Role of Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Monitoring Relapsing Polychondritis: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Sep;26(3):202-5 | Epub 16 Aug 2023
Role of Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Monitoring Relapsing Polychondritis: A Case Report
DWK Chan, EYP Lee
Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong SAR, China
Correspondence: Dr EYP Lee, Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong SAR, China. Email: eyplee77@hku.hk
Submitted: 11 Aug 2022; Accepted: 15 Nov 2022.
Contributors: Both authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: DWKC has disclosed no conflicts of interest. As an editor of the journal, EYPL was not involved in the peer review process.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: HKWC-2022-026). The patient has provided written informed consent for all treatments, procedures, and publication.
INTRODUCTION
Relapsing polychondritis (RP) is a rare autoimmune
inflammatory disease that affects cartilaginous tissue
with consequent recurrent inflammation and deformation
of the involved structures. Although the aetiology
remains unknown, it is often associated with autoimmune
disorders, with rheumatoid arthritis (RA) being the most
common.[1] [2] The sites that are first affected at disease onset
include the auricular and nasal cartilages. Nonetheless
other proteoglycan-rich structures including the eyes,
heart valves and blood vessels can be involved. Due
to its non-specific presentation and the lack of specific
diagnostic methods, RP has a high risk of misdiagnosis,
with a mean diagnostic delay of 2.9 years.[1]
There is no gold standard test or imaging to monitor RP.
Although laboratory investigations such as erythrocyte
sedimentation rate and C-reactive protein level can
indicate active inflammation, they are neither sensitive
nor specific.[2] Imaging modalities such as computed tomography (CT), although useful in the diagnosis of
RP, are less sensitive for demonstrating the extent of
active disease.[2] Histological confirmation is hindered
by access difficulty and the associated complications of
invasive procedures.[2] Recently, the use of fluorine-18
fluorodeoxyglucose positron emission tomography/CT
(18F-FDG PET/CT) has been investigated in the
diagnosis, management and monitoring of RP. We
present a 55-year-old man in whom 18F-FDG PET/CT
was used to assess RP.
CASE REPORT
A 55-year-old man with RA presented with a 3-year
history of worsening shortness of breath and wheeze.
The patient also experienced episodic nasal bridge
pain and bilateral ear blockage. He had been treated
with steroids (prednisolone) and immunosuppressants
(sulphasalazine and azathioprine) for RA since 2018. In
early 2021, he was admitted with increased shortness of
breath, cough, and sputum. He had no chest pain or fever. Physical examination showed diffuse chest wheeze but
was otherwise unremarkable. Chest X-ray showed no
consolidation. Blood tests showed a normal white blood
cell count (6.5 × 109/L) but elevated C-reactive protein
level (14.4 mg/L).
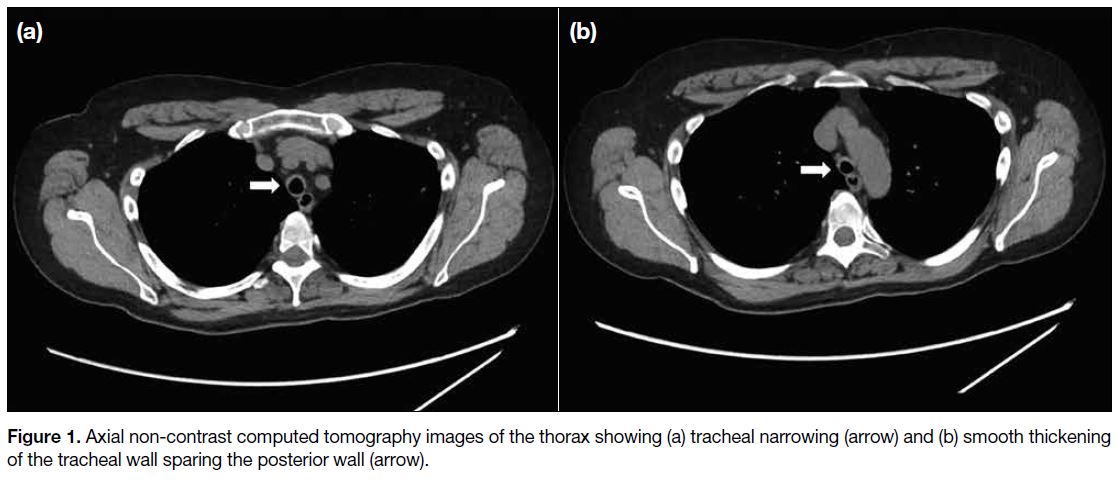
CT of the thorax revealed smooth narrowing of the
trachea and proximal bronchi and smooth tracheal wall
thickening sparing the posterior wall. Changes were
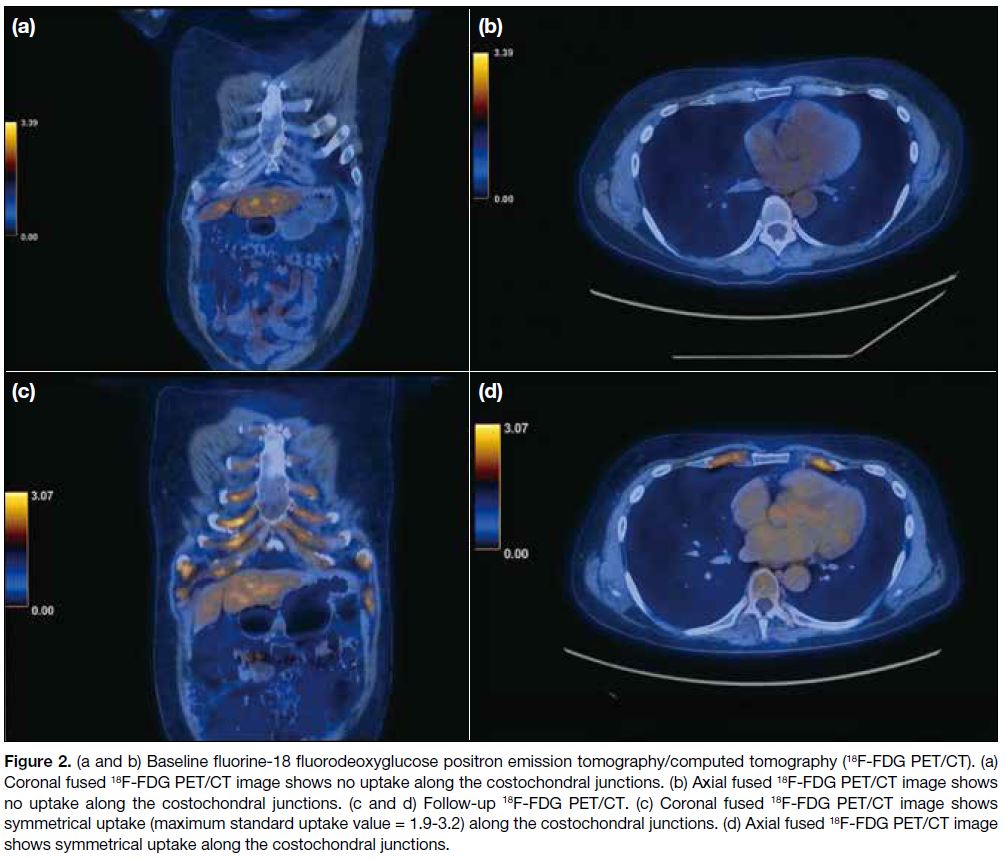
strongly suggestive of RP (Figure 1). A whole-body 18F-FDG PET/CT confirmed the changes but with no corresponding increased uptake or hypermetabolic disease elsewhere (Figure 2a and 2b). A diagnosis of RP was made and the patient was treated with prednisolone, methotrexate, and mycophenolate mofetil.
Figure 1. Axial non-contrast computed tomography images of the thorax showing (a) tracheal narrowing (arrow) and (b) smooth thickening of the tracheal wall sparing the posterior wall (arrow).
Figure 2. (a and b) Baseline fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). (a)
Coronal fused 18F-FDG PET/CT image shows no uptake along the costochondral junctions. (b) Axial fused 18F-FDG PET/CT image shows no uptake along the costochondral junctions. (c and d) Follow-up 18F-FDG PET/CT. (c) Coronal fused 18F-FDG PET/CT image shows symmetrical uptake (maximum standard uptake value = 1.9-3.2) along the costochondral junctions. (d) Axial fused 18F-FDG PET/CT image
shows symmetrical uptake along the costochondral junctions.
The patient complained of unresolved bony pain with
persistently elevated erythrocyte sedimentation rate
(46 mm/h) and C-reactive protein level (71 mg/L). A
follow-up 18F-FDG PET/CT 12 months later to assess
response to immunosuppressive therapy revealed new
symmetrical uptake along the costochondral junctions (Figure 2c and 2d), suggestive of active RP. In view
of the radiological findings and clinical progression,
the dosage of methotrexate was increased; biologics
would be considered if the disease remained refractory.
The patient’s symptoms subsequently improved with a
decreasing trend of serum inflammatory markers.
DISCUSSION
The 18F-FDG PET/CT was first reported in 2007 to provide metabolic information of an RP patient.[3] Since then, several case reports have shown 18F-FDG PET/CT
to be capable of determining organ involvement and
evaluating disease activity and therapeutic response.[4] [5] [6] [7]
The 18F-FDG PET/CT findings in patients with
RP comprise mainly airway wall thickening and
calcification, airway stenosis and malacia, and air
trapping.[1] The presence of symmetrically distributed
high FDG-uptake lesions may also be diagnostic of RP.[2]
Furthermore, 18F-FDG PET/CT has a role in targeting
biopsy sites, increasing remarkably the biopsy yield
rate.[2] Nonetheless a retrospective study[8] found that
in biopsy-proven auricular RP, or where there was
tracheal involvement, the sensitivity and specificity of
18F-FDG PET/CT were only 55.6% and 5.3%,
respectively, raising questions about its usefulness in
guiding biopsy.
A recent single-centre retrospective study compared
18F-FDG PET/CT in patients before and after treatment
and correlated the findings with clinical symptoms.[9]
Follow-up 18F-FDG PET/CT in most patients revealed a
favourable treatment response, with significantly reduced
visual scores and maximum standard uptake values in
cartilaginous tissue. Another advantage of 18F-FDG
PET/CT lies in its ability to identify areas that are not
clinically accessible but are of importance. In a patient
with asymptomatic involvement of the aorta, 18F-FDG
PET/CT was able to identify increased FDG-uptake in
the superior mesenteric artery and renal arteries.[9] Our
case demonstrates the usefulness of 18F-FDG PET/CT
in assessing RP, providing objective evidence of active activity and prompting treatment escalation.
This concurred with the clinical symptoms and serum
inflammatory markers.
Apart from 18F-FDG PET/CT, other imaging modalities have been investigated in the diagnosis and monitoring
of RP. Although expiratory CT abnormalities are present
in the majority of RP patients, only half demonstrate
abnormalities on routine inspiratory CT scans.[10] Dynamic
expiratory CT has been proposed as a routine diagnostic
component if there is clinical suspicion of airway
involvement.[10] In addition, studies have highlighted
the use of bone scintigraphy using technetium-99m—methylene diphosphonate and gallium-67 citrate to
evaluate disease activity and treatment response in RP patients.[11] In conclusion, 18F-FDG PET/CT is useful
in the follow-up of RP and in providing objective and
measurable metrics to monitor disease activity.
REFERENCES
1. Borgia F, Giuffrida R, Guarneri F, Cannavò SP. Relapsing polychondritis: an updated review. Biomedicines. 2018;6:84. Crossref
2. Lei W, Zeng H, Zeng DX, Zhang B, Zhu YH, Jiang JH, et al. 18F-FDG PET-CT: a powerful tool for the diagnosis and treatment of relapsing polychondritis. Br J Radiol. 2016;89:20150695. Crossref
3. Nishiyama Y, Yamamoto Y, Dobashi H, Kameda T, Satoh K, Ohkawa M. [18F]fluorodeoxyglucose positron emission tomography
imaging in a case of relapsing polychondritis. J Comput Assist
Tomogr. 2007;31:381-3. Crossref
4. Zhou H, Su M, Li L. 18F-FDG PET/CT imaging of relapsing polychondritis: a case report. Medicine (Baltimore). 2016;95:e4496. Crossref
5. Jain TK, Sood A, Sharma A, Basher RK, Bhattacharya A, Mittal BR. Response assessment in relapsing polychondritis with 18F-FDG PET/CT [in English, Spanish]. Rev Esp Med Nucl Imagen Mol. 2017;36:124-5. Crossref
6. Kamada H, Takanami K, Toyama Y, Saito M, Takase K. 18F-FDG PET/CT imaging of vasculitis complicated with relapsing polychondritis. Clin Nucl Med. 2020;45:e327-8. Crossref
7. Yokoyama T, Koyama N, Kodama K, Hagiwara K, Kanazawa M. 18F-fluorodeoxyglucose positron emission tomography for relapsing
polychondritis as a diagnostic approach and evaluation of disease
activity. BMJ Case Rep. 2009;2009:bcr02.2009.1591. Crossref
8. Zeng Y, Li M, Chen S, Lin L, Li S, He J, et al. Is 18F-FDG PET/CT useful for diagnosing relapsing polychondritis with airway involvement and monitoring response to steroid-based therapy? Arthritis Res Ther. 2019;21:282. Crossref
9. Sharma A, Kumar R, Mb A, Naidu GS, Sharma V, Sood A, et al.
Fluorodeoxyglucose positron emission tomography/computed
tomography in the diagnosis, assessment of disease activity and
therapeutic response in relapsing polychondritis. Rheumatology
(Oxford). 2020;59:99-106. Crossref
10. Lee KS, Ernst A, Trentham DE, Lunn W, Feller-Kopman DJ, Boiselle PM. Relapsing polychondritis: prevalence of expiratory CT airway abnormalities. Radiology. 2006;240:565-73. Crossref
11. Güngör F, Ozdemir T, Tunçdemir F, Paksoy N, Karayalçin B, Erkiliç M. Tc-99m MDP bone scintigraphy in relapsing
polychondritis. Clin Nucl Med. 1997;22:264-6. Crossref