Isolated Unilateral Facet Tuberculosis of the Lumbar Spine: A Case Report
CASE REPORT
Hong Kong J Radiol 2024 Jun;27(2):e106-11 | Epub 24 May 2024
Isolated Unilateral Facet Tuberculosis of the Lumbar Spine: A Case Report
HL Chan1, YH Sin2, KF Tam1
1 Department of Radiology, North District Hospital, Hong Kong SAR, China
2 Department of Orthopaedics and Traumatology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr HL Chan, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: chl284@ha.org.hk
Submitted: 19 July 2023; Accepted: 5 October 2023.
Contributors: All authors designed the study. HLC and YHS acquired the data. All authors analysed the data. HLC and YHS drafted the
manuscript. KFT critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki. Patient’s informed consent for the study and publication was obtained.
INTRODUCTION
Spinal tuberculosis without vertebral body involvement
is rare and can be challenging to diagnose given its non-specific
clinical symptoms and atypical radiological
features. We present a case of isolated unilateral facet
tuberculosis of the lumbar spine in a 70-year-old man
who presented with right lower limb radiating pain,
weight loss and dry cough. Subsequent radiological
and histopathological examination confirmed spinal
tuberculosis involving isolated unilateral facet.
CASE PRESENTATION
A 70-year-old man presented with a 3-month history of
back pain and radiating pain as well as impaired sensation
over the right buttock and lateral side of the right lower
limb since January 2023. He had no history of trauma or
injury but had lost 10 pounds in the last 6 months and had
a chronic dry cough with occasional shortness of breath
at rest. Medical history was otherwise unremarkable
with no history of primary neoplasm or family history
of malignancy. He was admitted to the orthopaedic ward after presenting to the emergency department due to unbearable pain.
Physical examination revealed fair general condition,
blood pressure 164/85 mm Hg, pulse rate 102 bpm, and
no fever. There was localised paraspinal muscle spasm
and tenderness at the right L4-L5 level. There was mild
reduced sensation over the right L3 to L5 dermatome and
right big toe dorsiflexion power reduced to grade 4/5 of
the Medical Research Council Scale for Muscle Strength
(corresponding to L5 myotome). Both lower limbs had
preserved power and limb reflexes were unremarkable.
Laboratory Investigation
Laboratory investigations revealed a microcytic
hypochromic anaemia with haemoglobin level 12.6 g/dL,
mean corpuscular volume 78.6 fL, mean corpuscular
haemoglobin level 25.4 pg, white blood cell count
8.4 × 109/L, and platelet count 266 × 109/L. Erythrocyte sedimentation rate was increased at 42 mm/h, and
C-reactive protein level was elevated at 20.1 mg/L. Liver and renal function tests were unremarkable. Blood
tests for tumour markers including carcinoembryonic
antigen, carbohydrate antigen 19-9, alpha-fetoprotein,
and prostate-specific antigen were all normal.
Radiological Investigations
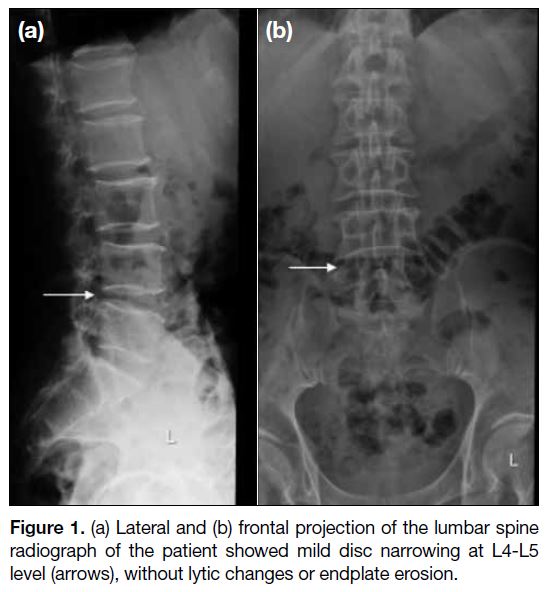
Lumbar spine radiograph showed mild disc narrowing at L4-L5 level with no lytic changes or endplate erosion
(Figure 1). No significant erosion or arthrosis at the facet
joints of the lumbar spine were noted.
Figure 1. (a) Lateral and (b) frontal projection of the lumbar spine
radiograph of the patient showed mild disc narrowing at L4-L5
level (arrows), without lytic changes or endplate erosion.
In view of the history of back pain and radiating right
lower limb pain, magnetic resonance imaging (MRI) of
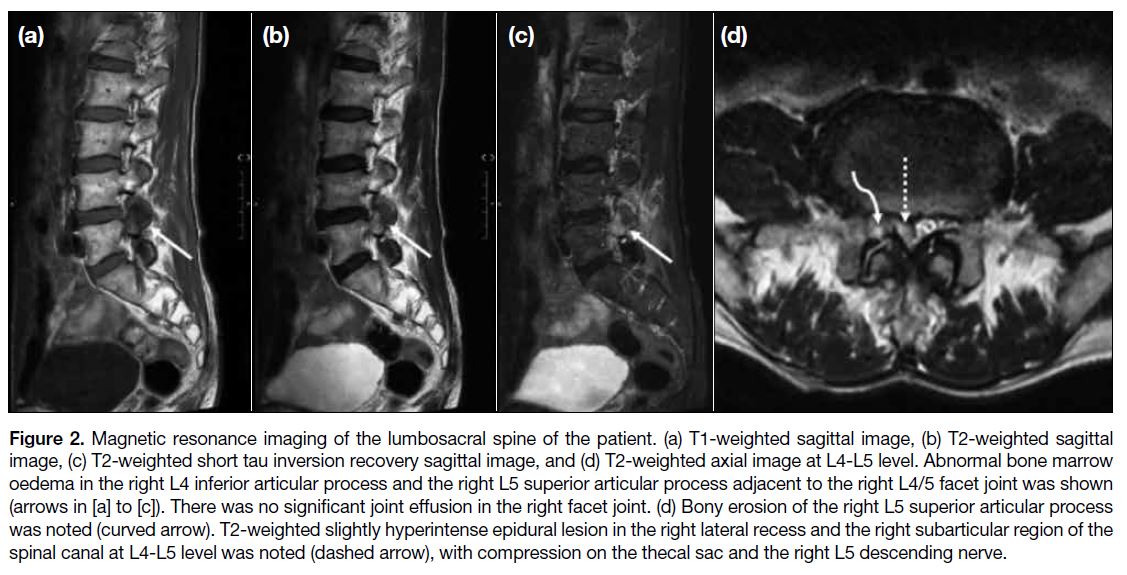
the lumbar spine was performed and revealed abnormal
bone marrow oedema in the right L4 inferior articular
process and the right L5 superior articular process
adjacent to the right L4/5 facet joint. No significant joint
effusion in the right facet joint was seen. Bony erosion
of the right L5 superior articular process was also noted.
There was a T2-weighted slightly hyperintense epidural
lesion in the right lateral recess and the right subarticular
region of the spinal canal at level L4-L5, compressing
the thecal sac and the right L5 descending nerve (Figure 2). The rest of the spine in T2-weighted sagittal screening was unremarkable.
Figure 2. Magnetic resonance imaging of the lumbosacral spine of the patient. (a) T1-weighted sagittal image, (b) T2-weighted sagittal image, (c) T2-weighted short tau inversion recovery sagittal image, and (d) T2-weighted axial image at L4-L5 level. Abnormal bone marrow oedema in the right L4 inferior articular process and the right L5 superior articular process adjacent to the right L4/5 facet joint was shown (arrows in [a] to [c]). There was no significant joint effusion in the right facet joint. (d) Bony erosion of the right L5 superior articular process was noted (curved arrow). T2-weighted slightly hyperintense epidural lesion in the right lateral recess and the right subarticular region of the spinal canal at L4-L5 level was noted (dashed arrow), with compression on the thecal sac and the right L5 descending nerve.
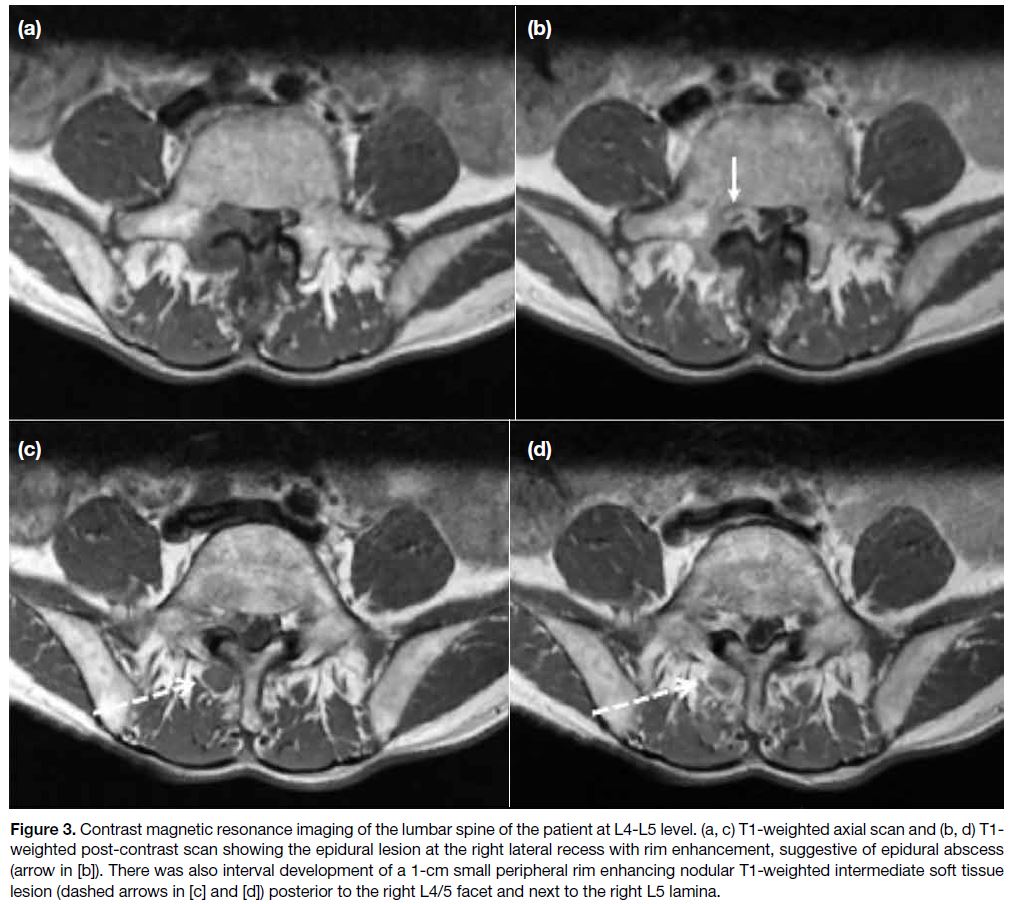
A contrast MRI (intravenous gadolinium injection) was
performed 10 days later (Figure 3), which confirmed
the epidural lesion at the right lateral recess with rim
enhancement, suggestive of an epidural abscess. There
was also interval development of a 1-cm small peripheral
rim-enhancing nodular T1-weighted intermediate soft
tissue lesion posterior to the right L4/5 facet, next to the
right L5 lamina.
Figure 3. Contrast magnetic resonance imaging of the lumbar spine of the patient at L4-L5 level. (a, c) T1-weighted axial scan and (b, d) T1-weighted post-contrast scan showing the epidural lesion at the right lateral recess with rim enhancement, suggestive of epidural abscess (arrow in [b]). There was also interval development of a 1-cm small peripheral rim enhancing nodular T1-weighted intermediate soft tissue lesion (dashed arrows in [c] and [d]) posterior to the right L4/5 facet and next to the right L5 lamina.
Dual-energy computed tomography (DECT) of the lumbar spine showed no internal calcification or
significant monosodium urate on colour-coded DECT.
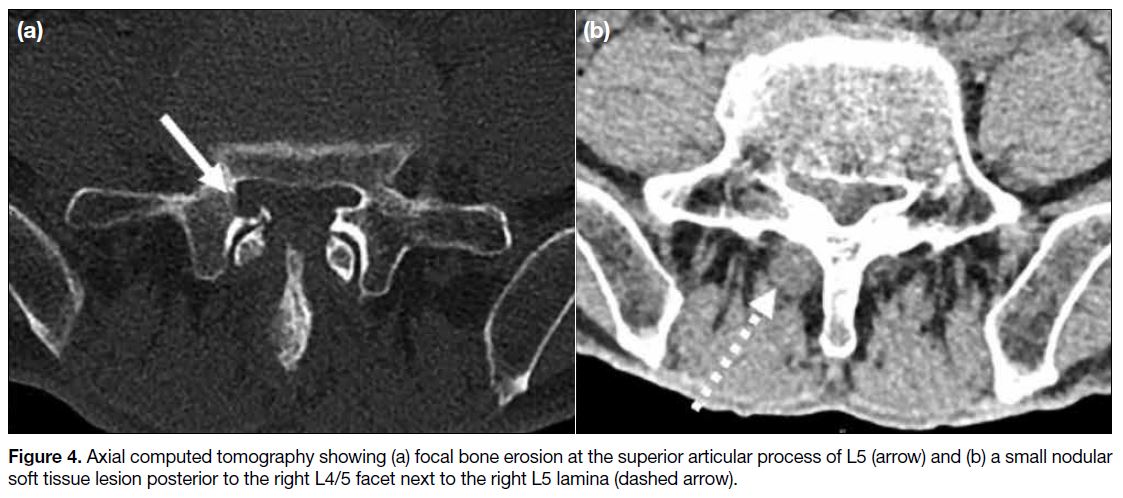
Computed tomography (CT) showed focal bone erosion
at the superior articular process of L5 and small nodular
soft tissue posterior to the right L4/5 facet next to the
right L5 lamina (Figure 4), corresponding to the MRI findings.
Figure 4. Axial computed tomography showing (a) focal bone erosion at the superior articular process of L5 (arrow) and (b) a small nodular soft tissue lesion posterior to the right L4/5 facet next to the right L5 lamina (dashed arrow).
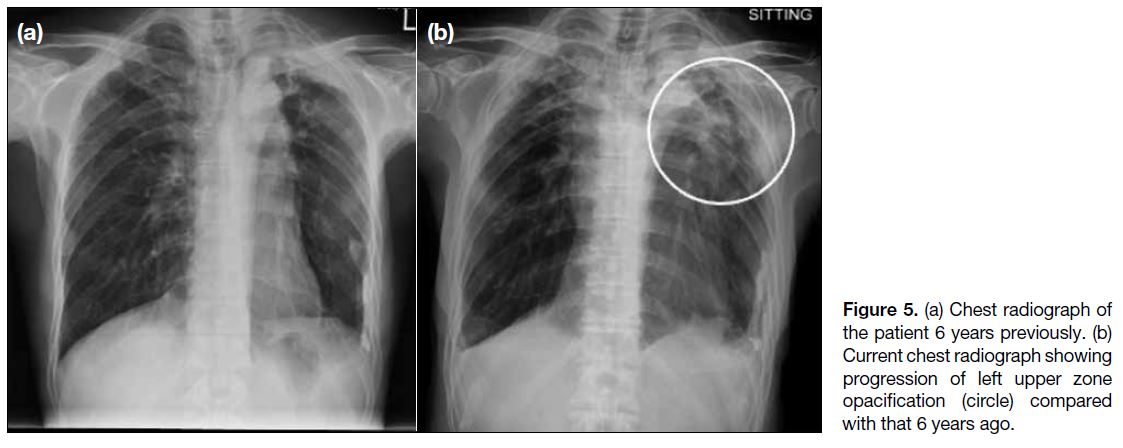
Chest radiograph revealed progression of left upper
zone opacification compared with an X-ray 6 years
previously (Figure 5). Sputum acid-fast bacillus smear
test was negative, but sputum culture 6 weeks later
grew Mycobacterium tuberculosis. At this stage the
top differential diagnosis was infective facet arthritis (particularly tuberculosis) but other possible differential
diagnoses included gouty arthritis (unlikely because of
negative urate deposition on DECT and no prior history
of gouty arthritis) and malignancy (but no personal
or family history of neoplasm and normal tumour
markers). Radiologically, the imaging features were
more suggestive of a joint disease with involvement of
both articular sides of the right L4/5 facet. Malignancy or
metastasis would be considered unlikely to cross the facet
joint. The interval development of a small peripheral
rim-enhancing nodular soft tissue lesion posterior to
the right L4/5 facet after 10 days (on contrast MRI)
also pointed to infection and made other differential
diagnoses unlikely.
Figure 5. (a) Chest radiograph of the patient 6 years previously. (b) Current chest radiograph showing progression of left upper zone opacification (circle) compared with that 6 years ago.
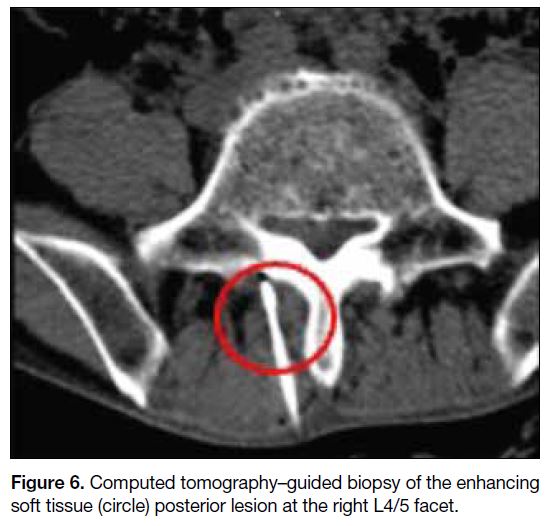
CT-guided biopsy of the enhancing soft tissue lesion
posterior to the right L4/5 facet was performed (Figure 6) using a co-axial system with a 17-gauge coaxial introducer (Merit Medical, South Jordan [UT], United
States) and an 18-gauge Temno needle (Merit Medical,
South Jordan [UT], United States). A total of four passes
of biopsy were performed. Pathology results showed
focal epithelioid granuloma formation. Scant acid-fast
bacilli were highlighted by Ziehl-Neelsen stain but no
fungal organisms were detected by periodic acid–Schiff
or Grocott’s methenamine silver stains. There was no
evidence of malignancy. Overall features suggested
mycobacterial infection.
Figure 6. Computed tomography–guided biopsy of the enhancing soft tissue (circle) posterior lesion at the right L4/5 facet.
Microbiology consultation suggested pulmonary
tuberculosis with bone and joint involvement. The
patient was commenced on antitubercular therapy of
isoniazid, rifampicin, pyrazinamide, and ethambutol. A
course of at least 9 to 12 months was planned in view of
bone involvement.
The patient’s neurological symptoms improved after
1 month of treatment. Laboratory tests also showed
erythrocyte sedimentation rate reduced to 35 mm/h and
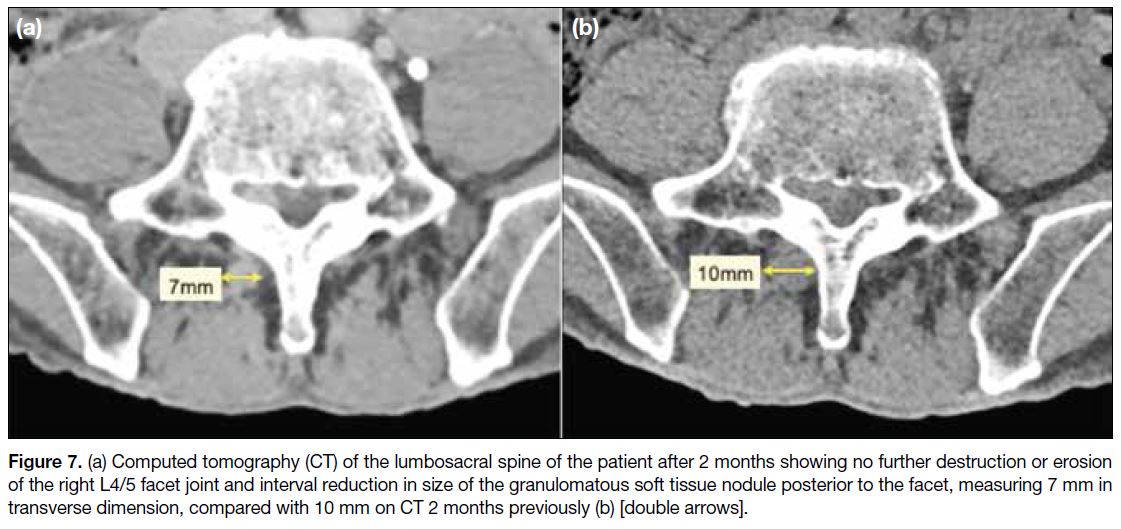
C-reactive protein level reduced to 6.2 mg/L. CT of the
lumbosacral spine after 2 months showed no further
destruction or erosion of the right L4/5 facet joint and interval reduction in size of the granulomatous soft
tissue nodule posterior to the right L4/5 facet
(Figure 7).
Figure 7. (a) Computed tomography (CT) of the lumbosacral spine of the patient after 2 months showing no further destruction or erosion of the right L4/5 facet joint and interval reduction in size of the granulomatous soft tissue nodule posterior to the facet, measuring 7 mm in transverse dimension, compared with 10 mm on CT 2 months previously (b) [double arrows].
DISCUSSION
Mycobacterium tuberculosis is a slow-growing
fastidious, aerobic bacillus. The primary site of
infection is usually the lungs with spinal infection
always secondary and occurring by hematogenous
dissemination.[1] Spinal tuberculosis accounts for
approximately 10% of extrapulmonary tuberculosis.[2] It is disseminated through the intercostal arteries, lumbar arteries and the vertebral venous plexus (Batson’s
plexus).[3]
Tuberculosis causes the development of granulomatous
inflammation that is characterised by the infiltration
of lymphocytes and epithelioid cells. These cells may
combine to form large Langhans-type giant cells and
ultimately lead to caseating necrosis of the affected
tissue, resulting in cold abscess formation.[1] Typical
histopathological features of spinal tuberculosis include
presence of tubercles, caseous necrosis, and tuberculous
granuloma, while occasional atypical features may be
encountered due to complex osteoclast development
via dysregulation of cytokines and chemokines.[4] [5] In
our case, pathology revealed granuloma formation, one
of the typical features, together with acid-fast bacilli
highlighted by Ziehl-Neelsen stain, confirming the
diagnosis of spinal tuberculosis.
Spinal tuberculosis most commonly manifests with
vertebral body involvement (anterior element) and is seen
in 90% to 95% of cases.[6] Posterior element involvement
is relatively rare and occurs by spread of infection via the
posterior vertebral venous plexus.[6] According to a study
by Narlawar et al[7] of patients with spinal tuberculosis,
only 3.06% (33/1076) showed isolated involvement
of posterior elements in the absence of any associated
involvement of anterior elements. Even more rare, only
1.3% (14/1076) showed isolated unilateral articular
process involvement.[7]
Posterior spinal tuberculosis is usually associated
with extradural granuloma and granulation tissue that
form near the dural sac with intraspinal extension
of tubercular granulation tissue or epidural abscess,
causing spinal canal stenosis.[8] The diagnosis of posterior
spinal tuberculosis can be challenging. The presenting
symptoms include localised back pain, radiating pain or
varying grades of paraplegia, along with other symptoms
related to primary tuberculosis such as weight loss
and chronic cough.[8] In our case, the patient presented
with right sciatica that is rather non-specific and can
mimic other musculoskeletal disorders (e.g., prolapsed
intervertebral disc). Nonetheless the history of weight
loss, chronic dry cough, and elevated inflammatory
markers prompted further investigation.
Imaging studies including MRI and DECT revealed
abnormal bone marrow oedema at the articular processes,
focal bony erosion, rim-enhancing epidural tissue and a
rim-enhancing nodular soft tissue lesion posterior to the
right L4/5 facet. These findings supported a diagnosis of
spinal tuberculosis,[9] later confirmed by histopathological
examination of the biopsy specimen.
The differential diagnoses of isolated unilateral facet
arthritis may include pyogenic arthritis, but no bacteria
were detected in the culture of the facet biopsy. Gouty
arthritis was also possible, but biopsy showed no gouty
deposit and DECT detected no urate deposit.
The treatment of spinal tuberculosis depends on the
severity of the disease and the presence of any neurological
deficits or spinal instability. In general, antitubercular
therapy is the mainstay of treatment. Surgery may be
required in cases where there is neurological deficit,
paravertebral abscess, spine instability or resistance to
antitubercular medications.[10]
The American Thoracic Society, the Centers for Disease
Control and Prevention, and the Infectious Diseases
Society of America recommend antitubercular therapy
for spinal tuberculosis comprised of an intensive phase
of 2 months of isoniazid, rifampicin, pyrazinamide, and
ethambutol, followed by a continuation phase for 7 to 10
months of isoniazid and rifampicin, with a total treatment
duration of 9 to 12 months.[11] This is longer than the
usual 6-month regimen for pulmonary tuberculosis. The
decision to use longer treatment for spinal tuberculosis is
because it is often associated with a higher risk of relapse
and treatment failure compared with other forms of tuberculosis. The prolonged therapy is thought to reduce
the risk of relapse and improve treatment outcomes.[12]
CONCLUSION
Isolated unilateral facet tuberculosis of the lumbar
spine is a rare form of spinal tuberculosis that poses
diagnostic challenges. Clinical and radiological features
are atypical. Diagnosis is usually based on a combination
of clinical, laboratory, and imaging findings, along with
a high index of suspicion. A multidisciplinary approach
involving orthopaedic surgeons, radiologists, and
microbiologists is necessary for optimal management
of spinal tuberculosis. Antitubercular therapy is the
mainstay of treatment, with surgery reserved for cases
with neurological deficits or spinal instability.
REFERENCES
1. Rajasekaran S, Soundararajan DC, Shetty AP, Kanna RM. Spinal tuberculosis: current concepts. Global Spine J. 2018;8(4 Suppl):96S-108S. Crossref
2. Batirel A. Tuberculous Spondylodiscitis. In: Sener A, Erdem H, editors. Extrapulmonary Tuberculosis. Cham: Springer International Publishing; 2019. p 83-99. Crossref
3. Shim HK, Cho HL, Lee SH. Spinal tuberculosis at the posterior element of spinal column: case report. Clin Neurol Neurosurg.
2014;124:146-50. Crossref
4. Hoshino A, Hanada S, Yamada H, Mii S, Takahashi M, Mitarai S, et al. Mycobacterium tuberculosis escapes from the phagosomes
of infected human osteoclasts reprograms osteoclast development
via dysregulation of cytokines and chemokines. Pathog Dis.
2014;70:28-39. Crossref
5. Li Y, Wang Y, Ding H, Zhang N, Ma A, Shi J, et al. Pathologic characteristics of spinal tuberculosis: analysis of 181 cases. Int J Clin Exp Pathol. 2020;13:1253-61.
6. Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical
perspective. Eur J Orthop Surg Traumatol. 2016;26:551-8. Crossref
7. Narlawar RS, Shah JR, Pimple MK, Patkar DP, Patankar T, Castillo M. Isolated tuberculosis of posterior elements of spine:
magnetic resonance imaging findings in 33 patients. Spine (Phila
Pa 1976). 2002;27:275-81. Crossref
8. Kumar K. Posterior spinal tuberculosis: a review. Mycobact Dis. 2017;7:1000243. Crossref
9. Boruah DK, Gogoi BB, Prakash A, Lal NR, Hazarika K, Borah KK. Magnetic resonance imaging evaluation of posterior
spinal tuberculosis: a cross-sectional study. Acta Radiol.
2021;62:1035-44. Crossref
10. Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal tuberculosis: diagnosis and management.
Asian Spine J. 2012;6:294-308. Crossref
11. Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers
for Disease Control and Prevention/Infectious Diseases Society of
America Clinical Practice Guidelines: treatment of drug-susceptible
tuberculosis. Clin Infect Dis. 2016;63:e147-95. Crossref
12. Pandita A, Madhuripan N, Pandita S, Hurtado RM. Challenges and controversies in the treatment of spinal tuberculosis. J Clin Tuberc Other Mycobact Dis. 2020;19:100151. Crossref