Kaposi Sarcoma of the Ankle Complicated by Emphysematous Osteomyelitis: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Dec;26(4):e29-32 | Epub 26 Oct 2023
Kaposi Sarcoma of the Ankle Complicated by Emphysematous Osteomyelitis: A Case Report
DS Poon, CSW Tang, DKM Mak, RP Houghton
Department of Radiology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
Correspondence: Dr DS Poon, Department of Radiology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom. Email: daniel.poon@kcl.ac.uk
Submitted: 24 Nov 2022; Accepted: 13 Mar 2023.
Contributors: DSP, DKMM and RPH designed the study. DSP, CSWT and DKMM acquired and analysed the data. DSP and CSWT drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki. The patient and her son provided consent for all treatment and procedures. Separate consent was provided by the patient specifically for the publication of this case.
INTRODUCTION
Emphysematous osteomyelitis (EO) is a rare skeletal
infection with a poor prognosis; mortality rate is estimated
to be approximately 32%.[1] Intraosseous osteomyelitis
was first described in 1981,[2] and only 34 cases have been
described in the literature.[3] Recognition of this condition
to facilitate prompt treatment is important for the best
possible outcome.
We present a case of endemic Kaposi sarcoma in a
human immunodeficiency virus–negative patient, with
disease recurrence complicated by EO.
CASE PRESENTATION
A 73-year-old female with known endemic Kaposi
sarcoma of the posterior left ankle diagnosed 4 years
previously via skin biopsy with human herpesvirus–8
positivity presented to our institution with rapidly
worsening hindfoot pain. At the time of initial diagnosis,
the patient underwent 20-Gy radiotherapy and two
further 8-Gy fractions over the following 2 years.
She also received six cycles of 20 mg/m2 pegylated
liposomal doxorubicin. There was an excellent response and her disease remained stable until the time
of her presentation 2 years later. She remained human
immunodeficiency virus negative throughout her clinical
encounters.
At presentation, the affected foot was extremely painful
to touch. An indurated ulcer was noted at the posterior
hindfoot, with the ulcer probing deep to bone; extensive
associated oedema circumferentially surrounding most
of the hindfoot was noted. The patient was pyrexic at
38.1℃ with mild tachycardia (with a heart rate of 110
beats per minute) on admission. Blood testing returned
a mild neutrophilia and a markedly elevated level of
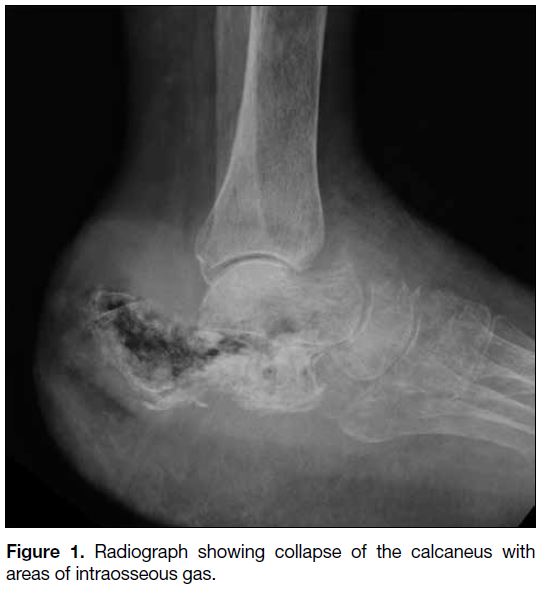
C-reactive protein. A hindfoot radiograph as part of
the patient’s initial workup revealed almost complete
collapse of the calcaneus with extensive intraosseous gas
(Figure 1).
Figure 1. Radiograph showing collapse of the calcaneus with areas of intraosseous gas.
The patient was immediately commenced on broadspectrum
antimicrobial therapy. The decision to perform
an emergent amputation was made following urgent
orthopaedic and oncology input on day of admission.
Preoperative contrast-enhanced magnetic resonance imaging was performed to aid surgical planning
and assess the extent of surrounding bone and soft
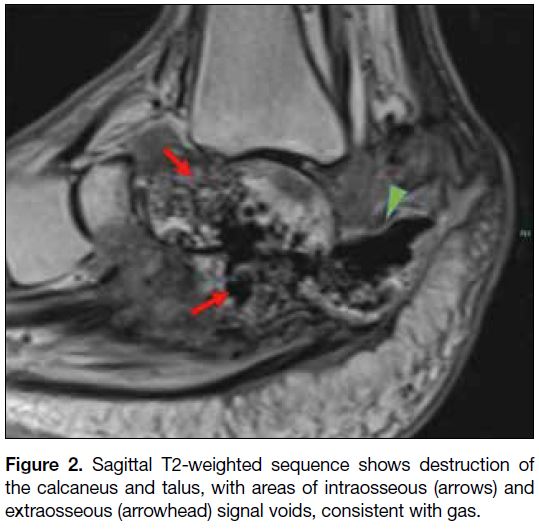
tissue involvement. The magnetic resonance imaging
confirmed extensive destruction of the calcaneus with
intraosseous gas as well as extraosseous gas surrounding
the posterior aspect of the remnant calcaneus; in addition,
destruction of the talus with intraosseous gas tracking
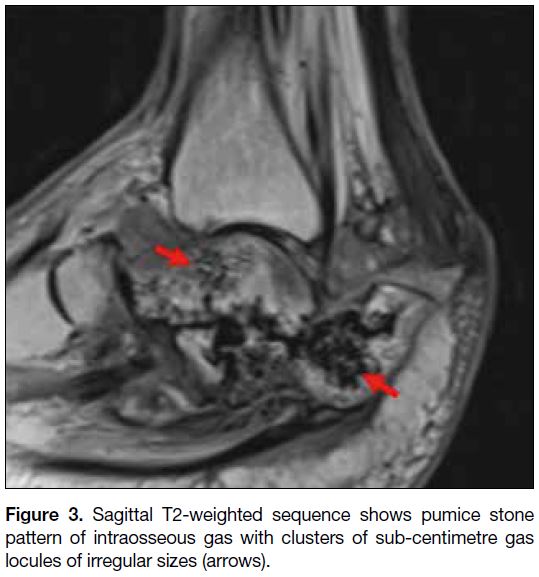
into the talar head/body was noted (Figure 2). A pumice
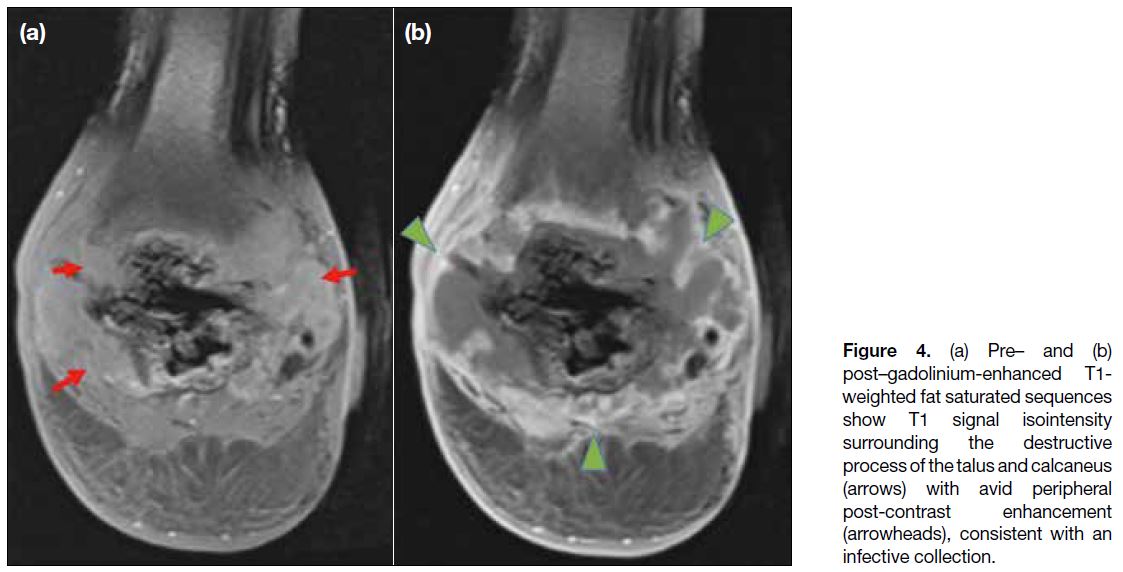
stone pattern of intraosseous gas was demonstrated (Figure 3). Following gadolinium administration, a large
peripherally enhancing collection surrounding the talus
and calcaneus was evident (Figure 4).
Figure 2. Sagittal T2-weighted sequence shows destruction of the calcaneus and talus, with areas of intraosseous (arrows) and extraosseous (arrowhead) signal voids, consistent with gas.
Figure 3. Sagittal T2-weighted sequence shows pumice stone pattern of intraosseous gas with clusters of sub-centimetre gas locules of irregular sizes (arrows).
Figure 4. (a) Pre– and (b)
post–gadolinium-enhanced T1-weighted fat saturated sequences show T1 signal isointensity surrounding the destructive process of the talus and calcaneus (arrows) with avid peripheral post-contrast enhancement (arrowheads), consistent with an infective collection.
The patient subsequently underwent a below-knee
amputation via a Bruckner’s approach. Perioperative
swabs of the ulcer and tissue sampling of the operative
specimen demonstrated extensive necrosis with
polymicrobial growth, including heavy growth of
Pseudomonas aeruginosa; local recurrence of the
Kaposi sarcoma was noted within the surrounding viable
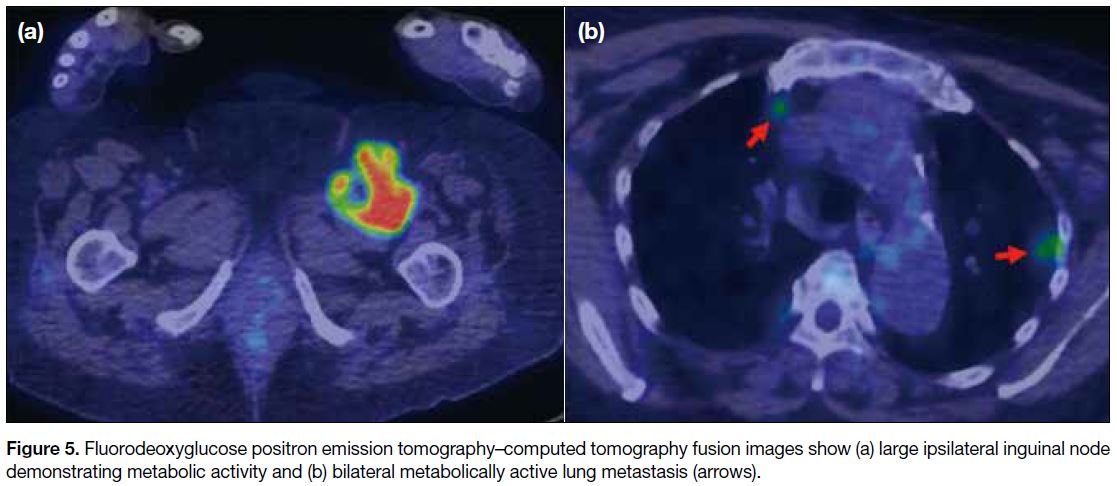
tissue. A subsequent postoperative fluorodeoxyglucose
positron emission tomography–computed tomography
showed metabolically active lung and ipsilateral inguinal
nodal metastases (Figure 5).
Figure 5. Fluorodeoxyglucose positron emission tomography–computed tomography fusion images show (a) large ipsilateral inguinal node demonstrating metabolic activity and (b) bilateral metabolically active lung metastasis (arrows).
The patient made a good postoperative recovery and
was discharged from hospital on the 23rd postoperative
day. A pre-discharge multidisciplinary case conference
opted for a further six cycles of liposomal doxorubicin
with palliative intent. The patient remains under joint
outpatient care in dermatology, oncology, and palliative
care.
DISCUSSION
To the best of our knowledge, there are no previously
described cases of EO developing as a result of Kaposi
sarcoma or its related treatment. EO is a rare and often
fatal skeletal infection, with <50 reported cases in the literature.[1] [3] [4] The most common sites of involvement
are the vertebrae followed by the lower extremities.[1]
Haematogenous and direct contiguous spread are welldescribed
routes of infection.[3]
The presence of intraosseous gas is a key diagnostic
feature of EO.[3] A pumice stone pattern of intramedullary
gas has been recently described as almost universal in
EO. This imaging feature is characterised by clusters of
intramedullary gas with irregular size[3]; our current case demonstrates this well. Further described secondary
features include the presence of adjacent soft tissue gas
and lack of cortical destruction, although these features
are not universal in EO.[3] Although the presence of
intraosseous gas is a prerequisite for diagnosis of EO,
it should be noted that alternative causes of intraosseous
gas, such as degenerative aetiologies, are much more
common, although these rarely give rise to the pumice
stone sign.[3] In cases of suspected EO, aggressive
empirical antimicrobial treatment is required given the prognosis; urgent surgical intervention remains the
mainstay of treatment for both spinal and extremity EO.
Radiotherapy is a well-established mode of treatment
for Kaposi sarcoma. As in our current case, radiotherapy
may serve as the main treatment in solitary cutaneous
lesions.[5] Late skin sequelae following radiotherapy are
common, and the possible risk of recurrence adjacent
to the radiotherapy field necessitates regular follow-up
and direct inspection by an experienced dermatologist.[5]
Despite regular reviews at 2 to 3 monthly intervals, our
patient unfortunately presented as an emergency with
an exquisitely painful foot and a rapidly progressive
ulcer. We postulate that the integrity of the skin was
initially compromised by rapid local disease recurrence
that ultimately led to an aggressive soft tissue and bone
infection via a direct route of polymicrobial seeding.
Our case highlights the importance of vigilance for rapid
progression of malignancies that have been previously stable. Furthermore, we wish to highlight the utility of a
cohesive multidisciplinary team in complex situations;
a team of radiologist, dermatologist, clinical oncologist,
orthopaedic surgeons, and infectious disease physicians
were involved in the emergent management of this
devastating foot infection.
REFERENCES
1. Luey C, Tooley D, Briggs S. Emphysematous osteomyelitis: a case report and review of the literature. Int J Infect Dis. 2012;16:e216-20. Crossref
2. Ram PC, Martinez S, Korobkin M, Breiman RS, Gallis HR, Harrelson JM. CT detection of intraosseous gas: a new sign of osteomyelitis. AJR Am J Roentgenol. 1981;137:721-3. Crossref
3. Small JE, Chea P, Shah N, Small KM. Diagnostic features of emphysematous osteomyelitis. Curr Probl Diag Radiol.
2022;51:666-72. Crossref
4. Sung S, Lee BH, Kim JH, Park Y, Ha JW, Moon SH, et al. Emphysematous osteomyelitis of the spine: a rare case report. Medicine (Baltimore). 2020;99:e21113. Crossref
5. Quéro L, Palich R, Valatin MA; On behalf of Cancervih Working Group. The role of radiotherapy in treating Kaposi’s sarcoma in HIV infected patients. Cancers (Basel). 2022;14:1915. Crossref