Ileo-Uterine Fistula Following Endometrial Aspiration with Imaging Investigations and Hysteroscopic Correlation: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Sep;26(3):e14-8 | Epub 23 Aug 2023
Ileo-Uterine Fistula Following Endometrial Aspiration with Imaging Investigations and Hysteroscopic Correlation: A Case Report
BYK Wong1, Carina Kwa2, KM Choi2, TKK Lai3
1 Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, Tseung Kwan O Hospital, Hong Kong SAR, China
3 Department of Radiology, Tseung Kwan O Hospital, Hong Kong SAR, China
Correspondence: Dr BYK Wong, Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China. Email: wyk300@ha.org.hk
Submitted: 7 Apr 2022; Accepted: 5 Aug 2022.
Contributors: All authors designed the study. BYKW and CK acquired and analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki and has provided informed consent for all procedures.
INTRODUCTION
Ileo-uterine fistula is a very rare condition sporadically
reported in the literature. Its occurrence following
endometrial sampling, which is a relatively minimally
invasive procedure, has not been reported. We present
a case of ileo-uterine fistula following endometrial
aspiration and describe its imaging features with
hysteroscopic correlation.
CASE REPORT
A 73-year-old female presented with a 2-week history
of post-menopausal brownish vaginal spotting. Physical
examination was unremarkable except for an atrophic
cervix. Transabdominal and transvaginal ultrasound
revealed a small, retroverted uterus. Endometrial
sampling with Pipelle identified chronic endometritis
and pyometra. Ultrasound examination was repeated
because of persistent foul-smelling vaginal discharge.
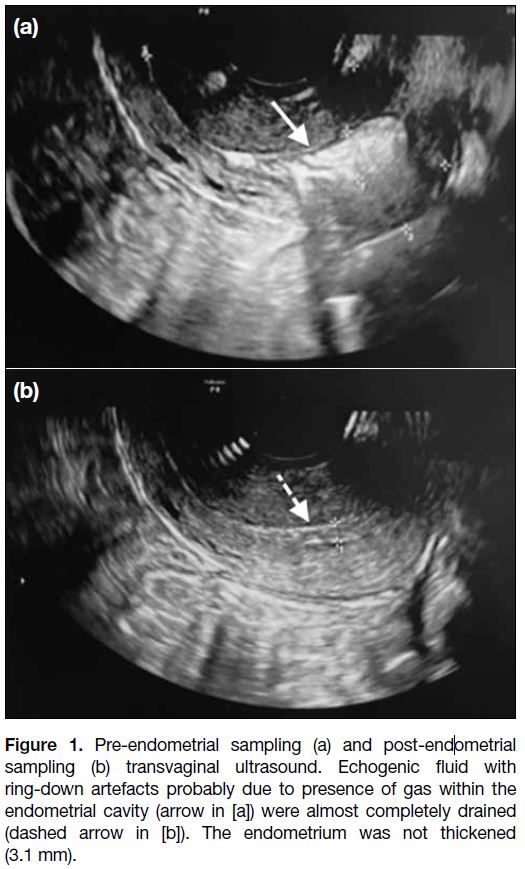
There was echogenic fluid with gaseous bubbles causing ring-down artefacts within the endometrial cavity. It was
drained by endometrial sampling and found to be pus
(Figure 1).
Figure 1. Pre-endometrial sampling (a) and post-endometrial
sampling (b) transvaginal ultrasound. Echogenic fluid with
ring-down artefacts probably due to presence of gas within the
endometrial cavity (arrow in [a]) were almost completely drained
(dashed arrow in [b]). The endometrium was not thickened
(3.1 mm).
Histology of the endometrial biopsy revealed
neutrophilic infiltrate, bacterial clumps, food particles,
intestinal epithelial fragments, and villi. There was no
evidence of malignancy. Diagnosis of pyometra was
made and perforation of uterus and/or intestinal-uterine
fistula were suspected.
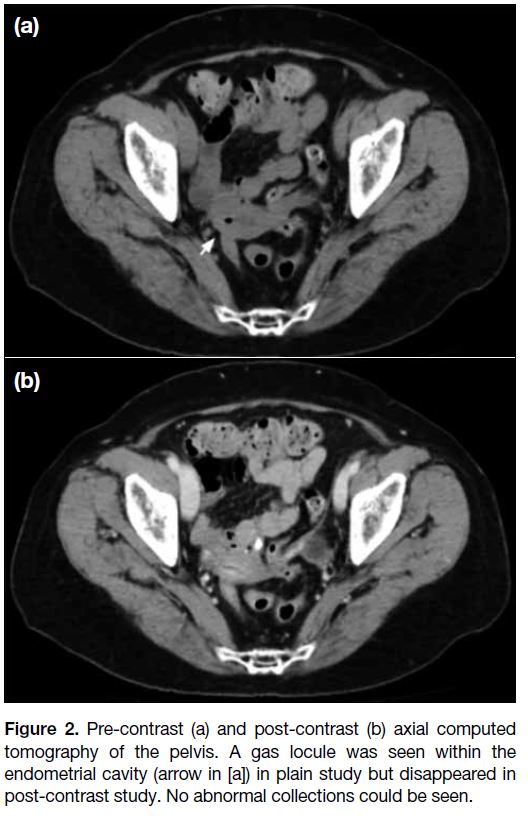
Urgent contrast-enhanced computed tomography (CT)
of the pelvis revealed an atrophic uterus. Some normal-looking
small bowel loops abutted the uterus. A tiny gas
locule was noted within the endometrial cavity on pre-contrast
study (Figure 2a) but was absent on post-contrast
study (Figure 2b). No definite enterouterine fistula was
found. There was no intra-abdominal collection, active
contrast extravasation or pneumoperitoneum.
Figure 2. Pre-contrast (a) and post-contrast (b) axial computed
tomography of the pelvis. A gas locule was seen within the
endometrial cavity (arrow in [a]) in plain study but disappeared in
post-contrast study. No abnormal collections could be seen.
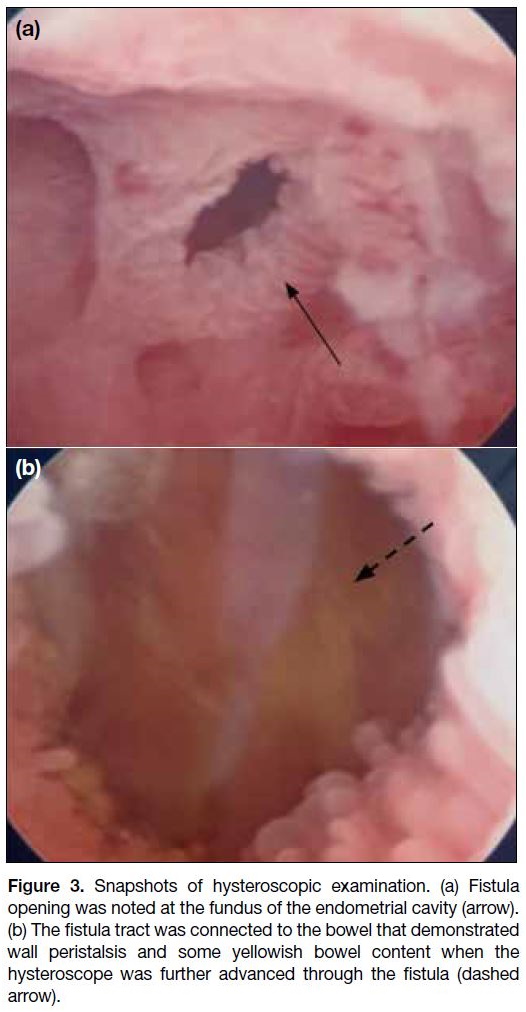
Diagnostic hysteroscopy revealed irregular and
oedematous endometrium with a 5-mm fistula opening
at the uterine fundus (Figure 3a). The hysteroscope was
able to pass through the fistula tract and enter the bowel
with bowel wall peristalsis and some yellowish bowel
content seen (Figure 3b). This confirmed the diagnosis
of enterouterine fistula, likely connecting the uterus and
the small bowel.
Figure 3. Snapshots of hysteroscopic examination. (a) Fistula
opening was noted at the fundus of the endometrial cavity (arrow).
(b) The fistula tract was connected to the bowel that demonstrated
wall peristalsis and some yellowish bowel content when the
hysteroscope was further advanced through the fistula (dashed
arrow).
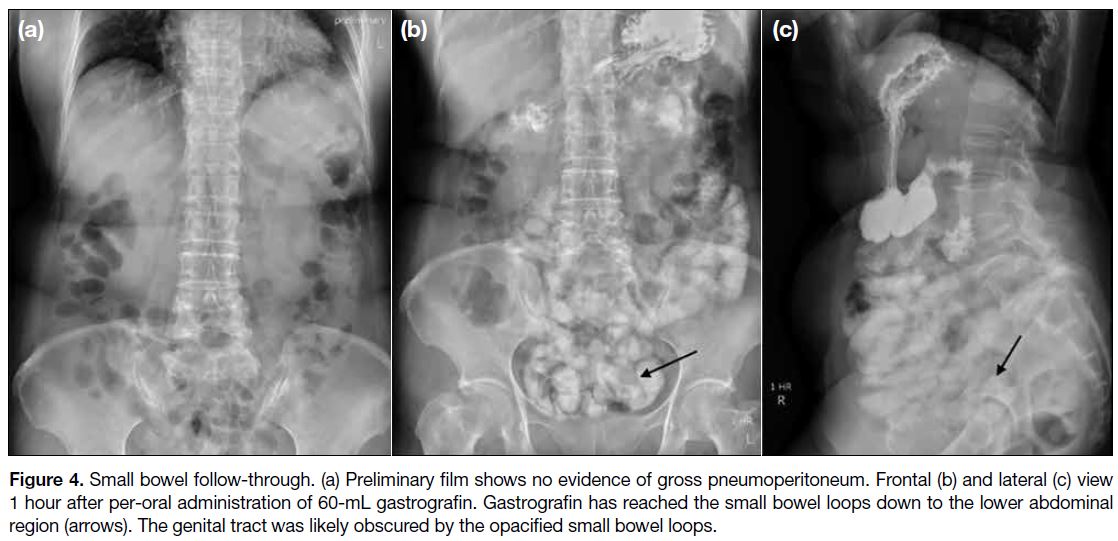
A small bowel follow-through was then arranged. The
preliminary film showed no gross pneumoperitoneum
(Figure 4a). Gastrografin reached the small bowel at
the lower abdomen on spot images taken 1 hour after
per-oral administration of gastrografin. The genital tract
was likely obscured by the opacified small bowel on
both frontal and lateral views (Figure 4b and c). The
patient complained of clear vaginal discharge 15 minutes
after the spot images, raising the clinical suspicion of
contrast material entering the genital tract via the fistula.
Figure 4. Small bowel follow-through. (a) Preliminary film shows no evidence of gross pneumoperitoneum. Frontal (b) and lateral (c) view
1 hour after per-oral administration of 60-mL gastrografin. Gastrografin has reached the small bowel loops down to the lower abdominal
region (arrows). The genital tract was likely obscured by the opacified small bowel loops.
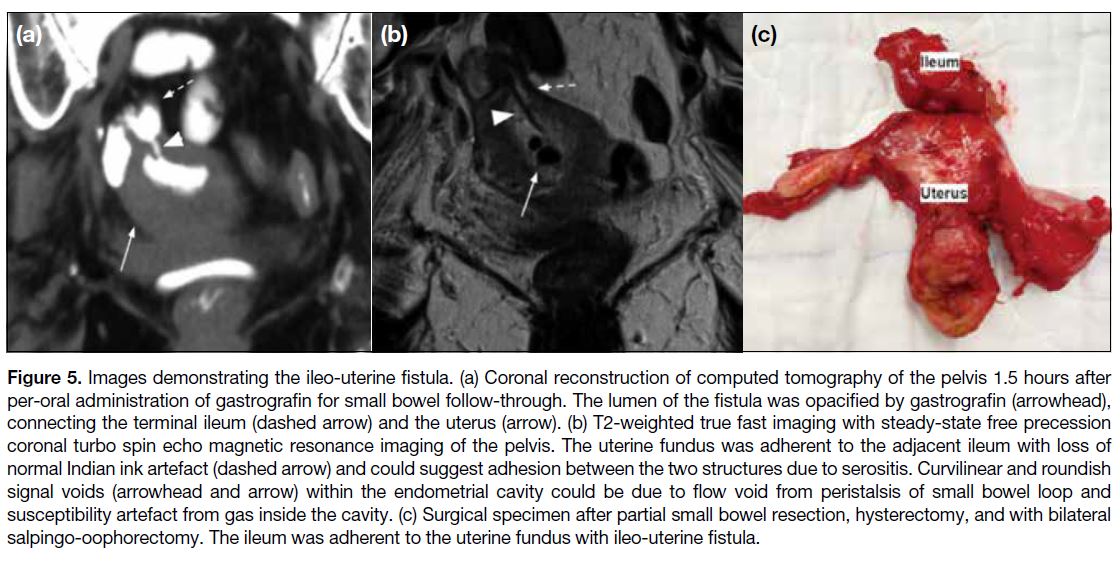
A supplementary plain CT of the pelvis identified a
contrast-opacified fistula tract between the terminal
ileum and the uterine fundus (Figure 5a).
Figure 5. Images demonstrating the ileo-uterine fistula. (a) Coronal reconstruction of computed tomography of the pelvis 1.5 hours after
per-oral administration of gastrografin for small bowel follow-through. The lumen of the fistula was opacified by gastrografin (arrowhead),
connecting the terminal ileum (dashed arrow) and the uterus (arrow). (b) T2-weighted true fast imaging with steady-state free precession
coronal turbo spin echo magnetic resonance imaging of the pelvis. The uterine fundus was adherent to the adjacent ileum with loss of
normal Indian ink artefact (dashed arrow) and could suggest adhesion between the two structures due to serositis. Curvilinear and roundish
signal voids (arrowhead and arrow) within the endometrial cavity could be due to flow void from peristalsis of small bowel loop and
susceptibility artefact from gas inside the cavity. (c) Surgical specimen after partial small bowel resection, hysterectomy, and with bilateral
salpingo-oophorectomy. The ileum was adherent to the uterine fundus with ileo-uterine fistula.
Magnetic resonance imaging (MRI) of the pelvis
with axial, sagittal, and coronal pre- and post-contrast
sequences including T1-weighted turbo spin echo and
T2-weighted true fast imaging with steady-state free
precession (trueFISP) imaging were performed. The
ileo-uterine fistula tract was not seen in most of the
sequences, except in the coronal T2-weighted trueFISP
image. The uterine fundus was adherent to the adjacent
ileum with loss of normal Indian ink artefact. The open
fistula tract contained T2-weighted hyperintense fluid
and a curvilinear hypointense signal, possibly due to
flow void. Roundish signal voids in the endometrial
cavity could be due to the gas locule inside the cavity
(Figure 5b). There were no contrast-enhanced septic foci
detected.
Total abdominal hysterectomy, bilateral salpingo-oophor-ectomy, and a partial small bowel resection of 5
cm of the distal ileum containing the fistula with end-to-end
anastomosis were performed. The surgical specimen
again clearly demonstrated the ileo-uterine fistula
connecting the ileum and the uterine fundus (Figure 5c).
Microscopic examination of the surgical specimen
showed herniation of small bowel mucosa into the
myometrium at the fistula opening and evidence of
endometritis. No endometrial hyperplasia or malignancy
was evident. The patient made an uneventful post-operative
recovery.
DISCUSSION
Ileo-uterine fistula usually presents with non-specific
symptoms such as abdominal pain and vaginal
discharge.[1] [2] [3] [4] Endometrial biopsy yielding abnormal
bowel tissue and food particles raises a suspicion of
enterouterine fistula.[5] Transvaginal ultrasonography may
reveal an intrauterine collection but rarely a fistula tract.
Diagnostic hysteroscopy can directly visualise a uterine
fistula opening.
Small enterouterine fistulas can be managed
conservatively by antibiotics and endometrial aspiration
but persistent symptoms warrant definitive surgery.[1] [3]
Further imaging investigations are important for pre-operative
planning.[1] [3] [6]
CT enables rapid assessment to exclude
pneumoperitoneum and gross pelvic abscess. It can
detect gas within the endometrial cavity, an indication
of enterouterine fistula.[7] [8] In this case, the gas within
the endometrial cavity was seen only transiently on
pre-contrast study. We hypothesise that this was due to
movement of gas to and from the fistula tract by bowel
peristalsis. This is supported by the bowel peristalsis
seen on hysteroscopy and the jet of flow void in coronal
T2-weighted trueFISP images. Gas due to recent
instrumentation would be expected to be consistently
seen in both pre- and post-contrast studies.
Fluoroscopy is the traditional imaging modality for
evaluating a pelvic fistula.[1] [2] [6] [9] Opacification of an ileo-uterine fistula tract can be achieved by small bowel follow-through
or hysterosalpingogram.[4] Hysterosalpingogram
has a higher chance of fistula opacification due to direct
manual injection of contrast into the uterine cavity but is
more invasive than small bowel follow-through. Contrast
opacification of the fistula tract in small bowel follow-through
suggests that bowel peristalsis alone builds up
sufficient pressure to open the fistula. This may predict a
likely need for surgical management due to a low chance
of spontaneous fistula closure. A complementary CT of
the pelvis right after fluoroscopy can better delineate
the three-dimensional anatomy of the contrast-opacified
fistula tract.[9]
MRI has the highest soft tissue contrast to delineate
a fistula tract. Rapid MRI sequences are essential to
minimise image degradation by bowel peristalsis.[6] [9]
We recommend T2-weighted trueFISP sequence to
look for hyperintense fluid and hypointense gas content
within the fistula. In addition, we propose that loss of normal Indian ink artefact at the fat-fluid interface in this
sequence can help detect the loss of fat plane between
the uterus and the bowel. The fistula tract was seen
only in one sequence since it opened only intermittently
under bowel peristalsis. Therefore, MRI images can be
obtained along the plane of the fistula with dynamic
study for better detection.[4]
Uterine perforation is an uncommon but known risk of
endometrial sampling with a reported incidence of 1 to 2
in 1,000 population.[10] Our patient had several risk factors
for uterine perforation including a small retroverted
uterus and chronic endometritis. The small bowel is
a freely mobile intraperitoneal structure and rarely
punctured during endometrial sampling. We hypothesise that the small bowel was relatively fixed to the uterus
due to serositis, evidenced by the loss of normal Indian
ink artefact between these structures in the coronal T2-weighted trueFISP MRI. The inflammation caused by
the presence of ileo-uterine fistula after endometrial
aspiration further led to a secondary pyometra.
In summary, ileo-uterine fistula is an extremely rare
complication following endometrial aspiration and can
cause great morbidity. A high index of clinical suspicion
should be maintained and appropriate investigations
should be carried out to guide early management.
REFERENCES
1. Hyde BJ, Byrnes JN, Occhino JA, Sheedy SP, VanBuren WM. MRI review of female pelvic fistulizing disease. J Magn Reson
Imaging. 2018;48:1172-84. Crossref
2. Fadel HE. Ileo-uterine fistula as a complication of myomectomy.
Case report. Br J Obstet Gynaecol. 1977;84:312-3. Crossref
3. Hawkes SZ. Enterouterine fistula; with a review of the literature and report of an unusual case. Am J Obstet Gynecol. 1946;52:150-3. Crossref
4. McFarlane ME, Plummer JM, Remy T, Christie L, Laws D, Richards H, et al. Jejunouterine fistula: a case report. Gynecol Surg. 2008;5:173-5. Crossref
5. Delara R, Cornella J, Young-Fadok T, Wasson M. Colouterine fistula presenting as postmenopausal endometritis. J Minim
Invasive Gynecol. 2020;27:1234-6. Crossref
6. Moon SG, Kim SH, Lee HJ, Moon MH, Myung JS. Pelvic fistulas complicating pelvic surgery or diseases: spectrum of imaging
findings. Korean J Radiol. 2001;2:97-104. Crossref
7. Behrouzi-Lak T, Aghamohammadi V, Afshari N, Kashefi S. Salpingo enteric fistula: a case report. Int Med Case Rep J.
2020;13:221-4. Crossref
8. Dewdney SB, Mani NB, Zuckerman DA, Thaker PH. Uteroenteric fistula after uterine artery embolization. Obstet Gynecol.
2011;118:434-6. Crossref
9. Pickhardt PJ, Bhalla S, Balfe DM. Acquired gastrointestinal fistulas: classification, etiologies, and imaging evaluation. Radiology. 2002;224:9-23. Crossref
10. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-44. Crossref