Application of Zero Echo Time Magnetic Resonance Angiography in Neuroimaging: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2024 Jun;27(2):e117-24 | Epub 22 May 2024
Application of Zero Echo Time Magnetic Resonance Angiography in Neuroimaging: A Pictorial Essay
WC Law1, BMH Lai1, CY Cheung2, KT Wong1, JM Abrigo1
1 Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr WC Law, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, The Chinese
University of Hong Kong, Hong Kong SAR, China. Email: lwc926@ha.org.hk
Submitted: 21 March 2023; Accepted: 29 September 2023.
Contributors: WCL and JMA designed the study. WCL acquired data. All authors analysed the data. WCL and JMA drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: 2022.396). Informed patient consent was waived by the Committee due to the retrospective nature of the study.
Acknowledgement: The authors thank Mr William Kin-ming Kwong, Senior Radiographer in the Department of Imaging and Interventional Radiology in Prince of Wales Hospital, for his technical support on magnetic resonance imaging protocols.
INTRODUCTION
Cerebrovascular malformations are characterised by
abnormal communications between high–velocity-flow
arteries and low–velocity-flow veins and venous sinuses
in the brain. Such gradient differences put cerebrovascular
malformations at risk for intracranial haemorrhage,
which is often associated with significant morbidity and
mortality. Digital subtraction angiography (DSA) is the
gold standard for imaging assessment because of its high
spatial resolution and dynamic information. However, it
is an invasive procedure and uses a high radiation dose,
making it less than ideal for screening and surveillance
purposes.
Magnetic resonance angiography (MRA) with time-of-flight (TOF) technique is widely used as a non-invasive
investigation to diagnose or follow up patients with cerebrovascular malformations. It does not require
contrast medium and utilises the inflow effect for
generation of images of vessels.[1] However, there are
some limitations of this technique. It is influenced by
haemodynamics; for instance, in areas with complex
vessel direction, such as the internal carotid artery (ICA)
siphon, the images may be suboptimal with heterogeneous
flow signal. It may also depict artefactual flow signal in
the cavernous sinus in patients without carotid cavernous
fistula (CCF)[2] or in the dural venous sinuses in patients
without dural arteriovenous fistula (AVF).[1] Magnetic
susceptibility artefacts and radiofrequency shielding
often lead to difficulty in evaluation of patients who
have undergone previous interventions such as coil
embolisation and stenting.
In clinical practice, contrast-enhanced MRA may be additionally performed for clarification. The multiphase
acquisition slightly increases the complexity of
interpretation but allows dynamic visualisation of early
venous drainage, confirming arteriovenous shunting. It
does require intravenous administration of gadolinium
contrast, which is contraindicated in patients with
renal impairment, and also makes it less than ideal for
surveillance screening purposes. In addition, the spatial
resolution of contrast-enhanced MRA is usually inferior
to that of TOF MRA.
Zero echo time (ZTE) MRA, also called silent MRA, is
a relatively new technique, which combines ultrashort
echo time (0.006 ms) with arterial spin labelling (ASL).
The ASL technique uses magnetically labelled blood as
endogenous contrast, and the final angiographic image
is generated by subtraction of pre- from post-labelled
images, achieving background suppression. ZTE has
minimal magnetic susceptibility artefact and the ASL
technique improves the detection of low-flow signal.
Several studies have reported the usefulness of silent
MRA for the characterisation of cerebrovascular diseases
(atherosclerotic steno-occlusions, moyamoya disease,
and arteriovenous malformations [AVMs])[3] and follow-up
of treated aneurysms.[4] [5] [6] It has also recently been
reported as being useful for dural AVFs and indirect
CCFs.[7] [8] In our experience, it is helpful for identification
and follow-up of different kinds of cerebrovascular
malformations, as it facilitates depiction of ‘arterialised’
veins/venous sinuses. Here we present a pictorial essay
of our experience, illustrating the applicability of ZTE
MRA in our clinical practice.
Study Population
The relevant MRA scans were retrospectively retrieved
from 1 January 2017 to 31 July 2022 using keyword
search of radiology reports in the Radiology Information
System, a system widely used by every radiology
department in Hong Kong. All patients with ZTE MRA
acquired for the evaluation or follow-up of vascular malformations with positive imaging findings were
included. Patients with cerebrovascular abnormalities
other than AVM and AVF were excluded. The
corresponding MRI images and DSA studies were also
reviewed.
Zero Echo Time Magnetic Resonance Imaging Protocol
MRI images were acquired using either a 1.5T (Ingenia;
Philips, Best, the Netherlands) or a 3T scanner (SIGNA
Architect; General Electric Healthcare, Milwaukee
[WI], United States) with 20- and 32-channel head coils,
respectively. The ZTE MRA can only be acquired in the
3T scanner. MRA scan parameters are listed in the Table.
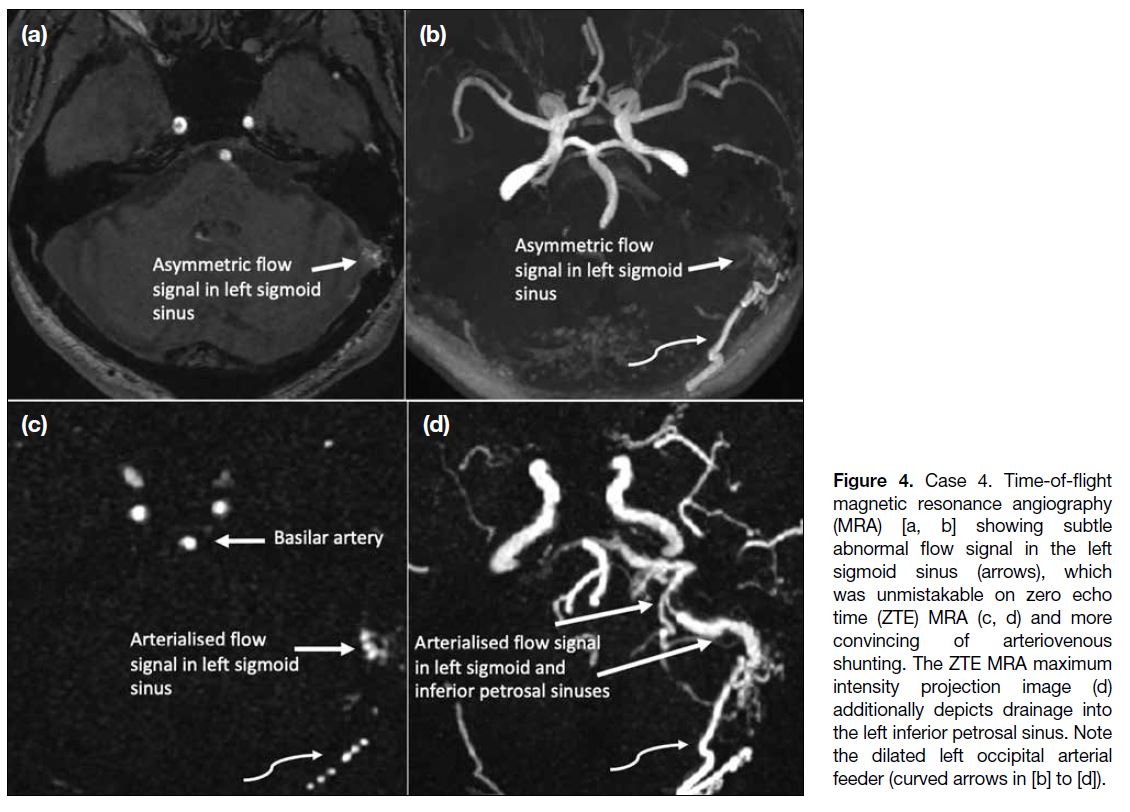
Table. Relative comparison of common cerebral magnetic resonance angiography techniques.
DSA was performed using a biplane angiography
system (Allura Xper FD 20/20; Phillips, Amsterdam,
the Netherlands, and ARTIS icono biplane; Siemens,
Erlangen, Germany), under local anaesthesia and using
femoral arterial access.
Case 1
A 59-year-old woman who presented with a left-sided
third cranial nerve palsy was diagnosed with a left-sided direct-type CCF and a left supraclinoid ICA saccular aneurysm on DSA. Both were treated simultaneously with placement of a flow diverter in the segment of
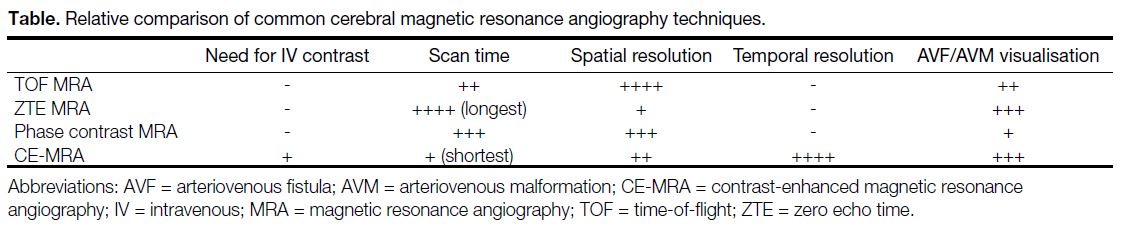
vessel from the cavernous ICA to the middle cerebral artery, with additional transvenous coil packing in the left cavernous sinus. She was noted to have residual CCF on follow-up DSA. On MRI, TOF MRA image (Figure 1a) showed coil-related magnetic susceptibility artefacts in the left cavernous sinus, which obscured the signal around the left ICA. ZTE MRA (Figure 1b and 1c) more clearly depicted abnormal extra-stent signal in the left cavernous sinus, corresponding to the residual CCF, and abnormal flow signal in the left pterygoid plexus, which correlated with the venous drainage on DSA (Figure 1d).
Figure 1. Case 1. Time-of-flight magnetic resonance angiography (MRA) image (a) showing susceptibility artefacts around the flow diverter (arrow). Zero echo time (ZTE) MRA image (b) clearly showing abnormal extra-stent flow signals in the left cavernous sinus, confirmed to be residual left direct carotid cavernous fistula (CCF) on digital subtraction angiography (DSA). A coronal reformat of maximum intensity projection ZTE MRA (c) shows the residual left CCF (straight arrow) and drainage into the left pterygoid plexus (curved arrow). Corresponding DSA image (d) also shows the drainage into the left pterygoid plexus (curved arrow).
Case 2
A 55-year-old woman presented with left 6th and 12th cranial nerve palsies. TOF MRA demonstrates heterogeneous flow signal within an abnormally dilated left condylar confluence (Figure 2a) and subtle flow signal around the left vertebral artery (Figure 2b). ZTE MRA more clearly depicts abnormal arterialised flow within the left condylar confluence (Figure 2d) and pronounced asymmetry in the left vertebral artery region, secondary to arterialised flow signal in the venous plexus surrounding the left vertebral artery (Figure 2e). ZTE MRA additionally showed abnormal signal in the left cavernous sinus and superior ophthalmic vein, which was suspected to be concomitant CCF (Figure 2f), with a differential diagnosis of retrograde flow from the condylar dural AVF. The abnormality in the left superior ophthalmic vein is not visible on TOF images (Figure 2c). DSA confirmed both the left condylar confluence dural AVF (Figure 2h) and concurrent left CCF (Figure 2g).
Figure 2. Case 2. Time-of-flight magnetic resonance angiography (MRA) [a-c] demonstrates an abnormally dilated left condylar confluence
with heterogeneous flow signal (a) [arrow] and subtle flow signal around the left vertebral artery (b) [arrow]. Zero echo time (ZTE) MRA (d-f) clearly showing abnormal arterialised flow within the left condylar confluence (d) and in the venous plexus surrounding the left vertebral artery (e). Additional abnormal signal in the left cavernous sinus and superior ophthalmic vein, which are more clearly shown on ZTE MRA (f), raises suspicion of concomitant carotid cavernous fistula (CCF) or retrograde flow from the condylar dural arteriovenous fistula (AVF). Lateral digital subtraction angiography (DSA) image (g) on left internal carotid artery injection confirms the concomitant left CCF with drainage into the superior ophthalmic vein (arrow). Frontal DSA image (h) on left external carotid artery injection showing the left condylar confluence dural AVF.
Case 3
A 62-year-old man presented with dizziness. DSA
revealed a dural AVF over the right occipital region,
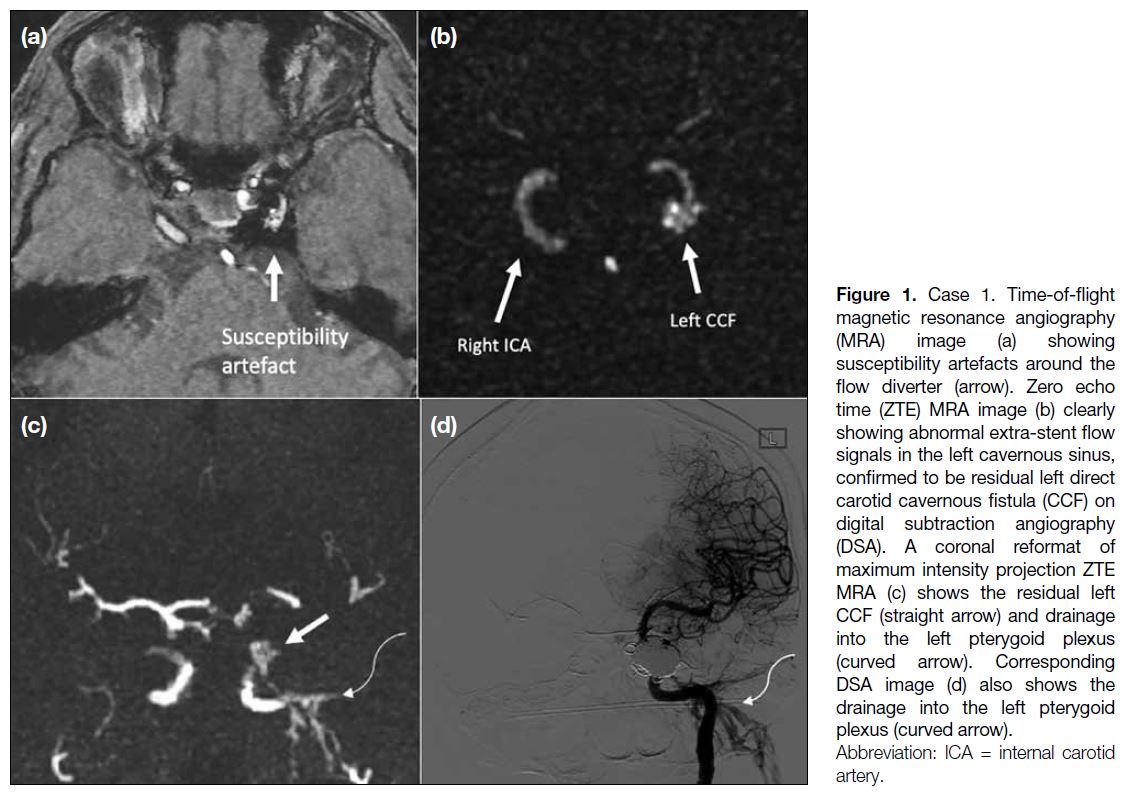
with arterial feeders from the right external carotid artery and the right vertebral artery, and venous drainage into the right sigmoid sinus with retrograde flow to the right transverse sinus. Transvenous embolisation was subsequently performed with complete occlusion of the dural AVF angiographically. Follow-up MRA (Figure 3a and 3b) showed evidence of recurrence of the dural AVF. Both TOF and ZTE MRA showed hypertrophied feeding arteries in the right occipital region and flow signal within the right sigmoid sinus suggestive of arteriovenous shunting. ZTE MRA shows additional
retrograde flow signal in the left transverse sinus. A second DSA (Figure 3c) confirmed the recurrent dural AVF and a second transvenous embolisation procedure was performed.
Figure 3. Case 3. Time-of-flight magnetic resonance angiography (MRA) [a] showing abnormal flow in the right transverse sinus (arrow),
while zero echo time MRA (b) showing arterialised flow in both transverse sinuses (arrows). Note the arterial feeders from the right posterior
auricular (curved arrows in [a] and [b]) and occipital arteries (arrowheads in [a] and [b]). Anteroposterior digital subtraction angiography image
(c) after right external carotid artery injection confirms the dural arteriovenous fistula, with drainage into both transverse sinuses (arrows).
Case 4
A 69-year-old man presented with acute haemorrhage
in the left temporooccipital region. DSA showed
evidence of dural AVF in the left occipital region, with
an arterial feeder from the left occipital artery, and
venous drainage into the left sigmoid sinus, left inferior
petrosal sinus, and left internal jugular vein. The patient declined embolisation and elected to undergo imaging
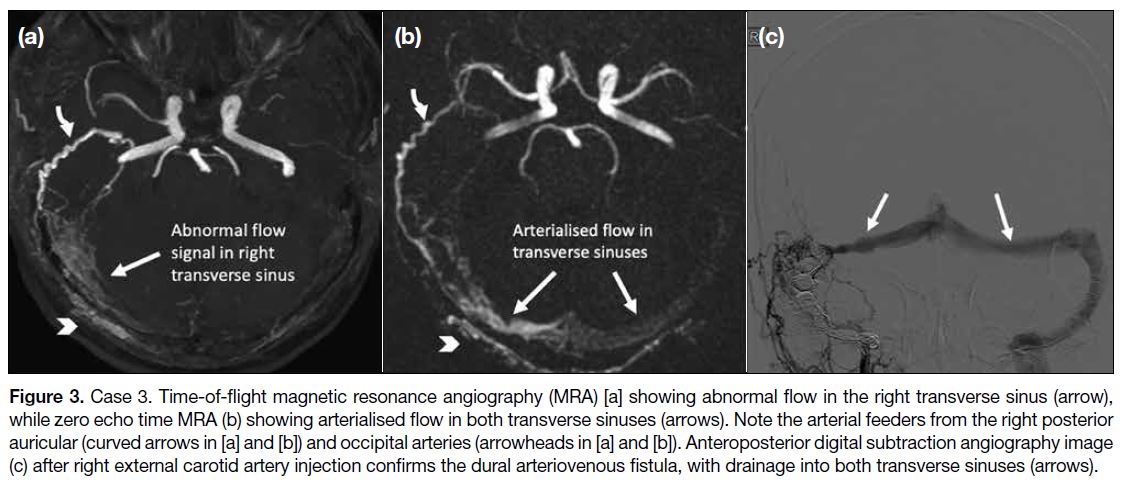
surveillance. TOF MRA showed subtle abnormal flow signal in the left sigmoid sinus (Figure 4a and 4b) which was unmistakable on the ZTE MRA (Figure 4c and 4d) and more convincing of arteriovenous shunting. Furthermore, ZTE MRA demonstrated venous drainage to the left inferior petrosal sinus (Figure 4d), which could not be appreciated on TOF MRA image.
Figure 4. Case 4. Time-of-flight
magnetic resonance angiography (MRA) [a, b] showing subtle abnormal flow signal in the left sigmoid sinus (arrows), which was unmistakable on zero echo time (ZTE) MRA (c, d) and more convincing of arteriovenous shunting. The ZTE MRA maximum intensity projection image (d) additionally depicts drainage into the left inferior petrosal sinus. Note the dilated left occipital arterial feeder (curved arrows in [b] to [d]).
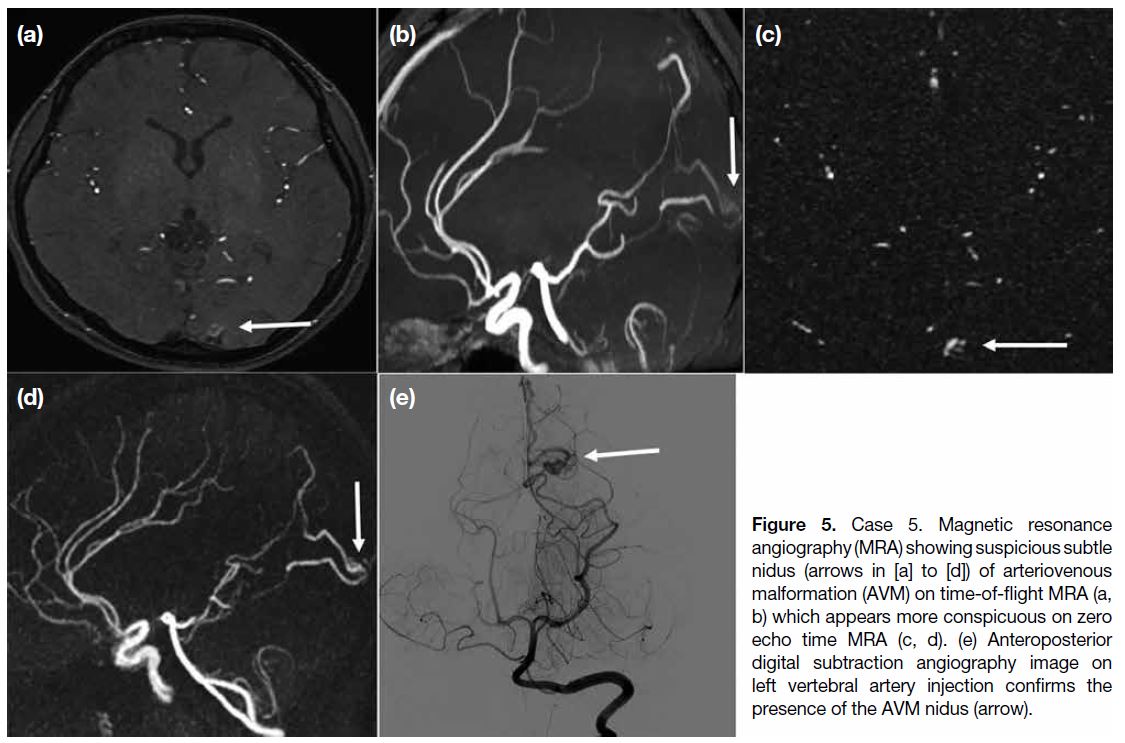
Case 5
A 27-year-old woman presented with left occipital lobe
haematoma. Investigation with MRA (Figure 5) showed
prominent vasculatures and possible nidus at the left occipital region, suspicious of an underlying AVM.
TOF MRA showed a prominent vessel in the region
which was more conspicuous in the ZTE MRA, owing
to background signal suppression of the technique.
Subsequent DSA (Figure 5e) confirmed a small left
occipital AVM, with arterial feeder from left posterior
cerebral artery and early venous drainage into superior
sagittal sinus. The patient was treated with transarterial
embolisation.
Figure 5. Case 5. Magnetic resonance
angiography (MRA) showing suspicious subtle nidus (arrows in [a] to [d]) of arteriovenous malformation (AVM) on time-of-flight MRA (a, b) which appears more conspicuous on zero echo time MRA (c, d). (e) Anteroposterior digital subtraction angiography image on left vertebral artery injection confirms the presence of the AVM nidus (arrow).
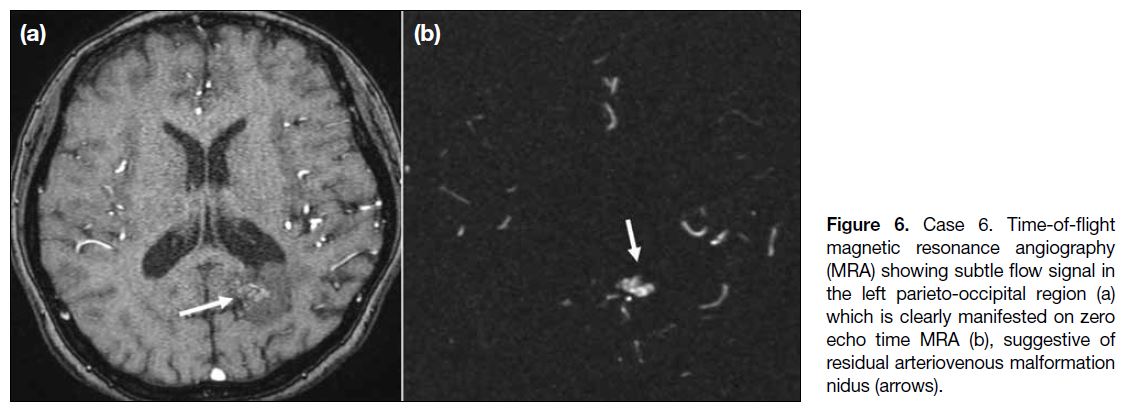
Case 6
A 53-year-old woman presented with headache. DSA
showed an AVM over the left parieto-occipital region,
with multiple arterial feeders from the left anterior
cerebral artery, middle cerebral artery, and posterior
cerebral artery and early venous drainage into the superior
sagittal and transverse sinuses. Two-stage treatment
was performed with embolisation of the left PCA and
subsequent radiosurgery. A small residual nidus was
noted on follow-up DSA. Further imaging surveillance
was performed with MRA, in which TOF MRA showed
subtle flow signal in the left parieto-occipital region (Figure 6a), likely corresponding to the residual nidus. The abnormality was more clearly manifested on ZTE MRA (Figure 6b).
Figure 6. Case 6. Time-of-flight
magnetic resonance angiography (MRA) showing subtle flow signal in the left parieto-occipital region (a) which is clearly manifested on zero echo time MRA (b), suggestive of residual arteriovenous malformation nidus (arrows).
DISCUSSION
We demonstrated the utility of ZTE MRA in the initial and follow-up evaluation of patients with cerebrovascular
malformations. In particular, ZTE MRA allowed for a
more confident diagnosis and better characterisation
of CCFs, dural AVFs, and AVMs. The imaging was
not constrained by artifacts from prior embolisation
or stenting. While most arteriovenous shunts could be
depicted on TOF MRA, occasionally, the abnormal
flow signals may be subtle, and ZTE MRA provided
additional strong supporting evidence to proceed to DSA
for final confirmation.
TOF MRA relies on the in-flow enhancement effect for
detection of signal and is dependent on the alignment
of vessels relative to the scan plane. If the direction of
flowing blood is parallel to the scan plane, the spins are
exposed to repetitive excitation pulses and the flow signal
may be saturated and lost.[9] It is also prone to magnetic
susceptibility artefacts and may demonstrate artefactual
flow signal in normal venous structures.
In ZTE MRA, the ZTE nature minimises the sensitivity
to magnetic field heterogeneity and the phase dispersion
of the labelled blood flow signal, hence decreasing
magnetic susceptibility artefacts.[10] The ASL technique uses the water molecules within arterial blood as an endogenous tracer. Hence, it is less influenced by the
blood flow state and is not affected by the blood flow
direction. The ultrashort echo time acquisition is a
defining feature of ZTE MRA that differentiates it from
other ASL-based MRA methodologies.
In normal circumstances, venous structures are not
expected to show a signal in ZTE MRA because only the
protons in the arteries are magnetically labelled. Hence,
the signal detected in these vascular regions would
suggest underlying arteriovenous shunting. This, too,
allows for easier image interpretation.
The background suppression achieved with ZTE MRA
also increases the sensitivity of detecting abnormal flow
against a dark background. In this sequence, ASL is used
as a preparation pulse. A control image is first obtained
before the labelling pulse and the labelled image is
acquired subsequently. Subtraction of the unlabelled from
the labelled images generates the angiographic images
and achieves background suppression.[11] Our experience
highlights such advantage, with abnormal flow signals
being more conspicuous on ZTE than TOF MRA,
allowing for their easy detection and straightforward
interpretation. Furthermore, the ZTE pulse sequence
minimises gradient switching, resulting in a quieter
scan, which could potentially improve patients’ comfort
during the exmination.[12]
ZTE MRA is not without disadvantages, however. First,
it requires longer imaging time (~6 mins vs. ~3 mins
for TOF) and thus is theoretically more susceptible to motion artefact. However, from our experience, most
patients were able to tolerate the prolonged acquisition.
Second, it has slightly lower spatial resolution due to
greater slice thickness and larger pixel size. Further, the
low background signal may lead to reduced anatomical
information as background structures are not well
delineated. In addition, ZTE MRA does not allow vessel
selectivity or time-resolved acquisition as in other ASL-based
MRA techniques, which could enhance shunt
characterisation; however, it still provides a robust
screening method prior to reference standard DSA.
Finally, ZTE MRA is limited by scanner and hardware
availability.
CONCLUSION
ZTE MRA is a useful adjunct to TOF MRA and has
good concordance with DSA, potentially obviating
the need for CE-MRA. ZTE MRA allows for easier
recognition of abnormal vascular shunts, increasing
confidence in making the diagnosis as well as improving
the characterisation of cerebrovascular malformations.
REFERENCES
1. Morelli, JN, Gerdes CM, Schmitt P, Ai T, Saettele MR, Runge VM,
et al. Technical considerations in MR angiography: an image-based
guide. J Magn Reson Imaging. 2013;37:1326-41. Crossref
2. Watanabe K, Kakeda S, Watanabe R, Ohnari N, Korogi Y.
Normal flow signal of the pterygoid plexus on 3T MRA in patients
without DAVF of the cavernous sinus. AJNR Am J Neuroradiol.
2013;34:1232-6. Crossref
3. Shang S, Ye J, Dou W, Luo X, Qu J, Zhu Q, et al. Validation of zero TE-MRA in the characterization of cerebrovascular diseases: a feasibility study. AJNR Am J Neuroradiol. 2019;40:1484-90. Crossref
4. Shang S, Ye J, Luo X, Qu J, Zhen Y, Wu J. Follow-up assessment of coiled intracranial aneurysms using ZTE MRA as compared with TOF MRA: a preliminary image quality study. Eur Radiol.
2017;27:4271-80. Crossref
5. Takano N, Suzuki M, Irie R, Yamamoto M, Teranishi K, Yatomi K,
et al. Non–contrast-enhanced silent scan MR angiography of
intracranial anterior circulation aneurysms treated with a low-profile
visualized intraluminal support device. AJNR Am J Neuroradiol.
2017;38:1610-6. Crossref
7. Balasubramanian AP, Kannath SK, Rajan JE, Singh G, Kesavadas C,
Thomas B. Utility of silent magnetic resonance angiography in the
evaluation and characterisation of intracranial dural arteriovenous
fistula. Clin Radiol. 2021;76:712.e1-8. Crossref
8. Kandasamy S, Kannath SK, Enakshy Rajana J, Kesavadas C,
Thomas B. Non-invasive angiographic analysis of dural carotid
cavernous fistula using time-of-flight MR angiography and silent
MR angiography: a comparative study. Acta Radiol. 2023;64:1290-7. Crossref
9. Azuma M, Hirai T, Shigematsu Y, Kitajima M, Kai Y, Yano S, et al.
Evaluation of intracranial dural arteriovenous fistulas: comparison
of unenhanced 3T 3D time-of-flight MR angiography with digital
subtraction angiography. Magn Reson Med Sci. 2015;14:285-93. Crossref
10. Ryu KH, Baek HJ, Moon JI, Choi BH, Park SE, Ha JY, et al.
Usefulness of noncontrast-enhanced silent magnetic resonance
angiography (MRA) for treated intracranial aneurysm follow-up in
comparison with time-of-flight MRA. Neurosurgery. 2020;87:220-8. Crossref
11. Irie R, Suzuki M, Yamamoto M, Takano N, Suga Y, Hori M,
et al. Assessing blood flow in an intracranial stent: a feasibility
study of MR angiography using a silent scan after stent-assisted
coil embolization for anterior circulation aneurysms. AJNR Am J
Neuroradiol. 2015;36:967-70. Crossref
12. Ljungberg E, Damestani NL, Wood TC, Lythgoe DJ, Zelaya F, Williams SC, et al. Silent zero TE MR neuroimaging: current state-of-the-art and future directions. Prog Nucl Magn Reson Spectrosc. 2021;123:73-93. Crossref