Sporadic Pulmonary Arteriovenous Malformation with a History of Stroke/Cerebrovascular Ischaemia Successfully Treated with Coil Embolisation: Two Case Reports
CASE REPORT
Hong Kong J Radiol 2023 Dec;26(4):e24-8 | Epub 7 Nov 2023
Sporadic Pulmonary Arteriovenous Malformation with a History of Stroke/Cerebrovascular Ischaemia Successfully Treated with Coil Embolisation: Two Case Reports
KO Cheung, CY Cheung, SW Sim, PSF Lee
Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr KO Cheung, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: cko398@ha.org.hk
Submitted: 5 Mar 2022; Accepted: 6 Nov 2022.
Contributors: All authors designed the study. KOC and SWS acquired and analysed the data. KOC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the patients for the purpose of the study.
CASE PRESENTATIONS
Case 1
A 64-year-old female, who had been a chronic smoker
for 47 years smoking one to five cigarettes per day, had
a history of ischaemic stroke at the age of 39. In 2019,
she was referred to the medical outpatient clinic of our
institution due to a 2-month history of cough, and a chest
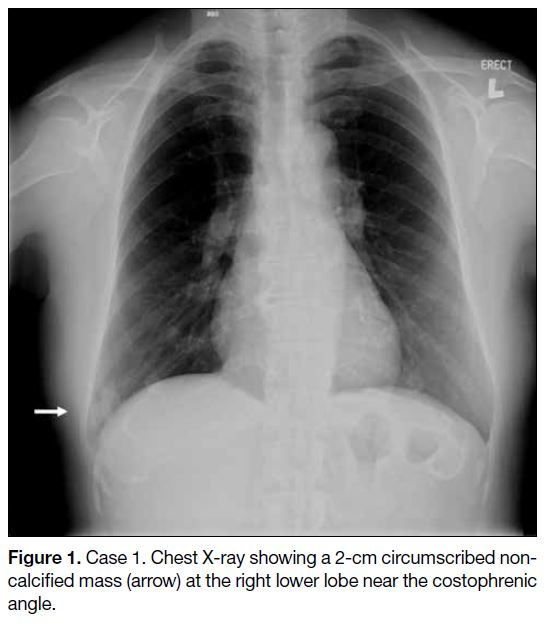
X-ray revealed an incidental finding of a 2-cm opacity
at the right lower zone (Figure 1). Subsequent contrast
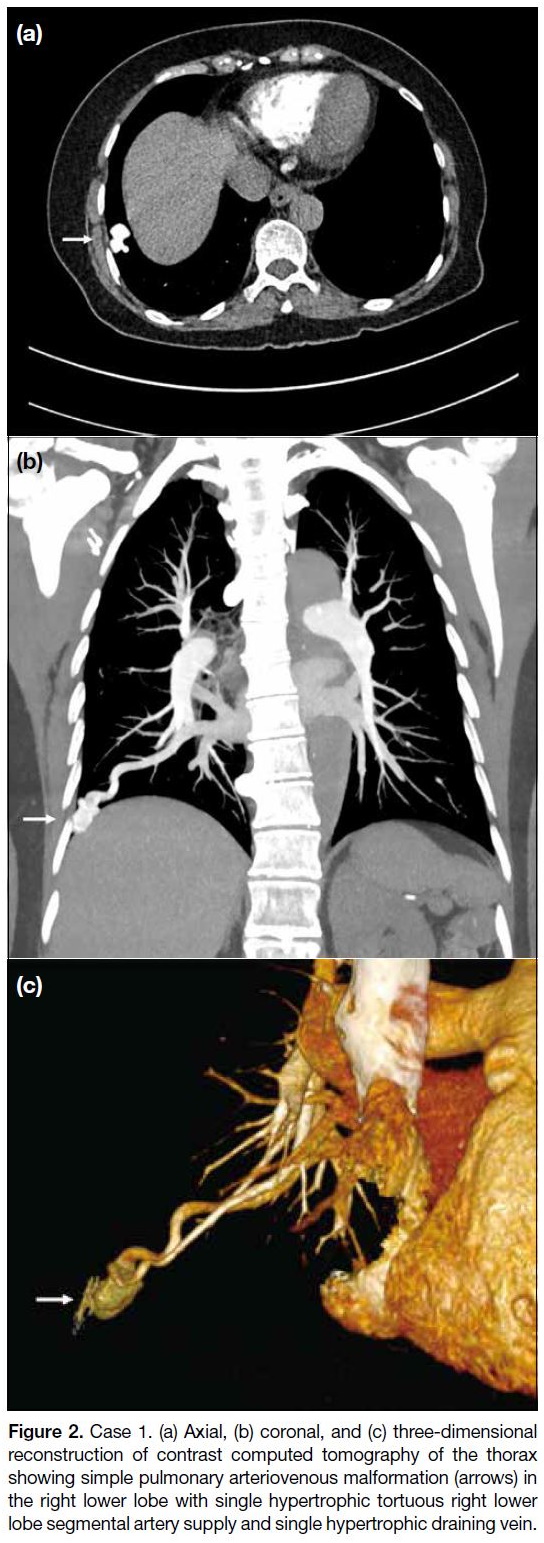
computed tomography (CT) of the thorax revealed a
2-cm avid arterial-enhancing lesion at the right lower
lobe near the costophrenic angle, corresponding to a
previous chest radiograph–detected lesion. With the
presence of a single hypertrophic tortuous pulmonary
artery supply and single hypertrophic early draining
pulmonary vein (Figure 2), a diagnosis of pulmonary
arteriovenous malformation (PAVM) was made.
Figure 1. Case 1. Chest X-ray showing a 2-cm circumscribed noncalcified mass (arrow) at the right lower lobe near the costophrenic angle.
Figure 2. Case 1. (a) Axial, (b) coronal, and (c) three-dimensional
reconstruction of contrast computed tomography of the thorax
showing simple pulmonary arteriovenous malformation (arrows) in
the right lower lobe with single hypertrophic tortuous right lower
lobe segmental artery supply and single hypertrophic draining vein.
The patient was treated with coil embolisation to the
PAVM in the right lower lobe, performed via a right antegrade common femoral vein approach. The right
lower lobe pulmonary artery was cannulated with
a 5-Fr multipurpose angiographic (MPA) catheter
(Cordis Corporation, Miami Lakes [FL], US). Digital
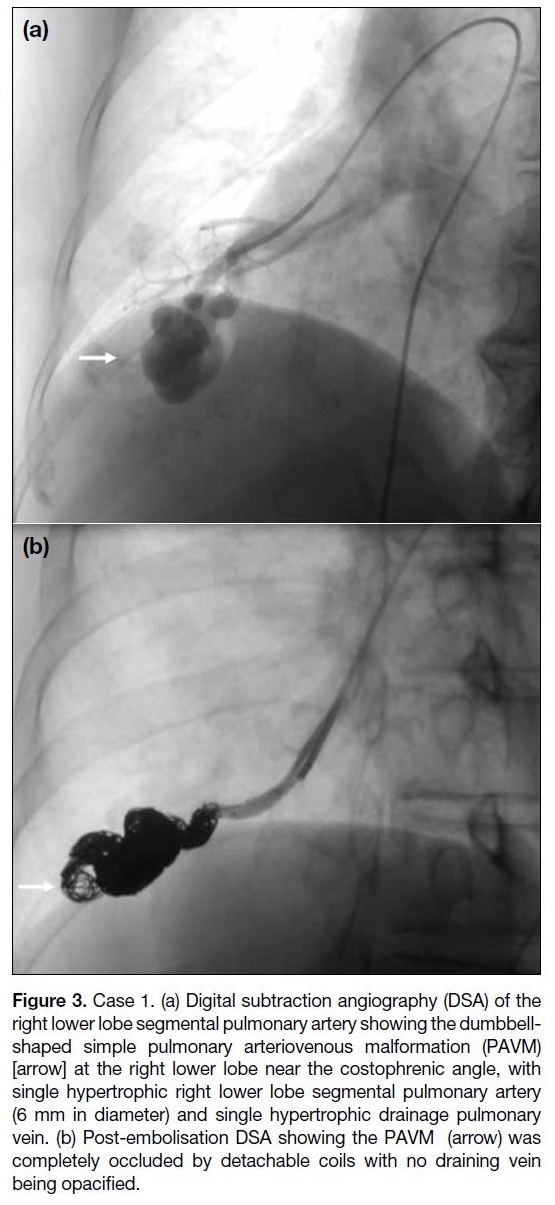
subtraction angiography (DSA) showed a dumbbell-shaped
simple PAVM with single hypertrophic right
lower lobe segmental pulmonary artery (6 mm in
diameter) and single hypertrophic draining right lower lobe pulmonary
vein (Figure 3a). The PAVM was selectively cannulated
with a 3-Fr Rebar Reinforced Microcatheter (Micro
Therapeutics Inc, Irvine [CA], US) through the 5-Fr
MPA catheter, and coil embolisation was performed
with 28 detachable coils (EV3 Concerto detachable
coils; Micro Therapeutics Inc, Irvine [CA], US). Post-embolisation
DSA revealed complete occlusion of the
PAVM (Figure 3b). No complication was encountered
and the patient was discharged home on the day of the
procedure. Nonetheless she defaulted her follow-up 2
months after embolisation.
Figure 3. Case 1. (a) Digital subtraction angiography (DSA) of the
right lower lobe segmental pulmonary artery showing the dumbbell-shaped
simple pulmonary arteriovenous malformation (PAVM)
[arrow] at the right lower lobe near the costophrenic angle, with
single hypertrophic right lower lobe segmental pulmonary artery
(6 mm in diameter) and single hypertrophic drainage pulmonary
vein. (b) Post-embolisation DSA showing the PAVM (arrow) was
completely occluded by detachable coils with no draining vein
being opacified.
Case 2
A 73-year-old female with controlled hypertension
was referred to the medical outpatient clinic of our
institution in 2019 for non-exertional chest pain. She
had a history of transient ischaemic attack at the age
of 64. Chest radiograph was unremarkable (Figure 4).
CT coronary angiogram incidentally revealed a cluster
of tortuous serpiginous vasculature at the anteromedial
retrosternal region of the right upper lobe, suspicious
of PAVM. Contrast CT of the thorax subsequently
confirmed this diagnosis (Figure 5).
Figure 4. Case 2. Chest radiograph was unremarkable. No focal
lung mass lesion was seen.
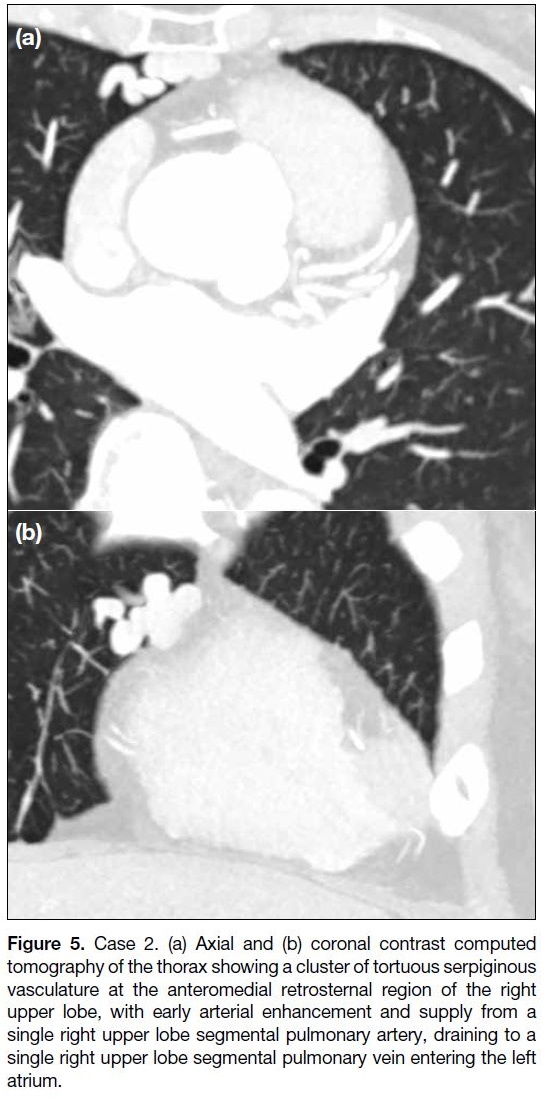
Figure 5. Case 2. (a) Axial and (b) coronal contrast computed
tomography of the thorax showing a cluster of tortuous serpiginous
vasculature at the anteromedial retrosternal region of the right
upper lobe, with early arterial enhancement and supply from a
single right upper lobe segmental pulmonary artery, draining to a
single right upper lobe segmental pulmonary vein entering the left
atrium.
The patient underwent coil embolisation to the PAVM in
the right upper lobe via a right antegrade common femoral
vein approach. The right upper lobe pulmonary artery
was cannulated with a 5-Fr MPA catheter. DSA showed
a 2.5 cm × 1.8 cm simple PAVM at the anteromedial
retrosternal region of the right upper lobe (Figure 6a and b). The PAVM was selectively cannulated with a 3-Fr
microcatheter through the 5-Fr MPA catheter, and coil
embolisation was performed with 21 detachable coils
(EV3 Concerto detachable coils).
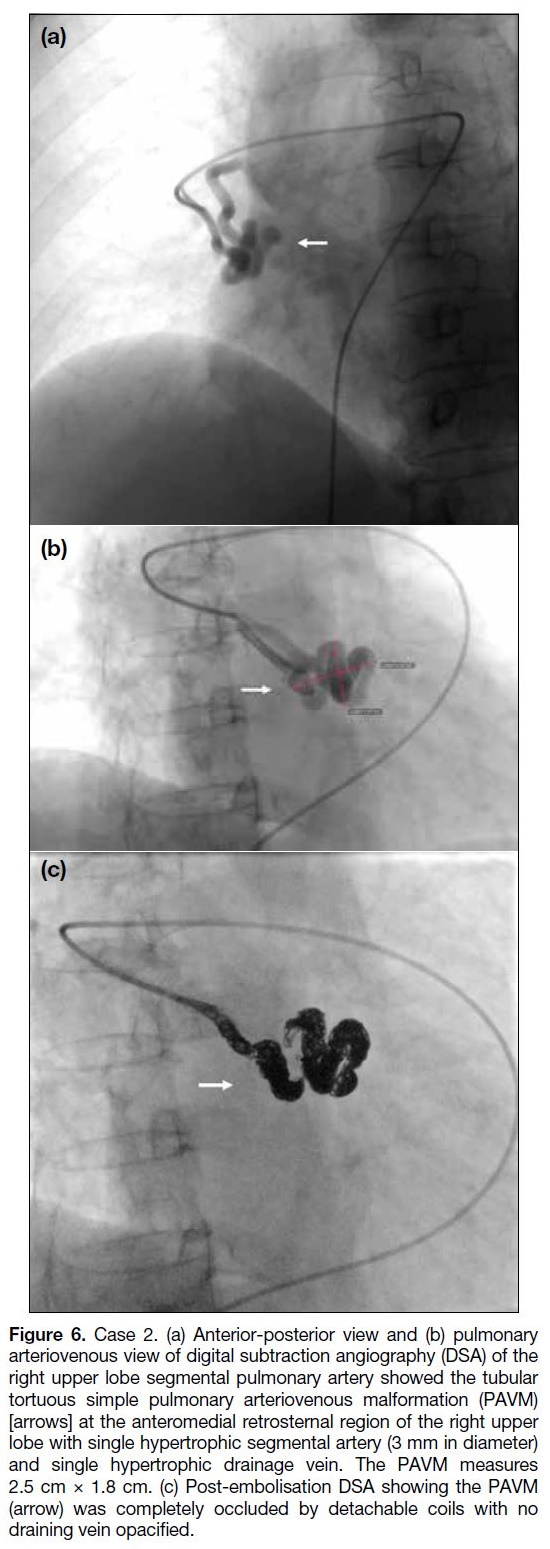
Figure 6. Case 2. (a) Anterior-posterior view and (b) pulmonary
arteriovenous view of digital subtraction angiography (DSA) of the
right upper lobe segmental pulmonary artery showed the tubular
tortuous simple pulmonary arteriovenous malformation (PAVM)
[arrows] at the anteromedial retrosternal region of the right upper
lobe with single hypertrophic segmental artery (3 mm in diameter)
and single hypertrophic drainage vein. The PAVM measures
2.5 cm × 1.8 cm. (c) Post-embolisation DSA showing the PAVM
(arrow) was completely occluded by detachable coils with no
draining vein opacified.
Post-embolisation DSA showed complete occlusion of
the PAVM (Figure 6c). No complication was encountered
and the patient was discharged home the next day. At
2-month follow-up, she remained asymptomatic and
had no exertional shortness of breath, ankle oedema or
haemoptysis. Follow-up radiological imaging was still
pending.
DISCUSSION
PAVM is a rare disease. It is a high-flow low-resistance
fistulous communication between the pulmonary artery
and pulmonary vein without interposition of a capillary
bed. Limited prevalence data suggest that PAVM
may affect up to 1 in 2600 individuals.[1] [2] The majority
(80%-90%) of PAVMs are congenital with concomitant
hereditary haemorrhagic telangiectasia (HHT), while the rest are sporadic. Sporadic PAVM is rarely reported
in the literature. According to Albitar et al,[3] sporadic
PAVMs occur more commonly in females, are most
often simple and single, have lower lobe predominance, and are associated with a high rate of neurological
complications. For our two patients, they had no
recurrent spontaneous epistaxis, mucosal telangiectasia
or family history of HHT, but with only visceral AVM.
Therefore, they had fewer than two of four Curaçao
diagnostic criteria, making HHT unlikely. They were
thus considered sporadic PAVM.
A PAVM can be asymptomatic or present with right-to-
left shunt symptoms, including dyspnoea, cyanosis,
haemoptysis and polycythaemia. The classic triad of dyspnoea on exertion, cyanosis, and clubbing should
alert the clinician to the possibility of a PAVM. Untreated
PAVMs can cause complications such as stroke, brain
abscess, rupture causing haemoptysis or haemothorax.
Both our patients had a history of stroke/cerebrovascular
ischaemia.
The preferred treatment for most patients with PAVM
is transcatheter embolisation. This has largely replaced
surgery because of its minimal invasiveness and short
recovery time. Surgical resection is reserved for PAVMs
not amenable to embolisation.
Treatment of PAVM can help prevent cerebral
complications such as transient ischaemic attack, stroke
or brain abscess; it can also help prevent pulmonary
complications such as lung haemorrhage, or decline in
exercise tolerance. A general rule may be to treat PAVMs
with feeding arteries >3 mm although more dedicated
centres will offer intervention when the feeding artery
exceeds 2 mm.[4] Nevertheless intervention is obviously
indicated when patients are symptomatic or when the
PAVM is enlarging.
A Grollman catheter is a right-angled pigtail catheter
with the curve on the reverse side of the angle. This
shape is most ideal to navigate through the heart or
to perform pulmonary angiogram. A reverse curve
catheter such as the Omni Flush catheter can also be
used to navigate through the right heart, but subsequent
exchange to an appropriate catheter for intervention is
needed. A steerable guiding sheath is another option. As
these catheters were not available at our centre, a 5-Fr
MPA catheter was used and we were able to successfully
navigate through the heart.
In DSA, we measure the diameter of arteriovenous
malformation to determine coil or plug size. An
undersized device should be avoided due to the risk of
distal migration. We may oversize the coil or plug by
20% to 50% relative to the feeding vessel.
The choice of embolic agents includes detachable
metallic coils, Amplatzer plug occluding device and
microvascular plug (MVP). Detachable metallic coils
enable more precise control. Coils should not be placed
directly into a PAVM because of the risk of paradoxical
coil embolisation. Instead, the tip of the coil should be
placed in a small side branch proximal to the target.
This allows it to prolapse into the feeding vessel. For
an Amplatzer plug occluding device, an appropriately sized sheath is placed into the feeding vessel and the
device is introduced via the sheath to the feeding vessel
proximal to the target. After confirming the position with
angiography, the device can be deployed by retracting
the sheath. For MVP, the MVP-5 system is advanced
into the feeding artery and the MVP plug is unsheathed
to occlude the target vessel. The position is confirmed by
selective angiogram with contrast injected via the rotator
haemostatic valve, followed by deployment of the MVP
using the detachment system.
Our patients were treated with detachable metallic coils. Heparin was given during the procedure to minimise the
risk of periprocedural paradoxical emboli, estimated at 1%.[4]
Complications of embolisation of PAVM are classified
as immediate, early or late. Immediate complications
include vascular injury and cardiac arrhythmia, but these
are rare. Air embolism is becoming rarer, probably due to
advances in technique and equipment. Precaution is also
taken to double-flush all catheters to avoid air bubbles and
clots. To avoid the complication of catheter entrapment
in a heart valve, extra care should be exercised when
withdrawing the catheter along the guidewire passing via
the heart valve post-embolisation. Pleurisy is considered
the most common early complication, occurring in up to
13% to 31% of patients.[5] It is usually self-limiting. Other
rarer early complications include pulmonary infarcts
and migration of embolic material. Late complications
include recanalisation and growth of new feeding arteries
due to collateralisation.[6] For both patients, they had no
post-embolisation complication detected.
After treatment of PAVMs, patients require long-term
follow-up imaging, preferably with a baseline scan 3 to
6 months after treatment to check if there is successful
sac involution. Follow-up imaging every 3 to 5 years
is necessary to capture any late collateralisation or recanalisation.[4]
CONCLUSION
Sporadic PAVM is rare and unlike congenital PAVM,
it is not associated with HHT. With embolisation being
minimally invasive and enabling faster recovery, it has
replaced surgery as the first-line treatment. We present
two cases of sporadic PAVM in middle-aged women with
a history of cerebrovascular accident. Both patients had
a single pulmonary artery supply and single pulmonary
vein drainage, and they were treated successfully with
coil embolisation without immediate complications.
REFERENCES
1. Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ; British Thoracic Society. British Thoracic Society Clinical Statement on Pulmonary Arteriovenous Malformations. Thorax. 2017;72:1154-63. Crossref
2. Nakayama M, Nawa T, Chonan T, Endo K, Morikawa S, Bando M, et al. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med. 2012;51:1677-81. Crossref
3. Albitar HA, Segraves JM, Almodallal Y, Pinto CA, De Moraes AG, Iyer VN. Pulmonary arteriovenous malformations in non-hereditary hemorrhagic telangiectasia patients: an 18-year retrospective study. Lung. 2020;198:679-86. Crossref
4. Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol. 2010;195:837-45. Crossref
5. Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158:643-61. Crossref
6. Hsu CC, Kwan GN, Evans-Barns H, van Driel ML. Embolisation for pulmonary arteriovenous malformation. Cochrane Database Syst Rev. 2018;1:CD008017. Crossref