Grouped Amorphous Microcalcifications on Mammography: A Single-Centre 8-Year Retrospective Cohort Study on an Asian Population
ORIGINAL ARTICLE
Hong Kong J Radiol 2024 Jun;27(2):e100-5 | Epub 21 May 2024
Grouped Amorphous Microcalcifications on Mammography: A Single-Centre 8-Year Retrospective Cohort Study on an Asian Population
JK Fung, AYT Lai, AHC Wong, CKM Mo, KH Chin, WWC Wong
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr JK Fung, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: fk100@ha.org.hk
Submitted: 14 November 2022; Accepted: 29 September 2023.
Contributors: All authors designed the study, acquired and analysed the data. JKF, AYTL, AHCW, CKMM and KHC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: HKECREC-2022-062). Informed patient consent was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
Grouped amorphous microcalcifications on mammography are classified as BI-RADS (Breast Imaging
Reporting and Data System) category 4B. While recent international studies support a lower subcategory (4A), we
sought to measure the malignancy rate of grouped amorphous microcalcifications classified as BI-RADS category
4A or above in the Asian population.
Methods
All cases at our hospital with any kind of suspicious microcalcifications underwent either stereotactic-guided
vacuum-assisted biopsy or excisional biopsy with stereotactic localisation from 2013 to 2020 were retrieved.
Cases with grouped amorphous microcalcifications as the most suspicious morphology on magnified views were
selected. Only cases with at least 2 years of follow-up were included. Final histological diagnosis was based on the
highest grade of tissue diagnosis at biopsy or excision.
Results
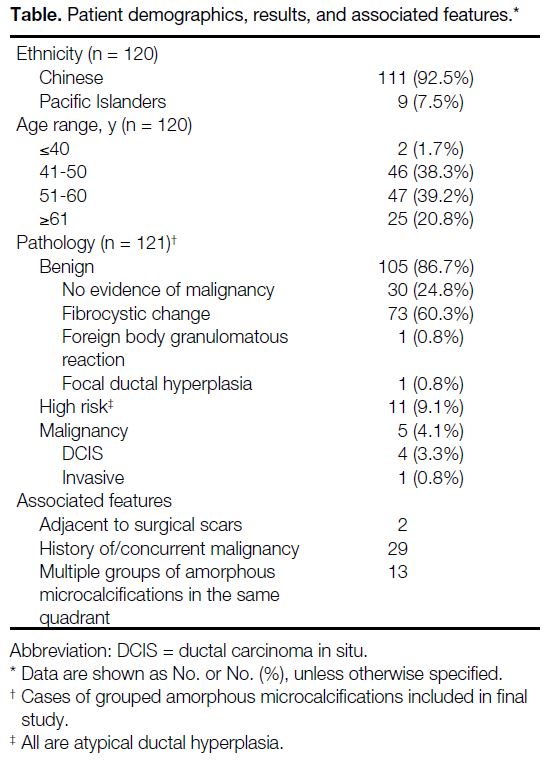
Among 333 biopsied cases, 121 were grouped amorphous microcalcifications. The majority of patients
(92.5%) were ethnic Chinese while the rest (7.5%) were Pacific Islanders. A total of 4.1% (n = 5) had malignant
final pathology, with four ductal carcinomas in situ (DCIS) and one invasive ductal carcinoma. A total of 9.1%
(n = 11) had high-risk pathology (all atypical ductal hyperplasia). In two cases, the microcalcifications were located
adjacent to surgical scars, with one diagnosed as DCIS.
Conclusion
The malignancy rate of grouped amorphous microcalcifications in our study is in line with recent studies,
providing support for classifying a BI-RADS category 4A for these calcifications. The majority of the malignant lesions
came back as DCIS, which carries promising post-treatment survival rates. Histological diagnosis remains indicated
for grouped amorphous microcalcifications, yet more nuanced management plans may be employed in the future.
Key Words: Breast; Calcinosis; Neoplasms
中文摘要
乳房X光檢查中的聚集無定形微鈣化:亞洲人群的單中心8年回顧性隊列研究
馮喬政、黎爾德、黃可澄、巫冠文、錢凱、黃慧中
引言
乳房X光檢查中的聚集無定形微鈣化歸類為 BI-RADS(乳房影像報告和數據系統)類別 4B。雖然最近的國際研究支持更低的子類別(4A),但我們嘗試評估亞洲人群中分類為BI-RADS 4A類或以上的聚集無定形微鈣化的惡性率。
方法
我們檢索於2013至2020年期間在本院接受立體定位真空輔助活檢或立體定位切除活檢的所有疑似微鈣化病例,並選擇放大視圖中最可疑的形態學為聚集無定形微鈣化的病例。我們僅納入至少追蹤兩年的病例。最終的組織學診斷是基於活檢或切除時組織學診斷的最高等級。
結果
333例活檢中,121例為無定形微鈣化。大多數患者(92.5%)是華裔,其餘患者(7.5%)是太平洋島民。總共4.1%(n = 5)最終病理為惡性,其中4例為導管原位癌,1例為浸潤性乳管癌。總共 9.1%(n = 11)有高風險病理(皆為非典型導管增生)。2例的微鈣化位於手術疤痕附近,其中1例被診斷為導管原位癌。
結論
本研究中分組的無定形微鈣化的惡性率與最近的研究一致,為這些鈣化的BI-RADS 4A 類分類提供了支持。大多數惡性病變以導管原位癌的形式復發,治療後的存活率良好。組織學診斷仍適用於聚集無定形微鈣化,但未來可能會採用更細緻的處理計劃。
INTRODUCTION
Amorphous morphology is categorised as 4B in the fifth
edition of BI-RADS (Breast Imaging Reporting and
Data System) of the American College of Radiology
(ACR), with a positive predictive value of malignancy
of approximately 20% based on older case series.[1]
Recent retrospective studies, including mostly Western
populations, have reported a malignancy rate of ≤10%
for grouped distribution, which may suggest a more
nuanced treatment approach.[2] [3]
Studies targeting grouped microcalcifications in the
Asian population are lacking. A relatively representative
study including 216 subjects by Iwase et al[4] reported a
malignancy rate of 2.8% in the Japanese population. The
objective of our study was to report the malignancy rate
of grouped amorphous microcalcifications in the Asian
population.
METHODS
Mammographically detected microcalcifications
classified as BI-RADS category ≥4A are routinely
scheduled for biopsy in our centre at Pamela Youde
Nethersole Eastern Hospital in Hong Kong. We retrieved all such cases that underwent either stereotactic-guided
vacuum-assisted biopsy or excisional biopsy with
stereotactic-guided localisation from 2013 to 2020 in our
centre from the electronic patient record.
Preprocedural mammograms were reviewed
independently by two breast radiologists (with 5 and
≥10 years of experience, respectively) on dedicated
5-megapixel breast imaging displays (MDMG-5221;
Barco NV, Kortrijk, Belgium). The radiologists were
blinded to the histological results.
All cases without dedicated magnification views
were excluded. Cases with grouped amorphous
microcalcifications, defined according to the fifth edition
of BI-RADS as the most suspicious morphology (i.e.,
either mammographically or sonographically having
a higher BI-RADS score, with category 4C being the
most suspicious), were documented by the radiologists.
Associated features, including proximity to previous
surgical scars, history of or concurrent malignancy,
and presence of multiple (≥3) groups of amorphous
calcifications in the same quadrant, were also recorded.
In the event of any discrepancies, a final decision was made by a third experienced breast radiologist (with ≥10
years of experience).
Only cases with ≥2 years’ follow-up in our institution
were included. Final histological diagnosis was obtained
from the electronic patient record and based on the
highest-grade tissue diagnosis at biopsy or excision. Final
statistical database was analysed with SPSS (Windows
version 29.0; IBM Corp, Armonk [NY], United States).
RESULTS
A total of 333 cases of mammographically-detected
suspicious microcalcifications underwent biopsy in our
centre across the 8-year span. Twenty-two cases without
dedicated magnification views were excluded. A total
of 130 clusters of amorphous microcalcifications in
130 cases were identified. Eight cases with <2 years of
follow-up in our institution were excluded (seven benign
and one high-risk pathology). One case of invasive
ductal carcinoma with architectural distortion as the
more suspicious feature was excluded from statistical
analysis.
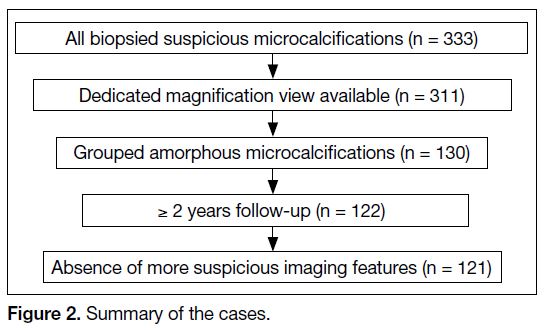
The final study included 121 groups of amorphous
microcalcifications in 120 patients (Figure 1). One
patient underwent two biopsies 6 years apart, for one
group of amorphous microcalcifications in each breast.
Both were proven benign. The majority of patients
(92.5%) were Chinese and the rest (7.5%) were Pacific
Islanders (Table).
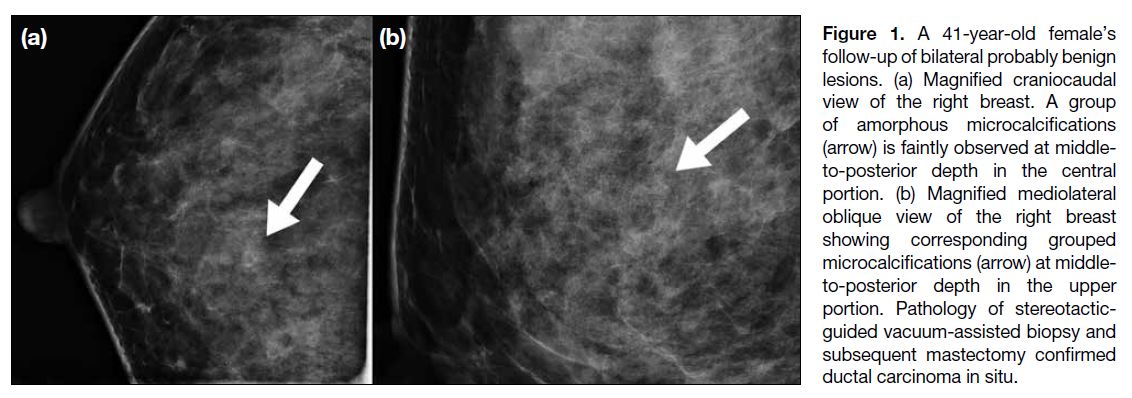
Figure 1. A 41-year-old female’s follow-up of bilateral probably benign lesions. (a) Magnified craniocaudal view of the right breast. A group of amorphous microcalcifications (arrow) is faintly observed at middle-to-posterior depth in the central portion. (b) Magnified mediolateral oblique view of the right breast showing corresponding grouped microcalcifications (arrow) at middle-to-posterior depth in the upper portion. Pathology of stereotactic-guided vacuum-assisted biopsy and subsequent mastectomy confirmed ductal carcinoma in situ.
Table. Patient demographics, results, and associated features.
Malignancy was found on pathology in 4.1% (n = 5) of
the cases. The majority of these cases (80%) came back
as ductal carcinoma in situ (DCIS) [Figure 2]. Two cases
underwent surgical excision, and two cases underwent
simple mastectomy. The remaining case was shown to
be invasive ductal carcinoma on the surgical specimen
of breast conservation treatment, which was upgraded
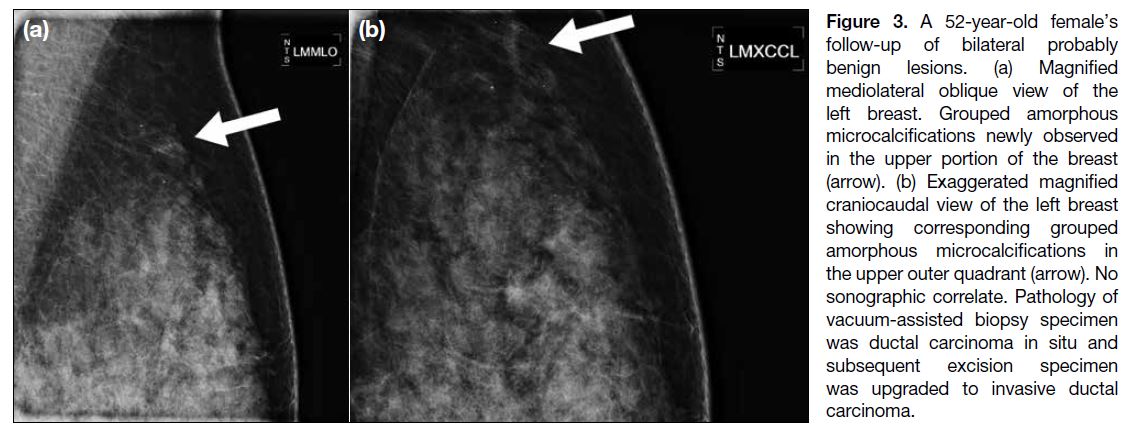
from DCIS on vacuum-assisted biopsy (Figure 3). It was
positive for oestrogen and progesterone receptors and
negative for human epidermal growth factor receptor 2.
Figure 2. Summary of the cases.
Figure 3. A 52-year-old female’s follow-up of bilateral probably benign lesions. (a) Magnified mediolateral oblique view of the left breast. Grouped amorphous microcalcifications newly observed in the upper portion of the breast (arrow). (b) Exaggerated magnified craniocaudal view of the left breast showing corresponding grouped amorphous microcalcifications in the upper outer quadrant (arrow). No sonographic correlate. Pathology of vacuum-assisted biopsy specimen was ductal carcinoma in situ and subsequent excision specimen was upgraded to invasive ductal carcinoma.
In all, 9.1% (n = 11) had high-risk pathology and all
yielded atypical ductal hyperplasia (ADH) [Table].
Eight cases underwent local excision and two underwent
wide local excision. There was no upgrade in the final
pathology of any of the 10 cases who underwent surgical
excision. One was concluded to have been completely
removed by vacuum-assisted excision in a joint
clinico-radiological-pathological meeting involving
pathologists, radiologists, and breast surgeons. Two
cases had the microcalcifications located adjacent to the
surgical scars for previous DCIS and invasive tubular
carcinoma and came back as benign foreign body
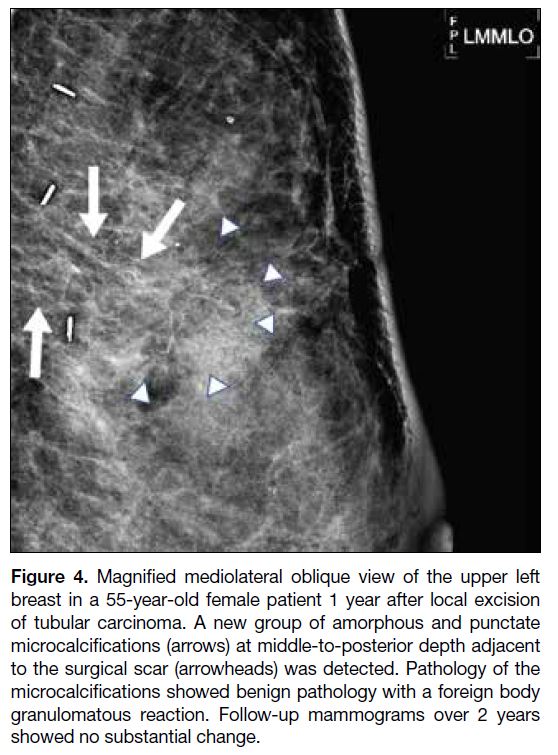
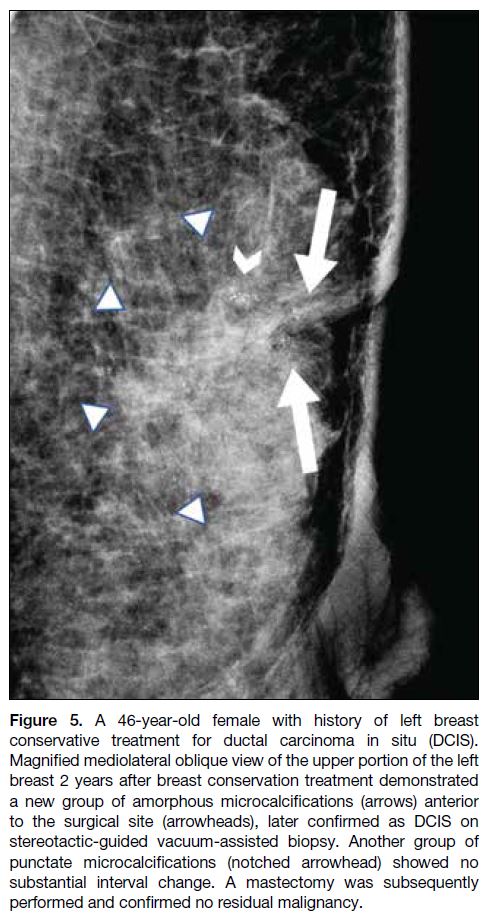
granulomatous reaction (Figure 4) and DCIS (Figure 5), respectively.
Figure 4. Magnified mediolateral oblique view of the upper left
breast in a 55-year-old female patient 1 year after local excision
of tubular carcinoma. A new group of amorphous and punctate
microcalcifications (arrows) at middle-to-posterior depth adjacent
to the surgical scar (arrowheads) was detected. Pathology of the
microcalcifications showed benign pathology with a foreign body
granulomatous reaction. Follow-up mammograms over 2 years
showed no substantial change.
Figure 5. A 46-year-old female with history of left breast
conservative treatment for ductal carcinoma in situ (DCIS).
Magnified mediolateral oblique view of the upper portion of the left
breast 2 years after breast conservation treatment demonstrated
a new group of amorphous microcalcifications (arrows) anterior
to the surgical site (arrowheads), later confirmed as DCIS on
stereotactic-guided vacuum-assisted biopsy. Another group of
punctate microcalcifications (notched arrowhead) showed no
substantial interval change. A mastectomy was subsequently
performed and confirmed no residual malignancy.
Within the 2-year follow-up period, there was no
recurrence in the malignant and high-risk groups. The
grouped macrocalcifications in the benign groups
all remained stable. As for the 13 cases with multiple
groups of amorphous microcalcifications, the other
groups also showed no substantial change, and thus were
not rebiopsied.
DISCUSSION
Amorphous microcalcifications with grouped
distribution are the most common type of suspicious
microcalcifications. Studies examining the malignancy
rate of grouped amorphous microcalcifications are limited. The single-institutional retrospective study
performed by Iwase et al4 is by far the relatively more
representative one covering the Asian population. Our
study sought to reveal the malignancy rate from an 8-year
database, with vast majority of Chinese ethnicity, and to
compare it with the reported rates of current international
studies.
The BI-RADS lexicon was developed by the ACR
to standardise the description and management
of mammographically detected findings. Various
combinations of microcalcification morphology and
distribution are subcategorised by their respective malignancy risks, aligned with positive predictive
values generated from ACR’s national mammography
database. BI-RADS category 4A and 4B lesions carry
malignancy risks of 2% to 10% and 10% to 50%,
respectively.[1] While biopsy is recommended for both
subcategories, BI-RADS category 4B lesions commonly
require a more comprehensive and meticulous
management plan, including rebiopsies in cases of
benign pathological findings, or even prophylactic
surgical excision subject to multiple factors and patient
preference. Other studies[2] [4] [5] have revealed malignancy
rates ranging from 2.8% to 7.6%, which fall within the
BI-RADS 4A category. This is in line with the 4.1%
malignancy rate (95% confidence interval = 1.4%-9.4%)
in our study, comprised of ethnic Chinese and Pacific
Islanders.
In recent years, screening programmes advocate a high
call-back biopsy rate, arousing controversies on the
acceptable malignancy risk and critics on unnecessary
biopsies and misallocated healthcare resources.[2] [3] [6]
Apart from recent evidence supporting a lower BI-RADS
subcategory as discussed above, the majority of
the malignancies come back as DCIS, which is known
to carry a high survival rate after surgical and optional
adjuvant treatments.[2] [3] This is also coherent with our
results, with 80% (n = 4) of malignant cases being
DCIS. Ongoing prospective trials such as the LORIS
trial (LOw RISk DCIS) and the COMET initiative (Core
Outcome Measures in Effectiveness Trials) are exploring
alternative treatment options including observation.[3]
Taking the latest evidence into consideration, a more
nuanced management plan may be employed in the
future.
Oligane et al[2] showed a higher risk of malignancy when
multiple amorphous groups are present in the same
quadrant. This feature was absent in the malignant cases
from our dataset. There is currently no agreed standard
management of the rest of the unbiopsied groups. In
our institution, when multiple groups of low-suspicion
microcalcifications are present in the same quadrant,
the largest group of the most suspicious morphology/distribution combination is targeted for biopsy, as
in the previous example in Figure 5. Majewski et al[7]
demonstrated a low malignancy rate in secondary biopsy
of other morphologically similar group(s) after initial
histopathology showing a high-risk lesion, meaning an
initial negative biopsy of one group is already useful to
predict negative outcome in the others. Hence, we also
advocate short follow-up intervals for the other groups and biopsy only if any increase in extent or suspicion are observed.
Limitations
There are a few limitations to this study. First, as
with other published studies, it retrospectively
included cases of suspicious microcalcifications from
histopathological results. There could be under-called
suspicious microcalcifications on initial mammograms
not subjected to biopsy. This also raises the issue of
inter-reader disagreement, particularly on calcification
descriptors such as morphology, as suggested by Lee
et al.[8] It is routine practice in our institution to perform
a preprocedural joint-specialty review for selected
ambiguous cases and for all external referrals to ensure
appropriate diagnosis and management.
Second, eight cases were excluded from the final study
due to lost or insufficient (<2 years) follow-up after
biopsy, with seven benign and one ADH on biopsy.
Oligane et al[2] reported a low (2.9%; all to DCIS) surgical
upgrade rate for high-risk lesions. In the very unlikely
situation that all eight cases turned out malignant 2 years
after the biopsy, the overall malignancy rate would be
10% (13/130), falling between the BI-RADS 4A and 4B
categories.
Third, our centre does not offer screening services. All
screening-detected cases in this study were referrals
from outside institutions. Of particular note, our centre
has a large volume of surveillance cases with a personal
history of breast malignancy or high-risk lesions, and
these have been a proven independent predictor of
higher malignancy risk by Oligane et al.[2] In our study,
29 cases (24%) of known or concurrent malignancy were
included (Table), where two of which were DCIS cases,
four of which were ADH, and the rest benign.
Finally, the single-centre setting may limit the external validity of the study.
CONCLUSION
The findings of this study are in line with recent studies
and support the BI-RADS category 4A for grouped
amorphous microcalcifications. The majority of the
malignant lesions also come back as DCIS, which carries
a promising post-treatment survival rate. Histological
diagnosis remains indicated, yet more nuanced
management plans may be implicated in future practice.
Further meta-analyses could be directed to exploring
differences in malignancy rates across ethnicities, age,
and breast density in order to tailor management.
REFERENCES
1. American College of Radiology. Breast Imaging Reporting and Data System, 5th edition. Reston, VA: American College of Radiology; 2013.
2. Oligane HC, Berg WA, Bandos AI, Chen SS, Sohrabi S, Anello M,
et al. Grouped amorphous calcifications at mammography:
frequently atypical but rarely associated with aggressive
malignancy. Radiology. 2018;288:671-9. Crossref
3. Moy L. Should we continue to biopsy all amorphous calcifications?
Radiology. 2018;288:680-1. Crossref
4. Iwase M, Tsunoda H, Nakayama K, Morishita E, Hayashi N, Suzuki K, et al. Overcalling low-risk findings: grouped amorphous calcifications found at screening mammography associated with
minimal cancer risk. Breast Cancer. 2017;24:579-84. Crossref
5. Kim SY, Kim HY, Kim EK, Kim MJ, Moon HJ, Yoon JH. Evaluation of malignancy risk stratification of microcalcifications detected on mammography: a study based on the 5th edition of BI-RADS. Ann Surg Oncol. 2015;22:2895-901. Crossref
6. Shen L, Ma X, Jiang T, Shen X, Yang W, You C, et al. Malignancy
risk stratification prediction of amorphous calcifications based
on clinical and mammographic features. Cancer Manag Res.
2021;13:235-45. Crossref
7. Majewski SA, Zuley ML, Pinnamaneni N, Ganott MA. Frequency
of carcinoma at secondary imaging-guided percutaneous breast
biopsy performed after a high-risk pathologic result at primary
biopsy. AJR Am J Roentgenol. 2013;201:439-47. Crossref
8. Lee AY, Wisner DJ, Aminololama-Shakeri S, Arasu VA, Feig SA,
Hargreaves J, et al. Inter-reader variability in the use of BI-RADS
descriptors for suspicious findings on diagnostic mammography:
a multi-institution study of 10 academic radiologists. Acad Radiol.
2017;24:60-6. Crossref