Dendriform Pulmonary Ossification in a Young Man: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Jun;27(2):e112-6 | Epub 28 May 2024
Dendriform Pulmonary Ossification in a Young Man: A Case Report
PL Lam1, KK Cheng2, KH Lee1, JCH Tsang3, ACL Chan3, DHY Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Radiology Department, Hong Kong Baptist Hospital, Hong Kong SAR, China
3 Department of Pathology, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr PL Lam, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China. Email: lpl404@ha.org.hk
Submitted: 21 June 2023; Accepted: 29 September 2023.
Contributors: All authors designed the study, acquired, and analysed the data. PLL drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided informed consent for all treatments and procedures, and consent for publication of this case report.
INTRODUCTION
Diffuse pulmonary ossification is characterised by
metaplastic mature bone formation in the lungs. There
are two distinct morphologies, namely, nodular and
dendriform. The more common nodular pulmonary
ossification occurs within alveolar spaces due to
organisation of intra-alveolar exudates. The underlying
culprits include chronic pulmonary congestion, such
as in mitral valve stenosis, as well as previous insults
causing haemosiderin accumulation.[1] On the contrary,
dendriform pulmonary ossification (DPO) describes
bony depositions in the alveolar interstitium that produce
a branching ‘dendriform’ pattern.[2] It is sparsely reported
with <100 cases recorded in the literature. In addition,
DPO is often diagnosed only during autopsy. It is
generally seen in older individuals aged >60 years.[3]
CASE PRESENTATION
A 23-year-old Chinese man with good past health
presented to the accident and emergency department
(AED) in June 2017 with acute abdominal pain, vomiting and diarrhoea for 1 day. Apart from obesity (body
mass index 32.5 kg/m2), his physical examination and
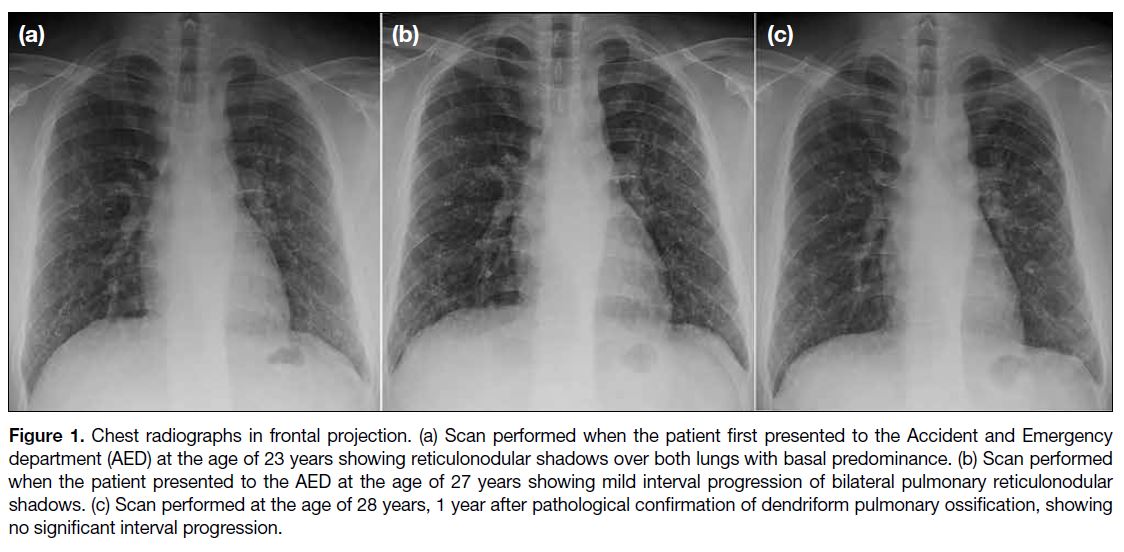
vital signs were normal. Chest radiograph incidentally
revealed reticulonodular shadows over both lungs with
basal predominance (Figure 1a). He was discharged 4
days later after recovering from acute gastroenteritis.
Computed tomography (CT) of the thorax and medical
specialist outpatient clinic consultation were arranged
but the patient defaulted from follow-up.
Figure 1. Chest radiographs in frontal projection. (a) Scan performed when the patient first presented to the Accident and Emergency department (AED) at the age of 23 years showing reticulonodular shadows over both lungs with basal predominance. (b) Scan performed when the patient presented to the AED at the age of 27 years showing mild interval progression of bilateral pulmonary reticulonodular shadows. (c) Scan performed at the age of 28 years, 1 year after pathological confirmation of dendriform pulmonary ossification, showing no significant interval progression.
Four years later, at the age of 27 years, the patient again presented to the AED with a 5-day history of mild right
ankle pain. His physical examination was normal other
than mild right ankle tenderness that quickly resolved
with analgesics. Nonetheless chest radiograph revealed
mild interval progression of bilateral pulmonary
reticulonodular shadows Figure 1b). The patient agreed
to further workup of his abnormal radiographic findings.
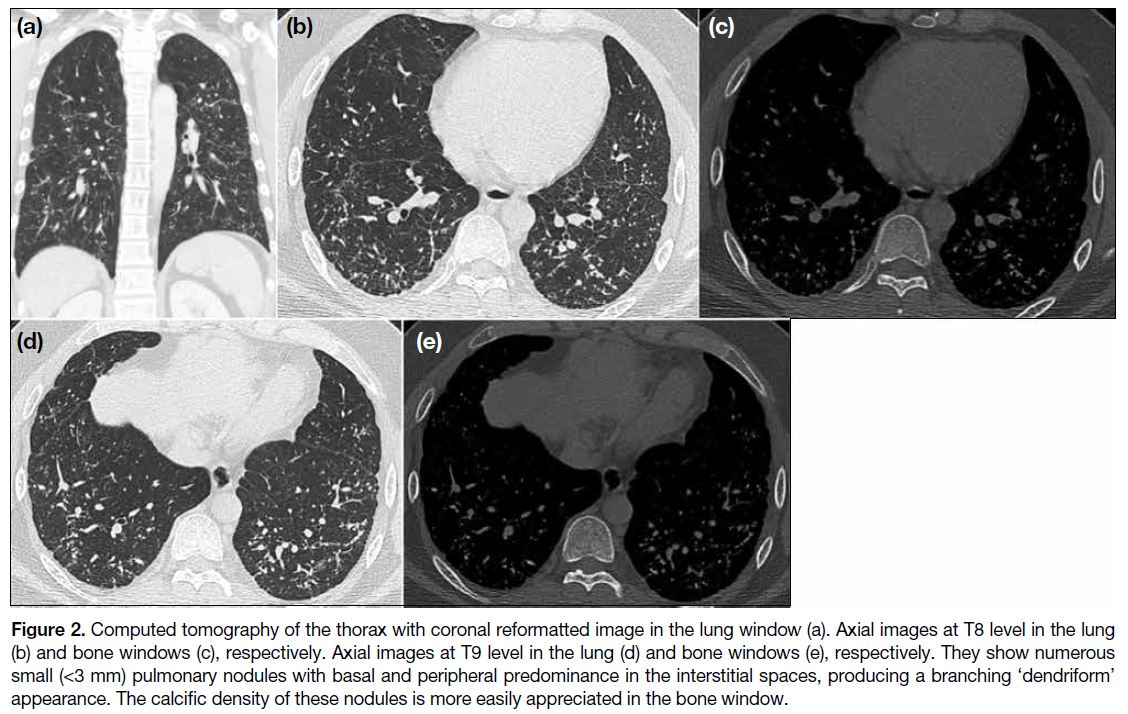
Early CT of the thorax was performed 4 days later
(Figure 2). There were numerous small (<3 mm) hyperdense nodules in the bronchovascular, interlobular
septal, perifissural and subpleural spaces of both
lungs with basal and peripheral predominance. These
interstitial nodules appeared to form contiguous
branching lines producing a ‘dendriform’ pattern. Lung volumes were preserved with no honeycombing
or traction bronchiectasis. There were no focal
consolidations, ground-glass opacities, pleural effusion
or pleural plaque. Mediastinal and hilar lymph nodes
were not enlarged or calcified. Trachea and main bronchi were patent without focal stenosis or wall calcifications.
Heart size was normal and pulmonary vasculature was
not dilated. DPO was considered as one of the primary
differential diagnoses but the young age at presentation
and scarcity of prior case reports hindered a definitive
radiological diagnosis.
Figure 2. Computed tomography of the thorax with coronal reformatted image in the lung window (a). Axial images at T8 level in the lung (b) and bone windows (c), respectively. Axial images at T9 level in the lung (d) and bone windows (e), respectively. They show numerous small (<3 mm) pulmonary nodules with basal and peripheral predominance in the interstitial spaces, producing a branching ‘dendriform’ appearance. The calcific density of these nodules is more easily appreciated in the bone window.
Upon further inquiry, the patient recalled a 1-minute
episode of heavy fume exposure after lighting
firecrackers during Chinese New Year at the age of 8
years. Otherwise, he could recollect no other occasion of
potential hazardous inhalation. He worked indoors as an
accountant and considered occupational risks unlikely.
He was also a non-smoker. Barring occasional snoring,
he experienced no breathing difficulties and had normal
exercise tolerance.
Lung function tests were normal but polysomnography
revealed mild-to-moderate obstructive sleep apnoea
(OSA) [sleep efficiency = 69%, respiratory disturbance
index = 9.1, and oxygen desaturation index = 13.3].
Blood tests, including complete blood count, coagulation profile, immunoglobulin patterns, liver, renal, and
endocrine functions were all within the normal reference
range. Biological markers for rheumatic diseases, such
as rheumatoid factor, anti-nuclear antibodies, anti-neutrophil
cytoplasmic antibody, C3, and C4, were
negative.
Microbiological examination showed past varicella
zoster virus infection with positive anti–varicella zoster
virus immunoglobulin G antibody. Sputum, serum and
urine cultures were negative. Bronchoalveolar lavage was negative for acid-fast bacilli, fungi, ova, and cysts.
Reverse transcription polymerase chain reaction of
throat saliva for coronavirus disease 2019 was negative.
Antibodies to human immunodeficiency virus were also
negative.
No malignant cells were detected on sputum cytology
or bronchoalveolar lavage. Transbronchial biopsy of the
right lower lobe was unable to establish a pathological diagnosis.
The patient was referred to the cardiothoracic team
for lung biopsy. Video-assisted thoracoscopic wedge
resections of the left upper and left lower lobes were
performed 6 months after the initial CT study. Gross
examination of the biopsied specimens showed lung
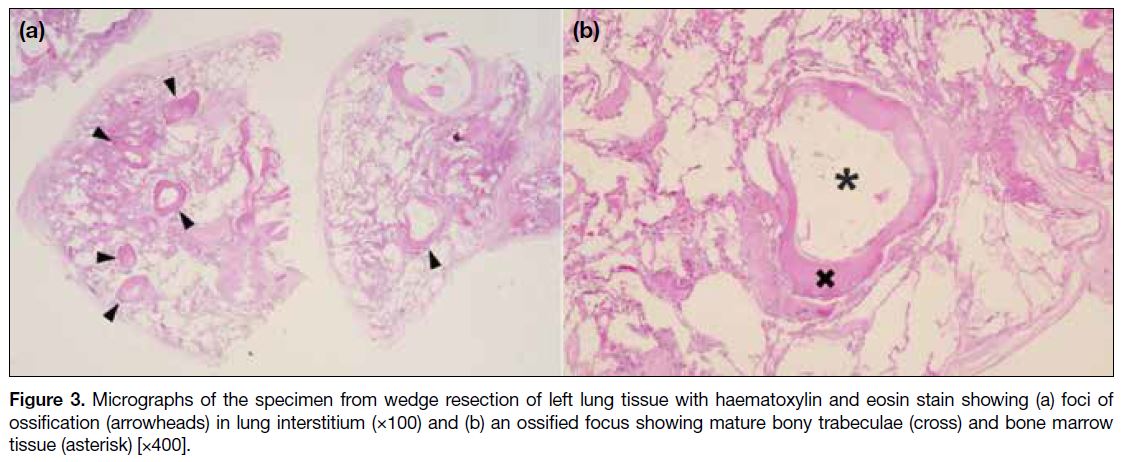
tissue with calcific gritty cut surfaces. Microscopic
sections revealed interstitial foci of ossification with
mature bony trabeculae and bone marrow tissue (Figure 3), consistent with DPO. There was no evidence of alveolar microlithiasis. Congo red stain showed no
amyloid deposition. Ziehl-Neelsen stain for acid-fast
bacilli and Grocott methenamine silver stain for fungi
were negative.
Figure 3. Micrographs of the specimen from wedge resection of left lung tissue with haematoxylin and eosin stain showing (a) foci of ossification (arrowheads) in lung interstitium (×100) and (b) an ossified focus showing mature bony trabeculae (cross) and bone marrow tissue (asterisk) [×400].
Since the patient was asymptomatic for DPO, he
opted for observation. He was also put on continuous
positive airway pressure machine for his OSA. Regular
consultation at medical specialist outpatient clinic and
follow-up chest radiograph at 6-month intervals were
arranged. He had no new complaints. The latest chest
radiograph performed over 1 year after the lung biopsy
showed no significant interval disease progression
(Figure 1c).
DISCUSSION
To the best of our knowledge, this is the youngest reported case of DPO in the literature. On chest radiograph, there
are reticulonodular shadows in both lungs, usually
with basal predominance.[4] There may be slow interval
disease progression.[5] The underlying pathological bony
deposition in the alveolar interstitium can be better
delineated by CT. Numerous small (usually <3 mm)
pulmonary nodules, typically with basal and peripheral
predominance, are seen in the interstitial spaces and
create the unique tree branch–like morphology of DPO.
The calcific density of these nodules can be more easily
appreciated in the bone window.[6]
One major diagnostic challenge to recognising DPO
lies in distinguishing it from concurrent pulmonary
disease. In several case reports, patients had concurrent
interstitial lung disease (ILD), especially usual interstitial
pneumonia.[1] [3] Although the pathophysiology of DPO remains to be elucidated, pulmonary ossifications are
often found in areas with more extensive fibrosis.[6] It has
been hypothesised that in injured lung tissue, pulmonary
fibroblasts and macrophages will undergo metaplasia into
osteoblasts and osteoclasts. This may give rise to ectopic
ossification.[7] ILD shares some common radiological
features with DPO, such as involvement of the alveolar
interstitium with basal and peripheral predominance.[8]
Reviewing the lung fields after adjusting to the bone
window is helpful to reveal the dendriform ossifications
amongst the labyrinthine reticulations in ILD.
Another challenge in reaching an unequivocal
radiological diagnosis of DPO is related to the long
list of differentials for diffuse hyperdense pulmonary
nodules. They include, but are not limited to, pulmonary
alveolar microlithiasis, previous infections (such as
healed varicella pneumonia, tuberculosis, and fungal
infections), pneumoconiosis, metastasis, hypercalcaemia,
sarcoidosis, and amyloidosis.[9] Although the branching
interstitial involvement in DPO is a key distinguishing
radiological feature,[10] it may not always be convincingly
identified. Other radiological features, e.g., the fine
sand–like microcalcifications in pulmonary alveolar
microlithiasis,[11] the random scatter and coalescence of
nodules in healed varicella pneumonia,[12] the presence of
mediastinal lymphadenopathy or calcified lymph nodes
in tuberculosis, histoplasmosis and sarcoidosis, can
provide clues to the correct diagnosis.[9] In addition, the
patient’s demographics, occupational risks, inhalation
exposure and medical history are often helpful to reduce
the possible differentials.
The biggest hurdle to a conclusive radiological
diagnosis for this patient was his young age of onset.
In a retrospective study by Gruden et al,[13] the mean age
of 52 patients with DPO was 78 years (range, 58-96).
The authors also proposed some potential risk factors,
two of which were present in our patient—OSA and
gender. OSA was found in 15 patients (28.8%) in this
study.[13] It was hypothesised that aspiration could be the
underlying pathogenic pathway for DPO, since other
possible risks factors included gastroesophageal reflux
disease and debilitating neurological conditions. As
for gender, DPO has a strong male predilection. In past
studies, the proportion of males ranged from >85%[3] to
100%13. Nonetheless while OSA is not uncommon in
young men, DPO is rare, if not unprecedented. A case
series by Baddini Martinez and Ramos[14] of three patients
diagnosed with DPO >20 years after transitory inhalation
of hydrocarbon combustion products may offer some
insight into our case. Our patient recounted a brief episode
of heavy fume exposure after lighting firecrackers during
his formative years. Baddini Martinez and Ramos[14]
hypothesised that these combustion products could
initiate an inflammatory process in lung tissue, causing
deposition of collagen and dystrophic calcification, and
subsequently inducing bone and marrow precursor cells.
There is no specific management guideline for DPO.
Some patients are asymptomatic while others require
symptomatic relief of respiratory complaints. Follow-up
monitoring by interval imaging may be helpful.[3] [10]
REFERENCES
1. Lara JF, Catroppo JF, Kim DU, da Costa D. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification: report of a 26-year autopsy experience. Arch Pathol Lab Med. 2005;129:348-53. Crossref
2. Müller KM, Friemann J, Stichnoth E. Dendriform pulmonary ossification. Pathol Res Pract. 1980;168:163-72. Crossref
3. Fernández-Bussy S, Labarca G, Pires Y, Díaz JC, Caviedes I. Dendriform pulmonary ossification. Respir Care. 2015;60:e64-7. Crossref
4. Reddy TL, von der Thüsen J, Walsh SL. Idiopathic dendriform pulmonary ossification. J Thorac Imaging. 2012;27:W108-10. Crossref
5. Felson B, Schwarz J, Lukin RR, Hawkins HH. Idiopathic pulmonary ossification. Radiology. 1984;153:303-10. Crossref
6. Kim TS, Han J, Chung MP, Chung MJ, Choi YS. Disseminated dendriform pulmonary ossification associated with usual interstitial pneumonia: incidence and thin-section CT-pathologic correlation. Eur Radiol. 2005;15:1581-5. Crossref
7. Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology. 2006;38:45-8. Crossref
8. Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med.
2018;6:138-53. Crossref
9. Marchiori E, Souza AS Jr, Franquet T, Müller NL. Diffuse high-attenuation pulmonary abnormalities: a pattern-oriented diagnostic approach on high-resolution CT. AJR Am J Roentgenol.
2005;184:273-82. Crossref
10. Jamjoom L, Meziane M, Renapurkar RD. Dendriform pulmonary ossification: report of two cases. Indian J Radiol Imaging. 2013;23:15-8. Crossref
11. Korn MA, Schurawitzki H, Klepetko W, Burghuber OC. Pulmonary
alveolar microlithiasis: findings on high-resolution CT. AJR Am J
Roentgenol. 1992;158:981-2. Crossref
12. Kim JS, Ryu CW, Lee SI, Sung DW, Park CK. High-resolution CT findings of varicella-zoster pneumonia. AJR Am J Roentgenol. 1999;172:113-6. Crossref
13. Gruden JF, Green DB, Legasto AC, Jensen EA, Panse PM. Dendriform pulmonary ossification in the absence of usual
interstitial pneumonia: CT features and possible association with
recurrent acid aspiration. AJR Am J Roentgenol. 2017;209:1209-15. Crossref
14. Baddini Martinez JA, Ramos SG. Inhalation of hydrocarbon
combustion products as a cause of dendriform pulmonary
ossification. Med Hypotheses. 2008;71:981-2. Crossref