenicity with indistinct margins and
posterior acoustic enhancement or shadowing (Figure 8).
No differentiating radiological features were found for
primary and secondary breast plasmacytoma. Given the
non-specific radiological features, the diagnosis of breast
plasmacytoma relies on clinical suspicion when there is
a history of multiple myeloma, and confirmation through
histopathology.[23]
Figure 8. A 59-year-old woman with a known history of multiple myeloma was incidentally found to have a left breast lesion (thin arrows in [a] to [e]) in a contrast-enhanced magnetic resonance imaging study of the liver. The nodule is (a) isointense on the pre-contrast T1-weighted axial image, (b) hyperintense on the T2-weighted fat-saturated axial image, and (c) contrast-enhanced on the post-contrast T1-weighted axial image. Cropped images focusing on the lesion in the (d) diffusion-weighted imaging and (e) apparent diffusion coefficient sequences demonstrate restricted diffusion. (f) Further evaluation with bilateral mammography showing multiple equal- to high-density oval and irregular masses with circumscribed to ill-defined margins (white open arrows). (g-i) Ultrasound of both breasts showing multiple oval circumscribed masses corresponding to the mammographically detected lesions (blue arrowheads). The masses are of variable echogenicity, ranging from hyperechoic, mixed heterogeneous to hypoechoic. (j) A suspicious irregular hypoechoic left axillary lymph node with loss of fatty hilum is also seen (blue curved arrow). (k) Dual-tracer 18F-fluorodeoxyglucose/11C-acetate positron emission tomography/computed tomography showing hypermetabolism in the bilateral breast masses (white curved arrow); left axillary lymph nodes; and multiple other lesions in the liver, abdominal cavity, subcutaneous tissue, and skeleton (white arrowheads), suggestive of multiple myeloma with extraosseous involvement. (l) Histology sections with haematoxylin and eosin stain in low-power view (×40) showing sheets of tumour cells. (m) High-power view (×400) showing tumour cells possessing eosinophilic cytoplasm with perinuclear hof (blue and white arrows) and hyperchromatic nuclei. Immunohistochemically (×200), the tumour cells are negative for epithelial marker AE1/AE3 (pancytokeratin) [n] and positive for plasma cell marker CD138 (o). In situ hybridisation (×200) for lambda (p) and kappa (q) showing lambda light chain restriction. Overall features are consistent with plasmacytoma. Reproduced and adapted with permission from the Hong Kong Academy of Medicine (a-f, k, n, and o)[24]; image courtesy of Dr TS Wong and Dr KC Leung, Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong (l-q).
There are only a few reports on the magnetic resonance
imaging features of breast plasmacytoma. Variable T1
and T2 signal intensities have been reported, including low-to-isointense signal on T1-weighted images and
intermediate-to-high signal on T2-weighted images
(Figure 8[24]).[25] [27] Restricted diffusion and homogenous
enhancement with delayed washout kinetics have also
been noted (Figure 8[24]).[25] [26] [27]
A limited number of studies have reported on 18F-FDG PET/CT findings in breast plasmacytoma.[26] [27] [28] [29] Majority
of these cases showed 18F-FDG avidity,[26] [27] [29] although
low-grade uptake has also been reported.[28] Nevertheless,
18F-FDG PET/CT is increasingly being recognised as
a valuable tool for the diagnosis and management of
patients with plasma cell disorders, such as multiple
myeloma and solitary plasmacytoma.[30] Several tracers
other than 18F-FDG for PET/CT have also been
investigated in patients with multiple myeloma.[30]
CONCLUSION
Although non-epithelial malignancies of the breast
show features different from those of epithelial breast
carcinoma, in general their radiological appearance
is generally variable and non-specific. Radiologists
must interpret the imaging findings in conjunction with
the clinical context. Biopsy remains the mainstay of
diagnosis and should be considered in suspected cases.
REFERENCES
1. Feder JM, de Paredes ES, Hogge JP, Wilken JJ. Unusual breast lesions: radiologic-pathologic correlation. Radiographics. 1999;19 Spec No:S11-26. Crossref
2. Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257-62. Crossref
3. Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977;129:673-6. Crossref
4. Bartella L, Kaye J, Perry NM, Malhotra A, Evans D, Ryan D, et al. Metastases to the breast revisited: radiological-histopathological correlation. Clin Radiol. 2003;58:524-31. Crossref
5. Krishnan EU, Phillips AK, Randell A, Taylor B, Garg SK. Bilateral metastatic inflammatory carcinoma in the breast from primary ovarian cancer. Obstet Gynecol. 1980;55(3 Suppl):94S-96S. Crossref
6. Vergier B, Trojani M, de Mascarel I, Coindre JM, Le Treut A. Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol. 1991;48:112-6. Crossref
7. WHO Classification of Tumours Editorial Board. WHO Classification of Breast Tumours: WHO Classification of Tumours,
5th Edition. World Health Organization; 2019. pp 261-5.
8. Lim SZ, Ong KW, Tan BK, Selvarajan S, Tan PH. Sarcoma of the breast: an update on a rare entity. J Clin Pathol. 2016;69:373-81. Crossref
9. Bousquet G, Confavreux C, Magné N, de Lara CT, Poortmans P, Senkus E, et al. Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol.
2007;85:355-61. Crossref
10. Matsumoto RA, Hsieh SJ, Chala LF, de Mello GG, de Barros N. Sarcomas of the breast: findings on mammography, ultrasound, and magnetic resonance imaging. Radiol Bras. 2018;51:401-6. Crossref
11. Smith TB, Gilcrease MZ, Santiago L, Hunt KK, Yang WT. Imaging features of primary breast sarcoma. AJR Am J Roentgenol. 2012;198:W386-93. Crossref
12. Berg WA, Leung JW. Diagnostic Imaging: Breast (3rd Edition). Philadelphia: Elsevier Health Sciences; 2019. pp 730-5.
13. Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in
24 patients. Radiology. 2007;242:725-34. Crossref
14. Shim E, Song SE, Seo BK, Kim YS, Son GS. Lymphoma affecting the breast: a pictorial review of multimodal imaging findings. J Breast Cancer. 2013;16:254-65. Crossref
15. Raj SD, Shurafa M, Shah Z, Raj KM, Fishman MD, Dialani VM. Primary and secondary breast lymphoma: clinical, pathologic, and multimodality imaging review. Radiographics. 2019;39:610-25. Crossref
16. Surov A, Holzhausen HJ, Wienke A, Schmidt J, Thomssen C, Arnold D, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. 2012;85:e195-205. Crossref
17. Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007;245:692-702. Crossref
18. Au W, Chan AC, Chow LW, Liang R. Lymphoma of the breast in Hong Kong Chinese. Hematol Oncol. 1997;15:33-8. Crossref
19. Picasso R, Tagliafico A, Calabrese M, Martinoli C, Pistoia F, Rossi A, et al. Primary and secondary breast lymphoma: focus on epidemiology and imaging features. Pathol Oncol Res.
2020;26:1483-8. Crossref
20. Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25-30. Crossref
21. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-68. Crossref
22. Santra A, Kumar R, Reddy R, Halanaik D, Kumar R, Bal CS, et al. FDG PET-CT in the management of primary breast lymphoma. Clin Nucl Med. 2009;34:848-53. Crossref
23. Surov A, Holzhausen HJ, Ruschke K, Arnold D, Spielmann RP. Breast plasmacytoma. Acta Radiol 2010;51:498-504. Crossref
24. Mo CK, Lai AY, Lo SS, Wong TS, Wong WW. Bilateral breast multiple myeloma: a case report. Hong Kong Med J. 2022;28:488-90. Crossref
25. Neuhaus T, Hess T. Bilateral extramedullary plasmacytoma of the breast. Breast J. 2014;20:315-8. Crossref
26. Park YM. Imaging findings of plasmacytoma of both breasts as a preceding manifestation of multiple myeloma. Case Rep Med. 2016;2016:6595610. Crossref
27. Vong S, Navarro SM, Darrow M, Aminololama-Shakeri S.
Extramedullary plasmacytoma of the breast in a patient with
multiple myeloma. J Radiol Case Rep. 2020;14:14-23. Crossref
28. Ginat DT, Puri S. FDG PET/CT manifestations of hematopoietic
malignancies of the breast. Acad Radiol. 2010;17:1026-30. Crossref
29. Rachh S, Puj K, Parikh A. 18F-FDG PET/CT in the evaluation of solitary extramedullary plasmacytoma: a case series. Asia Ocean J Nucl Med Biol. 2021;9:56-61. Crossref
30. Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S,
et al. Role of 18F-FDG PET/CT in the diagnosis and management
of multiple myeloma and other plasma cell disorders: a consensus
statement by the International Myeloma Working Group. Lancet
Oncol. 2017;18:e206-17. Crossref