Antibody-Mediated Striatal Encephalitis and Aseptic Meningitis in A Child with Neuropsychiatric Lupus: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Sep;26(3):198-201 | Epub 31 Aug 2023
Antibody-Mediated Striatal Encephalitis and Aseptic Meningitis in A Child with Neuropsychiatric Lupus: A Case Report
SM Yu1, TY Yuen1, KC Chan1, KM Yam2, WP Sze2, ACH Ho2
1 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Paediatrics, Prince of Wales Hospital, Hong Kong SAR, China
Correspondence: Dr Dr SM Yu, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR,
China. Email: fayeyupwr@gmail.com
Submitted: 10 Feb 2022; Accepted: 15 Jul 2022.
Contributors: SMY designed the study. All authors acquired and analysed the data. SMY, KMY, WPS and ACHH drafted the manuscript. SMY
and ACHH critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors declared no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki. Verbal consent for publication was obtained from the
patient’s parents.
INTRODUCTION
Neuropsychiatric systemic lupus erythematosus
(NPSLE) refers to the neurological and psychiatric
symptoms that are thought to be related to systemic
lupus erythematosus (SLE). NPSLE can be devastating,
responsible for high rates of morbidity and mortality.
It can occur any time in SLE. A recent large cohort
study showed that NPSLE affects up to 25% of patients
with juvenile-onset SLE.[1] The aetiology is thought
to be multifactorial but vasculopathy, autoantibody
production, and cytokine-induced neurotoxicity play a
major role in the pathogenesis.[2]
Our clinical report highlights two infrequent subtypes
of NPSLE–striatal encephalitis and aseptic meningitis,
with substantial clinical and radiological response to
immunosuppressants. The radiological features and
treatment response of striatal encephalitis in our case are
strikingly similar to those of the subset of anti–N-methyl-D-aspartate receptor (anti-NMDAR) autoimmune
encephalitis involving the striatum.
CASE REPORT
An 11-year-old girl with known SLE presented to our
hospital with a 1-week history of difficulty in passing
urine, followed by acute-onset urinary retention. She was
also suspected to have depersonalisation-derealisation
syndrome for the last 1 year. She experienced progressive
worsening with avolition, apathetic, mental slowness,
and insomnia 4 weeks prior to this acute presentation.
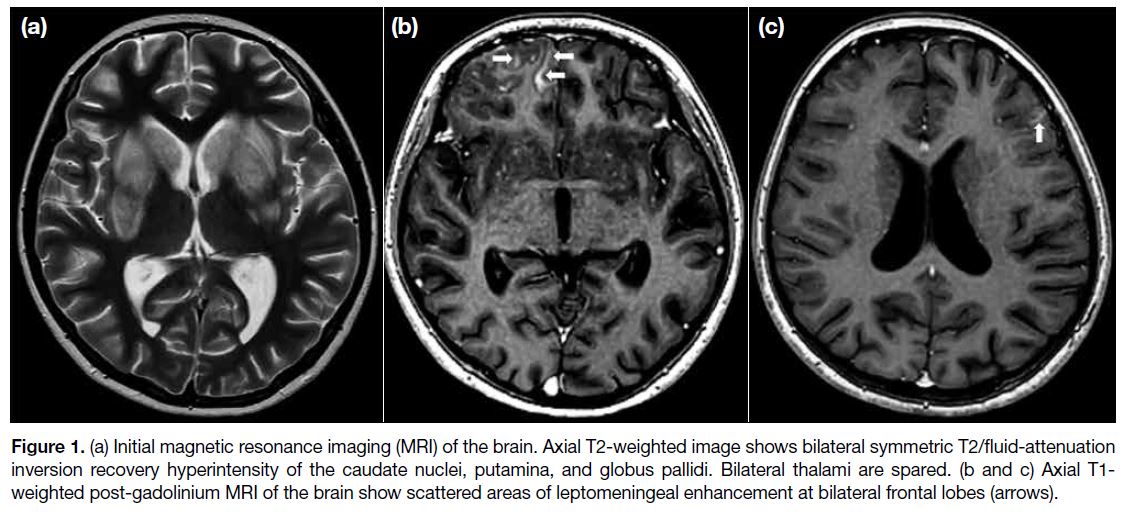
Urgent magnetic resonance imaging (MRI) brain
examination demonstrated bilateral symmetrical T2/fluid-attenuated
inversion recovery (FLAIR) hyperintensity of
the caudate nuclei, putamina, and globus pallidus without
contrast enhancement or restricted diffusion. Bilateral
thalami were spared (Figure 1a). Following intravenous
gadolinium injection, scattered areas of leptomeningeal
enhancement were present at bilateral frontal lobes and
the right cerebellar hemisphere (Figure 1b and 1c). MRI
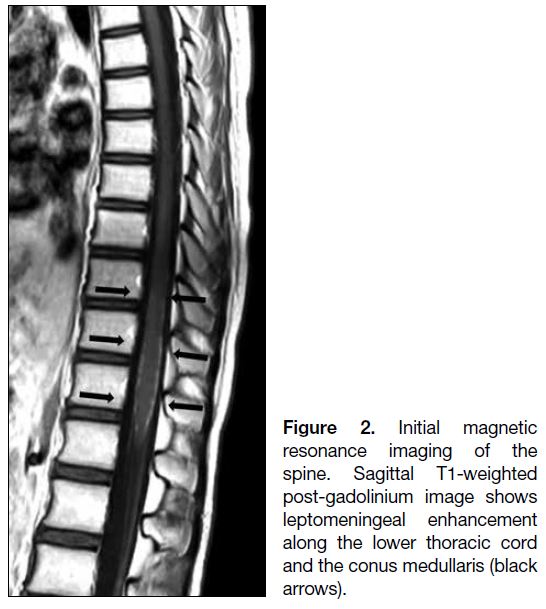
of the spine demonstrated leptomeningeal enhancement
along the lower thoracic cord and the conus medullaris
(Figure 2).
Figure 1. (a) Initial magnetic resonance imaging (MRI) of the brain. Axial T2-weighted image shows bilateral symmetric T2/fluid-attenuation
inversion recovery hyperintensity of the caudate nuclei, putamina, and globus pallidi. Bilateral thalami are spared. (b and c) Axial T1-weighted post-gadolinium MRI of the brain show scattered areas of leptomeningeal enhancement at bilateral frontal lobes (arrows).
Figure 2. Initial magnetic
resonance imaging of the spine. Sagittal T1-weighted post-gadolinium image shows leptomeningeal enhancement along the lower thoracic cord and the conus medullaris (black arrows).
The patient underwent extensive workup and was
positive for antinuclear antibodies, anti–double-stranded
DNA, anti-ribonucleoprotein, anti-extractable nuclear
antigen antibodies including anti-Ro, anti-La and
anti-Sm, as well as anti-ribosomal P antibodies; anti-NMDAR antibody was negative. Cerebrospinal fluid analysis revealed mildly elevated protein and normal leucocyte count. Cerebrospinal fluid gram stain, culture
and virology were negative.
The patient was empirically treated with intravenous
acyclovir, oral oseltamivir, and intravenous ciprofloxacin
at the time of presentation for 2 days without any clinical
improvement. Based on the clinical and characteristic
MRI features, a working diagnosis was made of
NPSLE with antibody-mediated striatal encephalitis
and aseptic meningitis. Antiviral and antibiotic
treatments were discontinued and immunosuppressive
treatment commenced. The patient received 5 days of
pulse intravenous methylprednisolone and intravenous
cyclophosphamide, followed by oral prednisolone and
mycophenolate mofetil.
Follow-up MRI of the brain was performed 3 days later
to guide clinical management. There was complete
resolution of the leptomeningeal enhancement but
persistent striatal T2/FLAIR hyperintensity. With
treatment, the clinical status of the patient gradually
improved. Foley was weaned off successfully. Her
mental state and sleep quality improved.
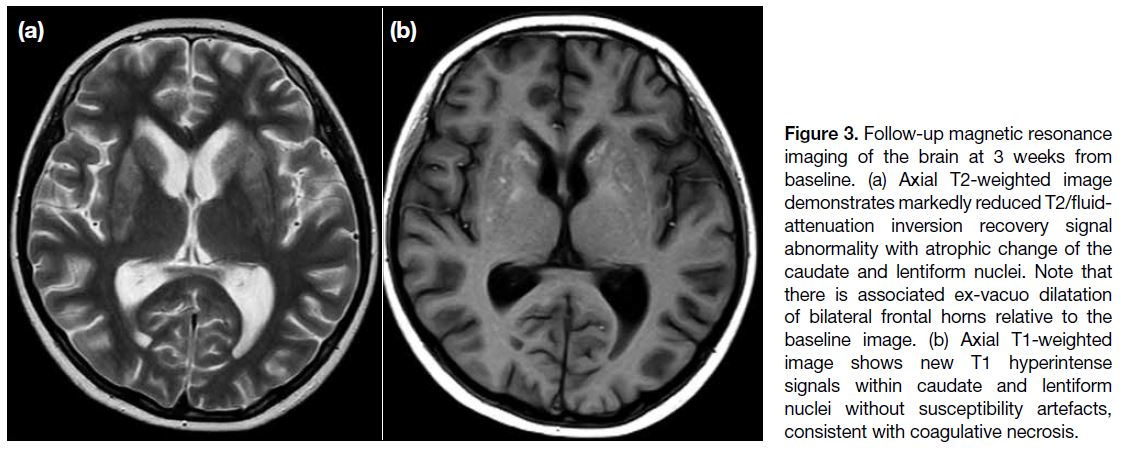
Another follow-up MRI of the brain 3 weeks from
baseline demonstrated reduction in the extent of
T2/FLAIR signal abnormality but interval development
of caudate and lentiform nuclei atrophy (Figure 3a).
There was also novel T1 hyperintensity within the
caudate and lentiform nuclei without associated susceptibility artefacts, consistent with coagulative necrosis (Figure 3b).
Figure 3. Follow-up magnetic resonance imaging of the brain at 3 weeks from baseline. (a) Axial T2-weighted image demonstrates markedly reduced T2/fluid-attenuation inversion recovery signal abnormality with atrophic change of the caudate and lentiform nuclei. Note that there is associated ex-vacuo dilatation of bilateral frontal horns relative to the baseline image. (b) Axial T1-weighted image shows new T1 hyperintense signals within caudate and lentiform nuclei without susceptibility artefacts, consistent with coagulative necrosis.
DISCUSSION
NPSLE describes a wide spectrum of peripheral and
central nervous system manifestations. The widely
quoted classification of NPSLE, made by an American
College of Rheumatology expert committee in 1999,
comprised 12 central and seven peripheral nervous system
manifestations.[3] Case definitions including diagnostic
criteria, exclusions, and methods of ascertainment were
developed for each specific neuropsychiatric syndrome.[3]
Among these, headache, mood disorders, cognitive
dysfunction, seizures, movement disorders, and
cerebrovascular disease are the most common NPSLE
manifestations.[1] [4] [5]
Neuroimaging plays a vital role in the diagnosis of
NPSLE and MRI is the preferred imaging modality for
assessment of neurological manifestations although a
negative scan does not exclude the presence of active
NPSLE. A wide range of radiological abnormalities has
been described but are often non-specific. In a single-centre
retrospective study by Al-Obaidi et al,[5] the major
MRI findings in juvenile-onset NPSLE were focal T2-weighted white matter hyperintensities (33%), brain
atrophy (18.5%), cortical grey matter lesions (3%),
and basilar artery territory infarction (3%), although a
majority of patients (59%) showed no MRI abnormalities
despite clinically active NPSLE. Striatal encephalitis
and aseptic meningitis have rarely been described as
characteristic manifestations of neuropsychiatric lupus,[6] [7] [8]
although they were coexistent in our patient.
Antibody-mediated striatal encephalitis is a rare
but characteristic manifestation of NPSLE.[6] [7] The
radiological features and clinical course of lupus-related
antibody-mediated striatal encephalitis closely
resemble those of anti-NMDAR striatal encephalitis. It
is suggested that peripheral anti–double-stranded DNA
antibodies, a specific serum autoantibody in SLE, may
enter the central nervous system to cross-react with
NMDAR antigens[6] [7] and mediate a non-thrombotic
and non-vasculitic pathology with features of neuronal
excitotoxicity.[6] Other autoantibodies thought to cause
NPSLE include anti-neuronal, anti-NMDAR2, anti-ribosomal
P, and anti-endothelial antibodies.[9]
The radiological pattern of bilateral symmetrical T2
hyperintensity involving lentiform and caudate nuclei
typically suggests systemic or metabolic causes. The
characteristic sparing of bilateral thalami and lack of
restricted diffusion and contrast enhancement provide
important clues for excluding multiple conditions
in the differential list including hypoglycaemia,
hypoxic ischaemic encephalopathy, extrapontine
myelinolysis, arterial occlusion, venous thrombosis, and
Creutzfeldt–Jakob disease. Other differentials including
organophosphate or methanol poisoning can be excluded
by clinical presentation and toxicology.
In an appropriate clinical setting, such as in our patient,
the presence of bilateral symmetrical T2 hyperintense
signal changes within the caudate and putamen without
evidence of restricted diffusion or contrast enhancement
was strongly suggestive of autoimmune encephalitis
of the striatum.[7] Intrinsic T1 hyperintensity within the striatum, also evident in our patient, may indicate a poor prognosis. This has been proposed to be related
to the development of coagulative necrosis secondary
to prolonged antibody-mediated inflammation and
excitatory glutamate neurotoxicity.[7]
Aseptic meningitis is another manifestation of NPSLE.
It is possibly mediated by a combination of mechanisms
such as anti-neuronal antibodies, antiphospholipid
antibodies, immune complex–mediated vasculitis, and
cytokines. Due to the non-specific radiological findings,
more common causes of leptomeningeal enhancement,
including infection and malignancy, must be excluded
before establishing the diagnosis.[4] Compared with
pyogenic meningitis, cerebrospinal fluid leucocytes
and proteins are usually lower in cases of lupus-related
aseptic meningitis.
In children with SLE, neuropsychiatric manifestation
is frequently associated with high overall SLE activity.
Aggressive treatment, including systemic corticosteroid
and immunosuppressants, is often required to control
the autoimmune process and avoid further damage.[4]
Close collaboration between the radiologist, paediatric
rheumatologist and neurologist is crucial to reach a
prompt diagnosis and maximise clinical outcomes in
these patients with NPSLE.
CONCLUSION
We present a patient with NPSLE presenting as
striatal encephalitis and aseptic meningitis who
demonstrated a good clinical and radiological response
to immunosuppressive therapy. The clinical course
and imaging features of antibody-mediated striated encephalitis in our patient closely resemble those of a
striatal variant of anti-NMDAR encephalitis.
Neuroimaging plays an important role in diagnosis,
monitoring treatment response and prognostication
by identifying complications such as brain atrophy or
intrinsic basal ganglionic T1 hyperintensity. Prompt
diagnosis and early treatment facilitates optimisation of
clinical outcomes.
REFERENCES
1. Giani T, Smith EM, Al-Abadi E, Armon K, Bailey K, Ciurtin C,
et al. Neuropsychiatric involvement in juvenile-onset systemic
lupus erythematosus: data from the UK juvenile-onset systemic
lupus erythematosus cohort study. Lupus. 2021;30:1955-65. Crossref
2. Hanly JG. Neuropsychiatric lupus. Curr Rheumatol Rep. 2001;3:205-12. Crossref
3. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes [editorial]. Arthritis Rheum. 1999;42:599-608. Crossref
4. Kivity S, Agmon-Levin N, Zandman-Goddard G, Chapman J, Shoenfeld Y. Neuropsychiatric lupus: a mosaic of clinical
presentations. BMC Med. 2015;13:43. Crossref
5. Al-Obaidi M, Saunders D, Brown S, Ramsden L, Martin N, Moraitis E, et al. Evaluation of magnetic resonance imaging
abnormalities in juvenile onset neuropsychiatric systemic lupus
erythematosus. Clin Rheumatol. 2016;35:2449-56. Crossref
6. DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT,
Diamond B. A subset of lupus anti-DNA antibodies cross-reacts
with the NR2 glutamate receptor in systemic lupus erythematosus.
Nat Med. 2001;7:1189-93. Crossref
7. Kelley BP, Corrigan JJ, Patel SC, Griffith BD. Neuropsychiatric lupus with antibody-mediated striatal encephalitis. AJNR Am J Neuroradiol. 2018;39:2263-69. Crossref
8. Canoso JJ, Cohen AS. Aseptic meningitis in systemic lupus erythematosus. Report of three cases. Arthritis Rheum. 1975;18:369-74. Crossref
9. Govoni M, Hanly JG. The management of neuropsychiatric lupus in the 21st century: still so many unmet needs? Rheumatology (Oxford). 2020;59(Suppl5):v52-62. Crossref