Molecular Classification and Respective Radiological Phenotypes of Breast Cancers: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2023 Sep;26(3):206-16 | Epub 11 Sep 2023
Molecular Classification and Respective Radiological Phenotypes of Breast Cancers: A Pictorial Essay
SM Yu1, YH Chan1, YS Chan1, C Tsoi1, GKF Tam2, EHY Hung1, WCW Chu1, HHL Chau1
1 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr HHL Chau, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China. Email:
Submitted: 30 Mar 2022; Accepted: 17 Jun 2022.
Contributors: SMY, WCWC and HHLC designed the study. SMY, YHC, YSC, CT and HHLC acquired and analysed the data. SMY and
HHLC drafted the manuscript. SMY, GKFT, EHYH, WCWC and HHLC critically revised the manuscript for important intellectual content. All
authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As editors of the journal, YHC and WCWC were not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2022.192). A waiver of informed consent of patients was granted by the Committee due to the retrospective nature of the study and no patient identifiers were used.
INTRODUCTION
According to the Centre for Health Protection, breast
cancer is the most common cancer among females in
Hong Kong.[1] In all, 4956 new cases of female breast
cancer were diagnosed in Hong Kong and the crude
incidence rate was 121.9 per 100,000 women in 2020.[1]
Breast cancer is traditionally classified based on the
clinicopathological analysis. In the past two decades,
identification of distinct gene expression profiling in
breast cancer has reshaped our understanding of breast
cancer biology. Unravelling the genetic heterogeneity
of breast cancer is fundamental to the development
of personalised medicine, which improves clinical
outcomes.
Full genomic analysis is costly and time-consuming,
therefore not widely available in routine practice; the
St Gallen International Expert Consensus panel has
suggested the analysis of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal
growth factor receptor 2 (HER2) by semiquantitative
immunohistochemistry (IHC) and the use of fluorescence
in situ hybridisation for HER2 for equivocal IHC to define
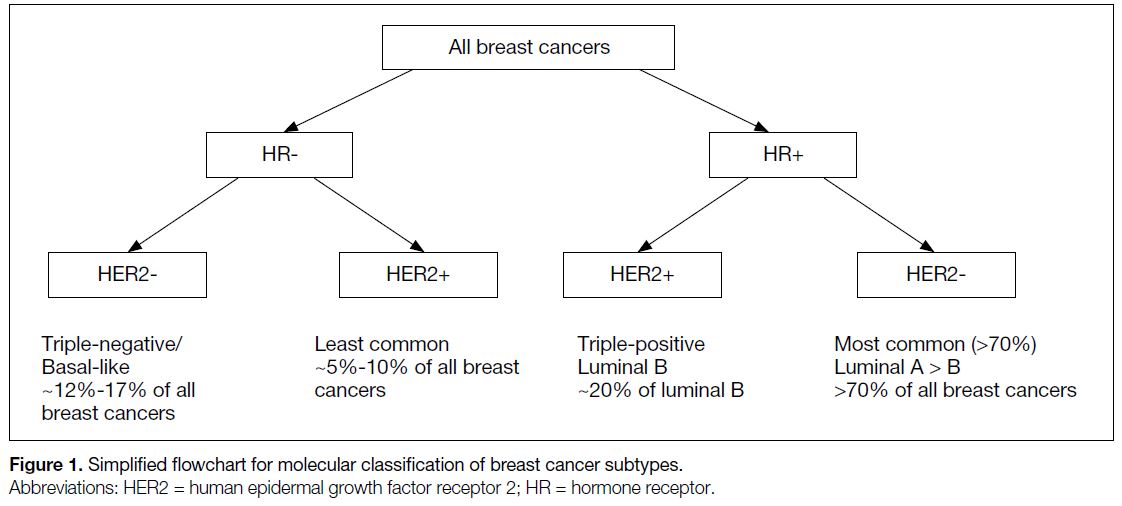
the four molecular subtypes of breast cancer (Figure 1).[2]
IHC analysis may not always accurately reflect the true
molecular subtypes of breast cancers. Discordance rates
between IHC analysis and genetic expression profiling
vary among different studies, but can be as high as 30%.[3]
Figure 1. Simplified flowchart for molecular classification of breast cancer subtypes.
The four intrinsic molecular subtypes of breast cancer
include luminal A, luminal B, HER2-enriched, and
basal-like (triple-negative). Each molecular subtype
shows different demographics, treatment responses,
preferential metastatic target organs, and prognoses.
Importantly, distinctive radiological features of each
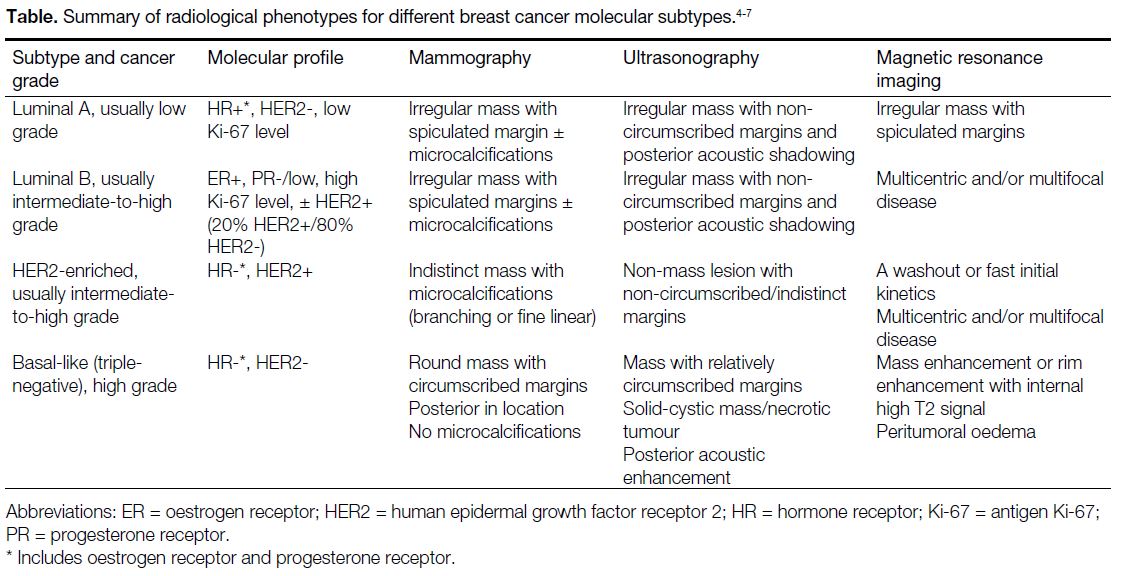
molecular subtype have been identified (Table).[4] [5] [6] [7]
Table. Summary of radiological phenotypes for different breast cancer molecular subtypes.
This article aims to provide radiologists with a pictorial exhibit on imaging phenotypes of breast cancer molecular
subtypes based on pathologically proven examples. It
also provides an overview of molecular classification
and clinical implications of each molecular subtype for
the field of precision medicine.
LUMINAL SUBTYPE (LUMINAL A AND LUMINAL B)
Genetic Expression and Clinical Implications
Luminal subtype is defined by the presence of ER and PR expression.[4] Luminal subtype is divided into two
distinct subgroups, namely luminal A and luminal
B. Approximately 70% of breast cancers are luminal
subtype breast cancers and they show a more favourable
prognosis than hormone receptor–negative breast
cancers.[5]
Luminal A subtypes are defined by expression of both
ER and PR without amplification of the HER2/neu proto-oncogene.
Patients with luminal A tumours have the best prognosis among all molecular subtypes.[4] [5] [6] Luminal A
tumours usually exhibit low histological grades with
higher expression of hormone receptors (ERs and PRs)
and lower proliferative activity, which can be assessed
through Ki-67 expression, in which the antigen Ki-67 is
a marker for cell proliferation (<14%).[4]
Luminal B subtypes also express ER and PR but they
have a higher Ki-67 expression (≥14%).[4] Luminal B
tumours are more often multifocal or multicentric and
more likely to metastasise to regional nodes than luminal
A tumours.[7] Patients with luminal B breast cancers often
have a poorer prognosis compared to patients harbouring
luminal A breast cancers.[5] [7] Furthermore, 20% of luminal
B tumours are HER2 positive by IHC analysis, referred to as the ‘luminal B HER2+’ subtype, which was shown
to be associated with a poorer prognosis and lower
10-year breast cancer–specific survival rate among the
luminal subtypes.[7]
The hormone receptor status is predictive of response to
hormone therapy, the mainstay of treatment for patients
with luminal breast cancers.[3] [6]
Imaging Characteristics
Luminal tumours typically demonstrate suspicious
mammographic features of breast cancer, namely
an irregular mass with spiculated or microlobulated
margins or a mass with suspicious microcalcifications
(Figures 2 and 3).[6] Associated architectural distortion is more commonly observed in luminal A tumours than
in luminal B tumours. The sonographic appearance
of luminal tumours is typically an irregular mass with
posterior acoustic shadowing (Figures 2 and 3).6 On
magnetic resonance imaging (MRI), luminal tumours
most commonly present as an enhancing irregular mass
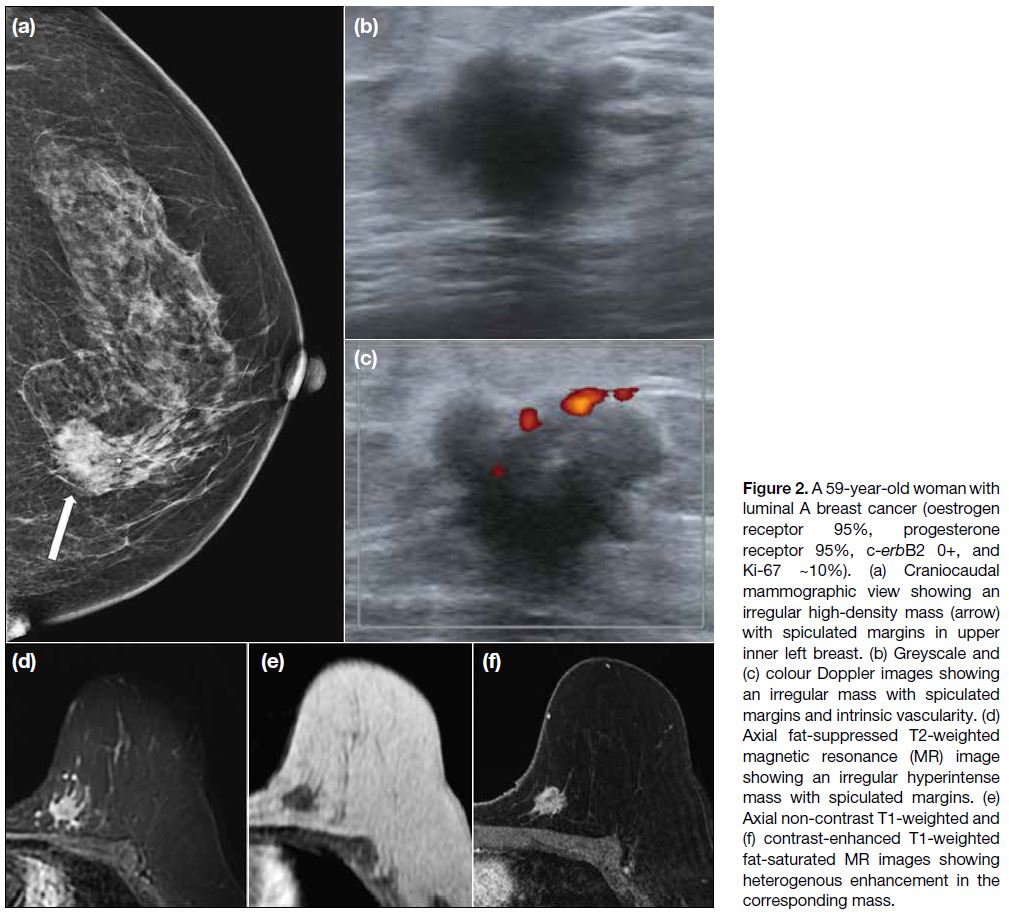
with spiculated margins (Figure 2).[8]
Figure 2. A 59-year-old woman with luminal A breast cancer (oestrogen receptor 95%, progesterone receptor 95%, c-erbB2 0+, and Ki-67 ~10%). (a) Craniocaudal mammographic view showing an irregular high-density mass (arrow) with spiculated margins in upper inner left breast. (b) Greyscale and (c) colour Doppler images showing an irregular mass with spiculated margins and intrinsic vascularity. (d) Axial fat-suppressed T2-weighted magnetic resonance (MR) image showing an irregular hyperintense mass with spiculated margins. (e) Axial non-contrast T1-weighted and (f) contrast-enhanced T1-weighted fat-saturated MR images showing heterogenous enhancement in the corresponding mass.
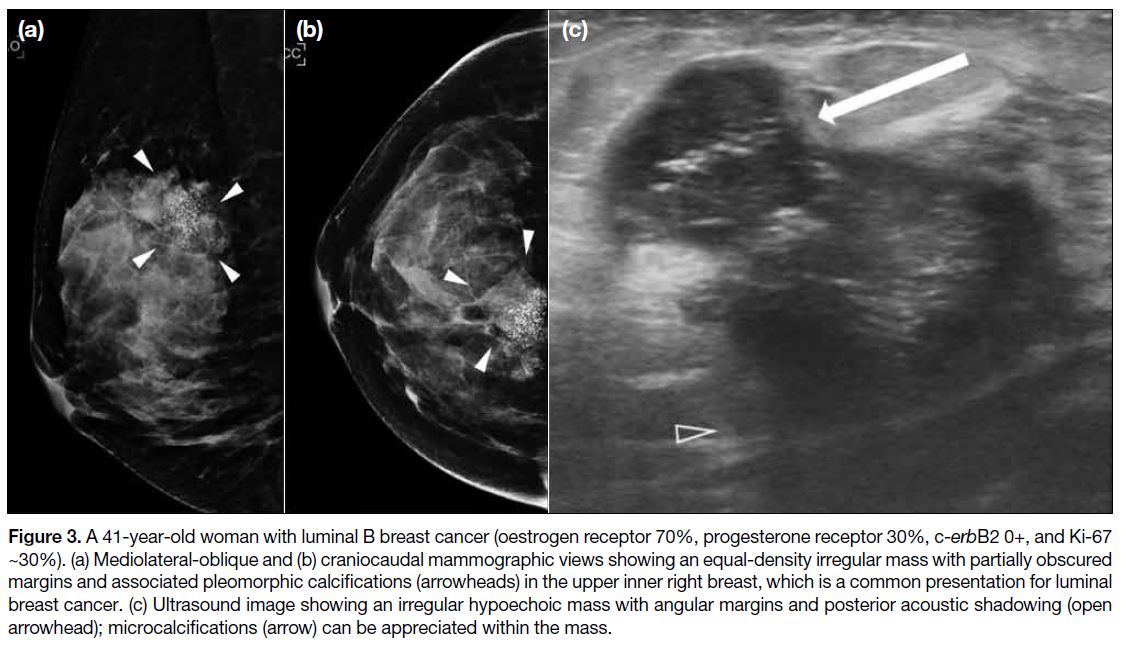
Figure 3. A 41-year-old woman with luminal B breast cancer (oestrogen receptor 70%, progesterone receptor 30%, c-erbB2 0+, and Ki-67
~30%). (a) Mediolateral-oblique and (b) craniocaudal mammographic views showing an equal-density irregular mass with partially obscured
margins and associated pleomorphic calcifications (arrowheads) in the upper inner right breast, which is a common presentation for luminal
breast cancer. (c) Ultrasound image showing an irregular hypoechoic mass with angular margins and posterior acoustic shadowing (open
arrowhead); microcalcifications (arrow) can be appreciated within the mass.
Luminal B tumours are more frequently associated with
axillary nodal metastases at the time of diagnosis than
luminal A tumours. Luminal B tumours more often
present with multifocal or multicentric disease on MRI.[3]
The ‘luminal B HER2+’ subtype has been reported to
be more likely to involve axillary lymph nodes and to
present with multifocal or multicentric disease on MRI
(Figure 4).[3]
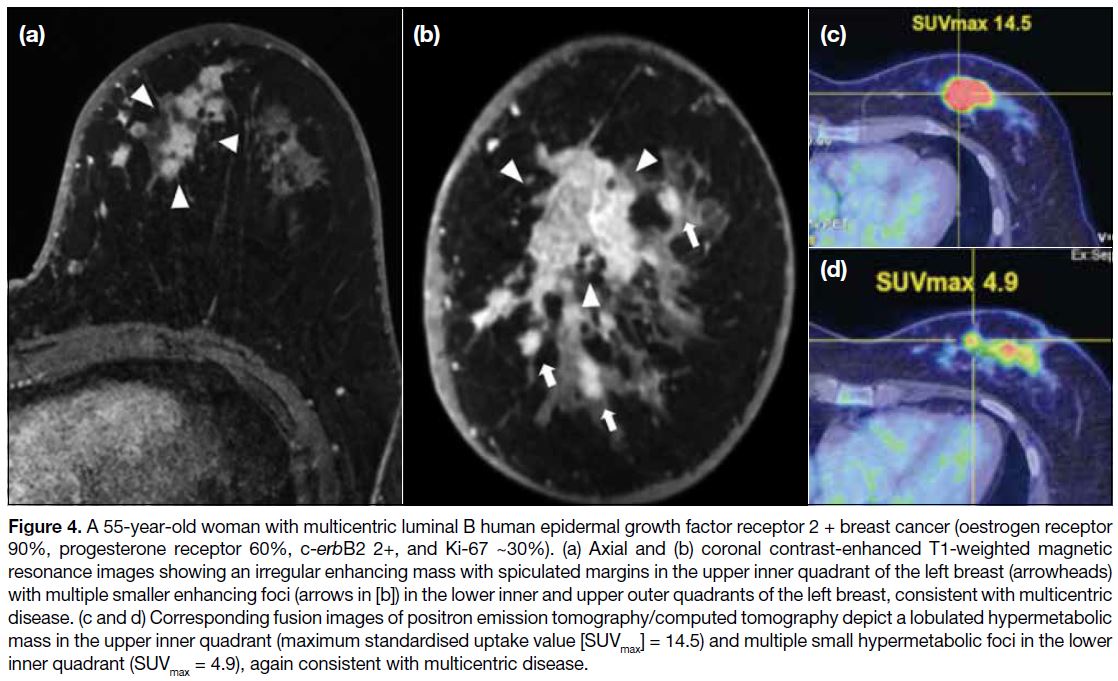
Figure 4. A 55-year-old woman with multicentric luminal B human epidermal growth factor receptor 2 + breast cancer (oestrogen receptor
90%, progesterone receptor 60%, c-erbB2 2+, and Ki-67 ~30%). (a) Axial and (b) coronal contrast-enhanced T1-weighted magnetic
resonance images showing an irregular enhancing mass with spiculated margins in the upper inner quadrant of the left breast (arrowheads)
with multiple smaller enhancing foci (arrows in [b]) in the lower inner and upper outer quadrants of the left breast, consistent with multicentric
disease. (c and d) Corresponding fusion images of positron emission tomography/computed tomography depict a lobulated hypermetabolic
mass in the upper inner quadrant (maximum standardised uptake value [SUVmax] = 14.5) and multiple small hypermetabolic foci in the lower inner quadrant (SUVmax = 4.9), again consistent with multicentric disease.
Distant metastasis in luminal breast cancers shows
a greater propensity to involve the skeletal system
compared to other breast cancer subtypes (Figure 5).[9] [10]
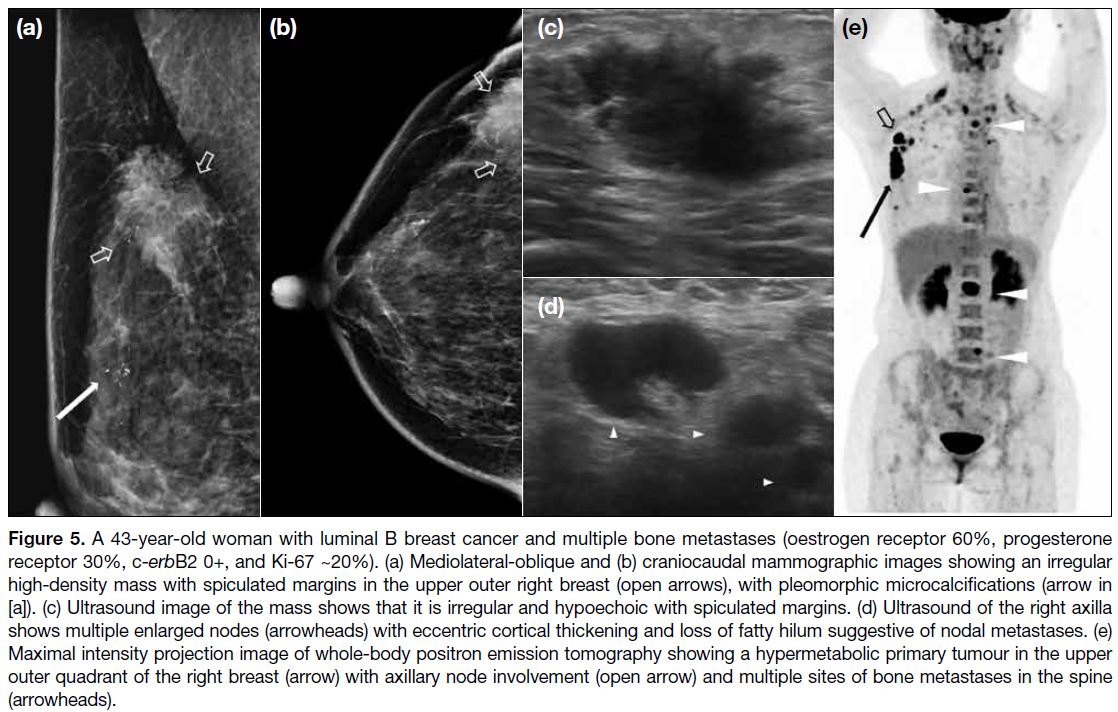
Figure 5. A 43-year-old woman with luminal B breast cancer and multiple bone metastases (oestrogen receptor 60%, progesterone receptor 30%, c-erbB2 0+, and Ki-67 ~20%). (a) Mediolateral-oblique and (b) craniocaudal mammographic images showing an irregular high-density mass with spiculated margins in the upper outer right breast (open arrows), with pleomorphic microcalcifications (arrow in
[a]). (c) Ultrasound image of the mass shows that it is irregular and hypoechoic with spiculated margins. (d) Ultrasound of the right axilla
shows multiple enlarged nodes (arrowheads) with eccentric cortical thickening and loss of fatty hilum suggestive of nodal metastases. (e)
Maximal intensity projection image of whole-body positron emission tomography showing a hypermetabolic primary tumour in the upper
outer quadrant of the right breast (arrow) with axillary node involvement (open arrow) and multiple sites of bone metastases in the spine
(arrowheads).
HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2–ENRICHED SUBTYPE
Genetic Expression and Clinical Implications
HER2-positive cancers are defined by overexpression
of the c-erbB2 (HER2/neu) gene, which encodes the
epidermal growth factor receptor type 2.[6] Of all HER2-positive tumours, 60% are HER2-enriched, characterised by HER2 positivity and ER and PR negativity.[6]
Being a proto-oncogene, amplification of c-erbB2
results in increased cellular aggressiveness and faster
growth.
HER2-enriched breast cancers are generally intermediate-to-high-grade tumours with an aggressive course, worse
survival rate, and higher recurrence rate compared to
luminal breast cancers.[5]
Fortunately, HER2-directed therapy has shown success
in improving clinical outcomes, and trastuzumab therapy
is a widely used and effective anti-HER2 agent.
Imaging Characteristics
HER2-enriched tumours most commonly present as a
mass associated with microcalcifications or as suspicious
microcalcifications alone on mammography (Figure 6).[6]
The margins of HER2-enriched tumours are usually
spiculated. Sonographically, HER2-enriched tumours
are usually iso- to hypoechoic with indistinct margins
and a high degree of vascularity (Figure 6).[6] On MRI,
a round mass with spiculated margins and non-mass
enhancement are the most frequent patterns in HER2-enriched subtype.[6] [8] HER2-enriched tumours are often
multifocal and/or multicentric on MRI and are frequently
associated with ductal carcinoma in situ (Figure 7).[6] [8]
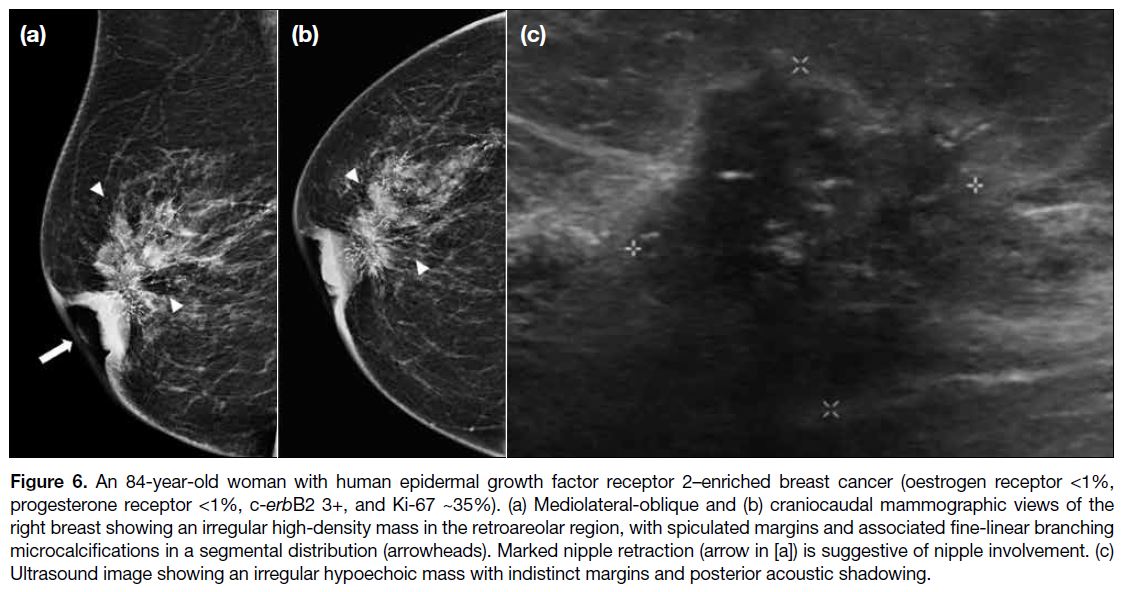
Figure 6. An 84-year-old woman with human epidermal growth factor receptor 2–enriched breast cancer (oestrogen receptor <1%,
progesterone receptor <1%, c-erbB2 3+, and Ki-67 ~35%). (a) Mediolateral-oblique and (b) craniocaudal mammographic views of the
right breast showing an irregular high-density mass in the retroareolar region, with spiculated margins and associated fine-linear branching
microcalcifications in a segmental distribution (arrowheads). Marked nipple retraction (arrow in [a]) is suggestive of nipple involvement. (c)
Ultrasound image showing an irregular hypoechoic mass with indistinct margins and posterior acoustic shadowing.
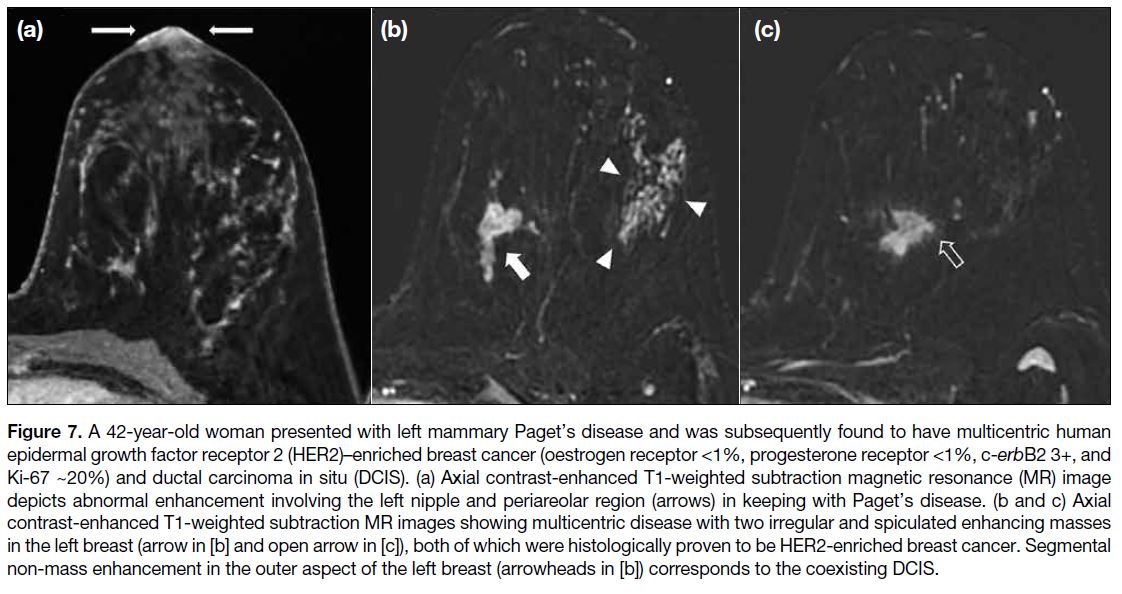
Figure 7. A 42-year-old woman presented with left mammary Paget’s disease and was subsequently found to have multicentric human
epidermal growth factor receptor 2 (HER2)–enriched breast cancer (oestrogen receptor <1%, progesterone receptor <1%, c-erbB2 3+, and
Ki-67 ~20%) and ductal carcinoma in situ (DCIS). (a) Axial contrast-enhanced T1-weighted subtraction magnetic resonance (MR) image
depicts abnormal enhancement involving the left nipple and periareolar region (arrows) in keeping with Paget’s disease. (b and c) Axial
contrast-enhanced T1-weighted subtraction MR images showing multicentric disease with two irregular and spiculated enhancing masses
in the left breast (arrow in [b] and open arrow in [c]), both of which were histologically proven to be HER2-enriched breast cancer. Segmental
non-mass enhancement in the outer aspect of the left breast (arrowheads in [b]) corresponds to the coexisting DCIS.
Distant metastases in HER2-enriched breast cancers
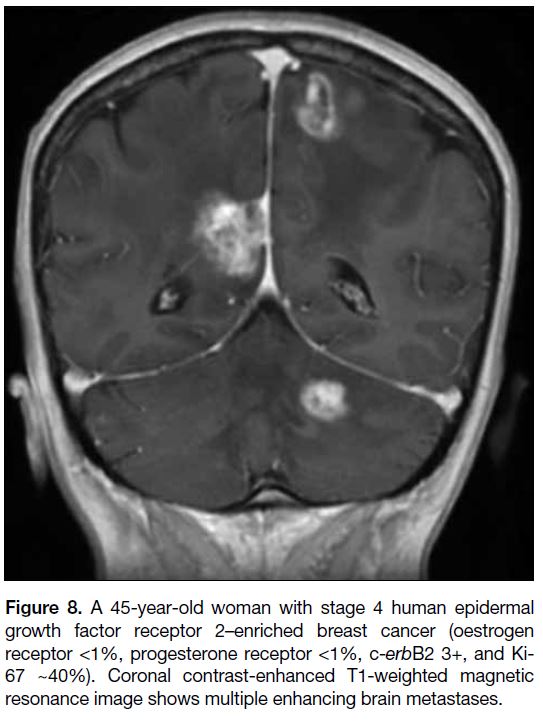
show a propensity to involve the brain (Figure 8).[9]
Figure 8. A 45-year-old woman with stage 4 human epidermal
growth factor receptor 2–enriched breast cancer (oestrogen
receptor <1%, progesterone receptor <1%, c-erbB2 3+, and Ki-67 ~40%). Coronal contrast-enhanced T1-weighted magnetic
resonance image shows multiple enhancing brain metastases.
The HER2-enriched subtype is found to be the most
frequent breast cancer molecular subtype associated with
mammary Paget’s disease (Figure 7).[11]
BASAL-LIKE SUBTYPE (TRIPLE-NEGATIVE)
Genetic Expression and Clinical Implications
Triple-negative breast cancer (TNBC) is defined by
lacking expression of ER, PR and HER2. The term
‘triple-negative’ is often used as a synonym for the ‘basal-like’ subtype because 86% of TNBC are the basal-like
subtype.[6] TNBC accounts for 12% to 17% of all
breast cancers,[12] [13] preferentially affecting young women
of African descent and carriers of germline breast cancer
susceptibility gene 1 (BRCA1) and partner and localiser
of BRCA2 (PALB2) mutations.[13]
TNBC has a tendency to develop early metastases to
lung and brain, leading to rapid progression.[10] [12] The
time from distant recurrence to death is much shorter
in triple-negative tumours than in other subtypes due to
the relatively high mortality associated with visceral soft
tissue metastases as compared to bone metastases in the
luminal subtypes.[10] When compared with other breast
cancer subtypes, patients with TNBC also experience a
higher frequency (34% vs. 20%) and earlier onset (2.6
vs. 5.0 years after diagnosis) of distant recurrence.[10]
Recurrences most commonly occur 1 to 4 years after
diagnosis and are rare beyond 8 years, in contrast to the
constant risk throughout the follow-up period in ER-positive
tumours.[10] Due to the aggressive tumour biology
and limited targeted therapy, patients harbouring TNBCs
show poorer prognosis than patients with hormone
receptor–positive tumours.[5] [10]
TNBC has been shown to have greater sensitivity to
neoadjuvant chemotherapy with a better pathological
complete response than luminal and HER2 subtypes.[12]
Conventional adjuvant chemotherapy using
anthracycline/taxane-based regimens remains the
standard of care for patients with TNBCs despite the
identification of several promising agents, such as
platinum.[12]
Imaging Characteristics
TNBC frequently presents as a palpable mass and often
lacks the classical suspicious mammographic features,
namely irregular mass, spiculated margins, and suspicious
microcalcifications. Unifocal disease, circumscribed
mass despite large size, necrotic mass with posterior
enhancement on ultrasound, and absent calcifications
on mammography are the imaging phenotype of TNBC
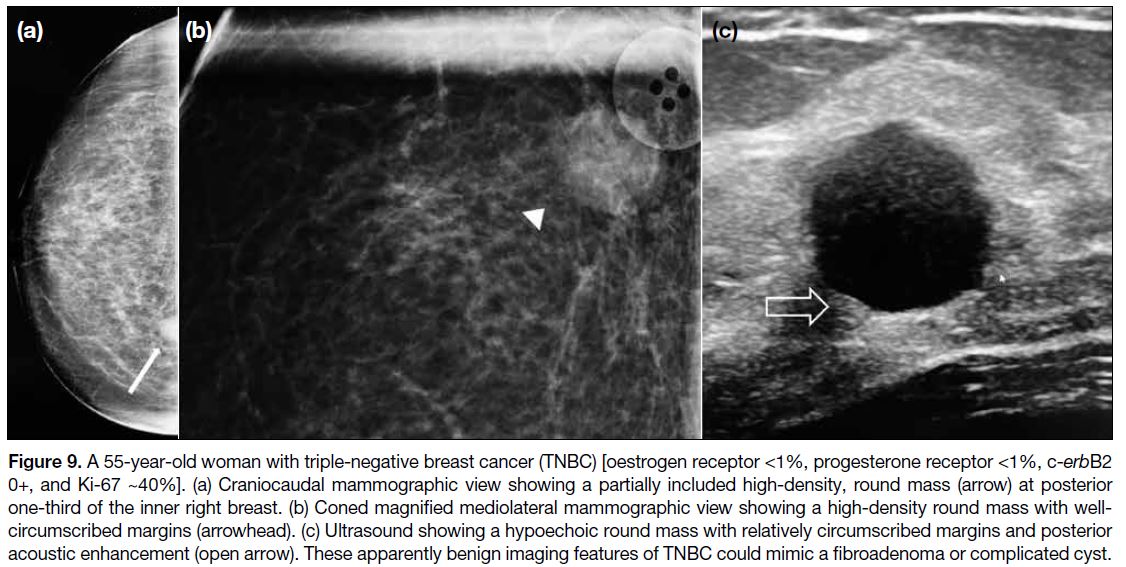
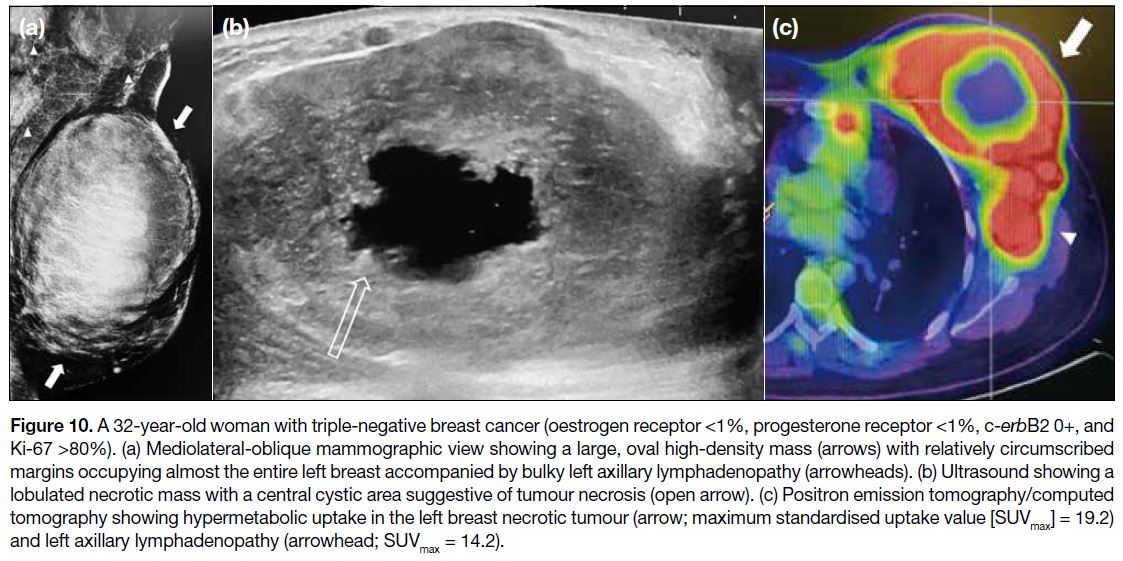
(Figures 9 and 10).[14] TNBCs also show tendency towards
a posterior or pre-pectoral location compared to other
breast cancer subtypes (Figure 9).[15] Due to the apparent
benign features on mammography and ultrasonography,
TNBC may be mistaken as a fibroadenoma or
complicated cyst (Figure 9). Besides the conventional
techniques of mammography and ultrasound, dynamic
contrast-enhanced MRI may serve as a useful adjunct to
distinguish them by analysis of their enhancement patterns
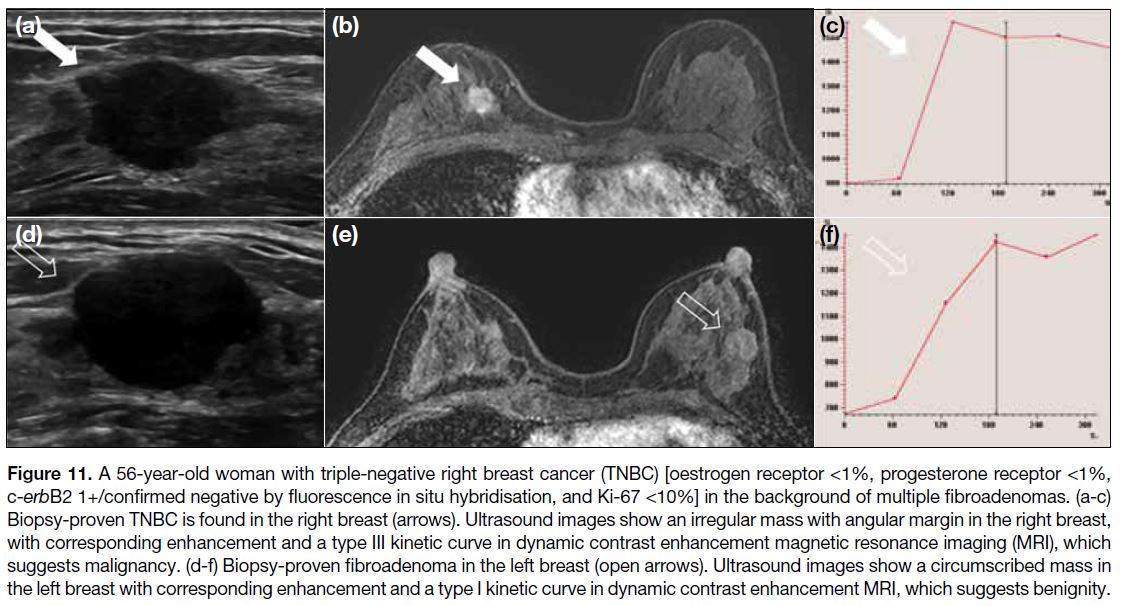
and respective kinetic curves (Figure 11). MRI breast
kinetic curves, categorised into type I (progressive), type
II (plateau), and type III (washout), offer distinguishing
patterns in malignancy suspicion. Progressive curves,
which demonstrate continuous enhancement over time,
typically denote benignity. Plateau curves, characterised
by initial enhancement followed by a plateau phase,
raise concerns for malignancy. Washout curves,
characterised by an initial uptake and subsequent
washout, strongly imply malignancy.
Figure 9. A 55-year-old woman with triple-negative breast cancer (TNBC) [oestrogen receptor <1%, progesterone receptor <1%, c-erbB2 0+, and Ki-67 ~40%]. (a) Craniocaudal mammographic view showing a partially included high-density, round mass (arrow) at posterior
one-third of the inner right breast. (b) Coned magnified mediolateral mammographic view showing a high-density round mass with well-circumscribed
margins (arrowhead). (c) Ultrasound showing a hypoechoic round mass with relatively circumscribed margins and posterior
acoustic enhancement (open arrow). These apparently benign imaging features of TNBC could mimic a fibroadenoma or complicated cyst.
Figure 10. A 32-year-old woman with triple-negative breast cancer (oestrogen receptor <1%, progesterone receptor <1%, c-erbB2 0+, and
Ki-67 >80%). (a) Mediolateral-oblique mammographic view showing a large, oval high-density mass (arrows) with relatively circumscribed
margins occupying almost the entire left breast accompanied by bulky left axillary lymphadenopathy (arrowheads). (b) Ultrasound showing a
lobulated necrotic mass with a central cystic area suggestive of tumour necrosis (open arrow). (c) Positron emission tomography/computed
tomography showing hypermetabolic uptake in the left breast necrotic tumour (arrow; maximum standardised uptake value [SUVmax] = 19.2)
and left axillary lymphadenopathy (arrowhead; SUVmax = 14.2).
Figure 11. A 56-year-old woman with triple-negative right breast cancer (TNBC) [oestrogen receptor <1%, progesterone receptor <1%, c-erbB2 1+/confirmed negative by fluorescence in situ hybridisation, and Ki-67 <10%] in the background of multiple fibroadenomas. (a-c) Biopsy-proven TNBC is found in the right breast (arrows). Ultrasound images show an irregular mass with angular margin in the right breast, with corresponding enhancement and a type III kinetic curve in dynamic contrast enhancement magnetic resonance
imaging (MRI), which suggests malignancy. (d-f) Biopsy-proven fibroadenoma in the left breast (open arrows). Ultrasound images show a
circumscribed mass in the left breast with corresponding enhancement and a type I kinetic curve in dynamic contrast enhancement MRI,
which suggests benignity.
TNBC has been shown to have a higher tumour
roundness score compared with the other subtypes,
reflecting a more biologically aggressive tumour
type (Figures 9 and 10).[14] In contrast to luminal and
HER2-enriched subtypes, there is no linear correlation
between tumour size and the likelihood of lymph node
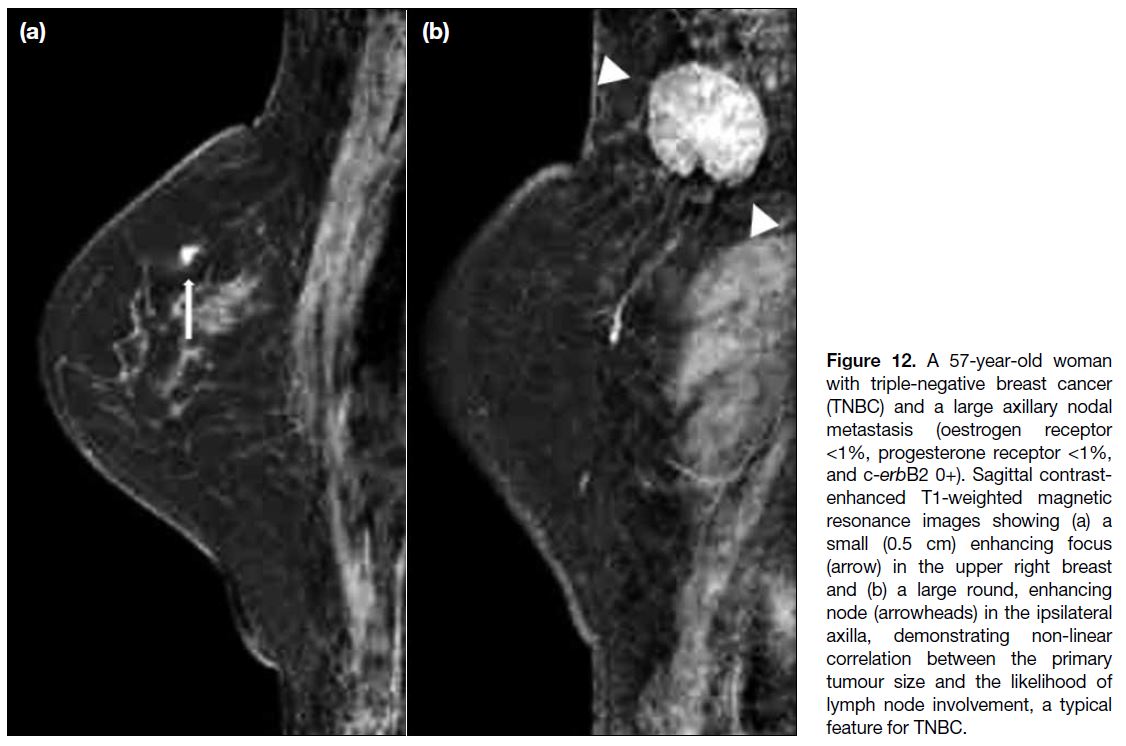
involvement (Figure 12).[10] [16]
Figure 12. A 57-year-old woman
with triple-negative breast cancer (TNBC) and a large axillary nodal metastasis (oestrogen receptor <1%, progesterone receptor <1%, and c-erbB2 0+). Sagittal contrast-enhanced T1-weighted magnetic resonance images showing (a) a small (0.5 cm) enhancing focus (arrow) in the upper right breast and (b) a large round, enhancing node (arrowheads) in the ipsilateral axilla, demonstrating non-linear correlation between the primary tumour size and the likelihood of lymph node involvement, a typical feature for TNBC.
Typical MRI findings in TNBC are mass enhancement
and rim enhancement, which are related to tumour
necrosis in these high-grade and fast-growing
tumours.[8] [14] The presence of peritumoral oedema was
found to be the only significant variable associated with
worse recurrence-free survival in patients with TNBC.[17]
MRI is the most sensitive imaging modality in the early
prediction of neoadjuvant chemotherapy response and
pathological complete response in patients harbouring
TNBCs.[14] Most of the studies have shown that TNBCs are
relatively chemosensitive compared to other subtypes.
Placement of a marker clip within the TNBC prior to
neoadjuvant chemotherapy allows precise localisation of
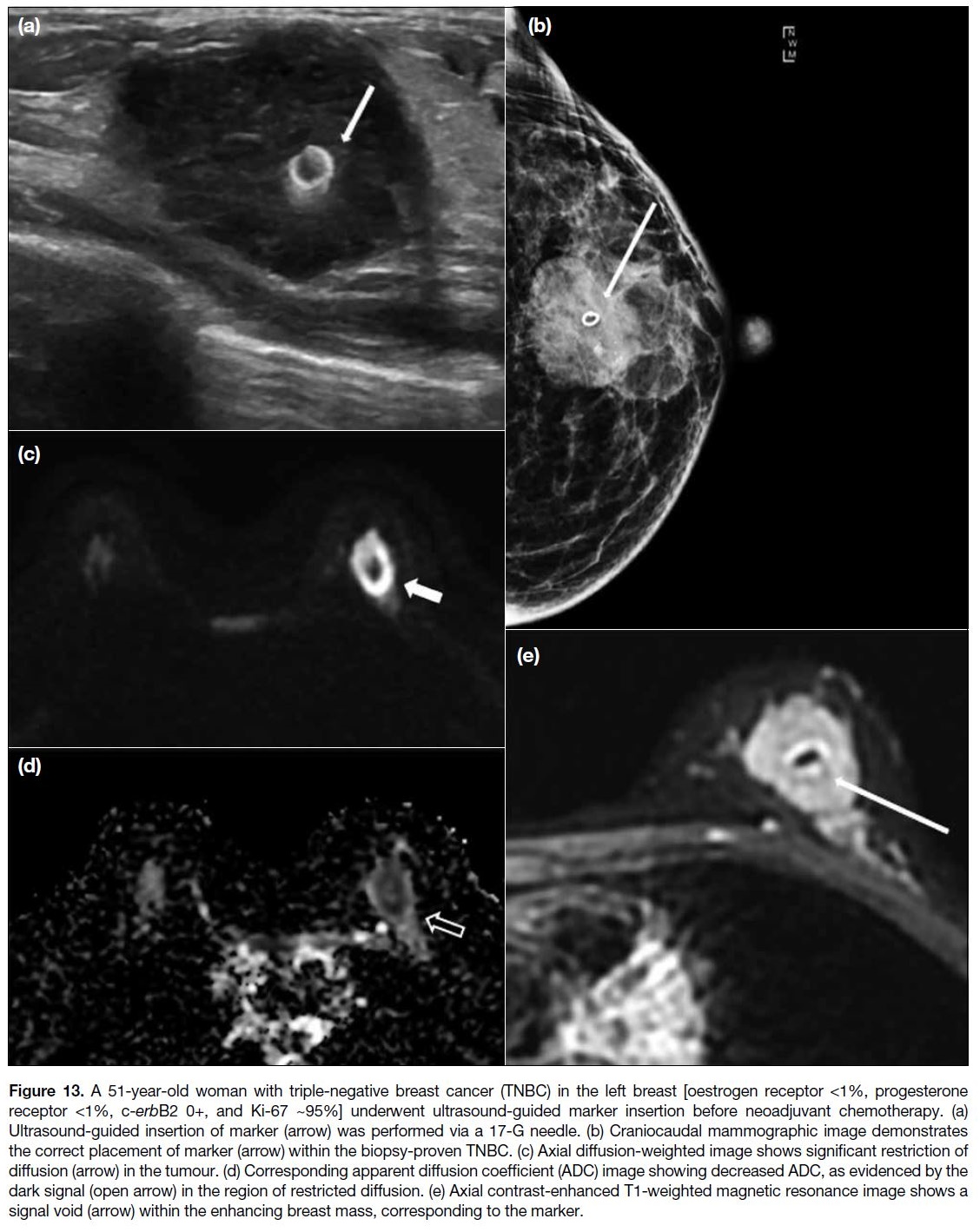
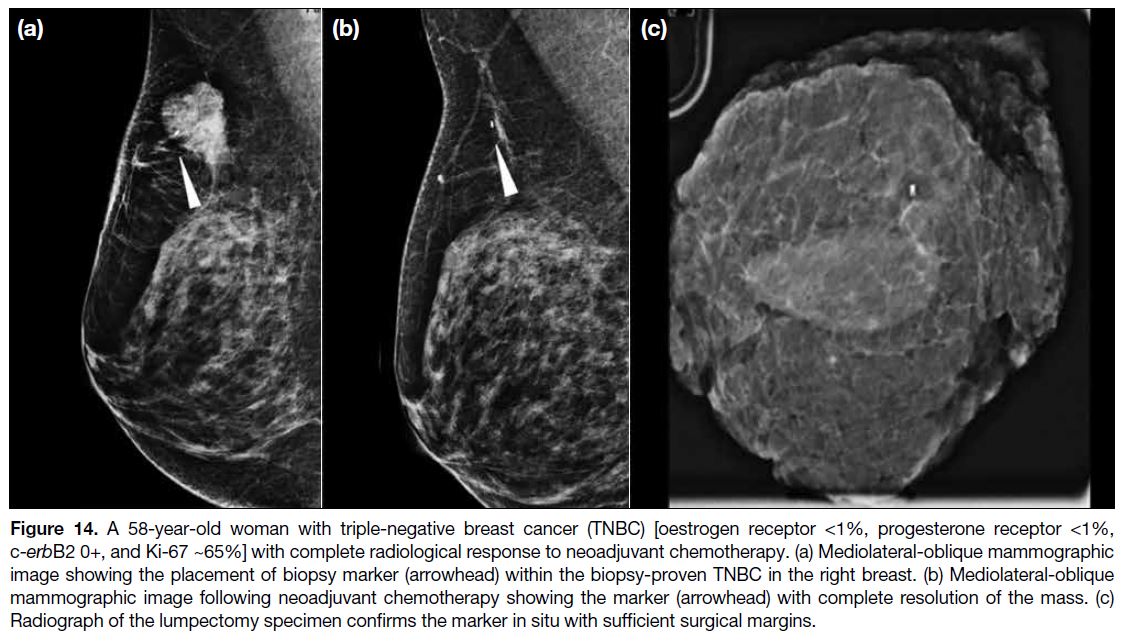
the tumour for subsequent operation (Figures 13 and 14).
Figure 13. A 51-year-old woman with triple-negative breast cancer (TNBC) in the left breast [oestrogen receptor <1%, progesterone
receptor <1%, c-erbB2 0+, and Ki-67 ~95%] underwent ultrasound-guided marker insertion before neoadjuvant chemotherapy. (a)
Ultrasound-guided insertion of marker (arrow) was performed via a 17-G needle. (b) Craniocaudal mammographic image demonstrates
the correct placement of marker (arrow) within the biopsy-proven TNBC. (c) Axial diffusion-weighted image shows significant restriction of
diffusion (arrow) in the tumour. (d) Corresponding apparent diffusion coefficient (ADC) image showing decreased ADC, as evidenced by the
dark signal (open arrow) in the region of restricted diffusion. (e) Axial contrast-enhanced T1-weighted magnetic resonance image shows a
signal void (arrow) within the enhancing breast mass, corresponding to the marker.
Figure 14. A 58-year-old woman with triple-negative breast cancer (TNBC) [oestrogen receptor <1%, progesterone receptor <1%,
c-erbB2 0+, and Ki-67 ~65%] with complete radiological response to neoadjuvant chemotherapy. (a) Mediolateral-oblique mammographic
image showing the placement of biopsy marker (arrowhead) within the biopsy-proven TNBC in the right breast. (b) Mediolateral-oblique
mammographic image following neoadjuvant chemotherapy showing the marker (arrowhead) with complete resolution of the mass. (c)
Radiograph of the lumpectomy specimen confirms the marker in situ with sufficient surgical margins.
CONCLUSION
Knowledge of molecular classification of breast cancers
allows better understanding of the clinical behaviour and
prognosis of the different breast cancer subtypes, thus
facilitating implementation of individualised therapies.
Precision medicine is the emerging approach for
individualising patient treatment, which is the standard of care for breast cancer management in the current
era. The role of radiologists is no longer limited to
establishing the diagnosis, staging, and surveillance
of breast cancers. They play a vital role in guiding the
investigation options based on the specific biology of
breast cancer, assessment of neoadjuvant treatment
response, and facilitating clinicians in optimising
individualised patient care. Thorough understanding of
breast cancer molecular subtypes is one of the biggest
steppingstones amongst breast radiologists to participate
in precision medicine practice.
REFERENCES
1. Centre for Health Protection, Department of Health, Hong Kong
SAR Government. Breast cancer. Available from: https://www.chp.gov.hk/en/healthtopics/content/25/53.html. Accessed 4 Sep 2023.
2. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011.
Ann Oncol. 2011;22:1736-47. Crossref
3. Johnson KS, Conant EF, Soo MS. Molecular subtypes of breast cancer: a review for breast radiologists. J Breast Imaging. 2021;3:12-24. Crossref
4. Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D,
de Azambuja E, et al. Luminal B breast cancer: molecular
characterization, clinical management, and future perspectives. J
Clin Oncol. 2014;32:2794-803. Crossref
5. Fallahpour S, Navaneelan T, De P, Borgo A. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open. 2017;5:E734-9. Crossref
6. Trop I, LeBlanc SM, David J, Lalonde L, Tran-Thanh D, Labelle M, et al. Molecular classification of infiltrating breast cancer: toward personalized therapy. Radiographics. 2014;34:1178-95. Crossref
7. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 Index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736-50. Crossref
8. Navarro Vilar L, Alandete Germán SP, Medina García R, Blanc García E, Camarasa Lillo N, Vilar Samper J. MR imaging findings in molecular subtypes of breast cancer according to
BI-RADS system. Breast J. 2017;23:421-8. Crossref
9. Bertos NR, Park M. Breast cancer—one term, many entities? J Clin Invest. 2011;121:3789-96. Crossref
10. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-34. Crossref
11. Arafah M, Arain SA, Raddaoui EM, Tulba A, Alkhawaja FH, Al Shedoukhy A. Molecular subtyping of mammary Paget’s disease using immunohistochemistry. Saudi Med J. 2019;40:440-6. Crossref
12. Newman LA, Reis-Filho JS, Morrow M, Carey LA, King TA.
The 2014 Society of Surgical Oncology Susan G. Komen for the
Cure Symposium: triple-negative breast cancer. Ann Surg Oncol.
2015;22:874-82. Crossref
13. Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16. Crossref
14. Dogan BE, Turnbull LW. Imaging of triple-negative breast cancer.
Ann Oncol. 2012;23 Suppl 6:vi23-9. Crossref
15. Kim WH, Han W, Chang JM, Cho N, Park IA, Moon WK. Location of triple-negative breast cancers: comparison with
estrogen receptor-positive breast cancers on MR imaging. PLoS
One. 2015;10:e0116344. Crossref
16. Foulkes WD, Metcalfe K, Hanna W, Lynch HT, Ghadirian P, Tung N, et al. Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer. 2003;98:1569-77. Crossref
17. Bae MS, Shin SU, Ryu HS, Han W, Im SA, Park IA, et al. Pretreatment MR imaging features of triple-negative breast cancer: association with response to neoadjuvant chemotherapy and
recurrence-free survival. Radiology. 2016;281:392-400. Crossref