Four-Dimensional Computed Tomography for Localisation of Parathyroid Adenomas
PERSPECTIVE
Hong Kong J Radiol 2023 Sep;26(3):185-93 | Epub 18 Sep 2023
Four-Dimensional Computed Tomography for Localisation of Parathyroid Adenomas
HS Leung1, SYW Liu2, KT Wong1, SM Yu1, AD King1
1 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Surgery, Prince of Wales Hospital, Hong Kong SAR, China
Correspondence: Dr HS Leung, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China. Email: lhs655@ha.org.hk
Submitted: 5 Feb 2022; Accepted: 17 May 2022.
Contributors: HSL and KTW designed the study. HSL, KTW and SMY acquired the data. HSL and SMY analysed the data. HSL and ADK drafted the manuscript. SYWL, KTW, SMY and ADK critically revised the manuscript for important intellectual content. All authors had full
access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2022.069). A waiver for obtaining patient consent was granted by the Committee due to the retrospective nature of the study.
Abstract
Primary hyperparathyroidism is a relatively common disorder with a myriad of end-organ manifestations, and
surgical treatment remains the only curative option. Minimally invasive parathyroidectomy has replaced bilateral
neck exploration as the standard technique for the majority of patients with solitary adenomas. Preoperative imaging
is essential for accurate localisation of parathyroid adenomas. Four-dimensional computed tomography of the
parathyroids has emerged as a useful problem-solving tool in the initial workup. This article reviews the underlying
imaging principles, techniques, and practical tips on image interpretation and reporting, all of which are essential
in successful preoperative localisation of parathyroid adenomas.
Key Words: Hyperparathyroidism; Hyperparathyroidism, primary; Parathyroid neoplasms; Parathyroidectomy
中文摘要
用於副甲狀腺腺瘤定位的四維電腦斷層掃描
梁皓生、廖玉華、黃嘉德、于雪梅、金雅桃
原發性副甲狀腺功能亢進症是一種相對常見的疾病,並會影響多重器官功能,手術治療仍然是唯一的治癒選擇。微創副甲狀腺切除術已取代雙側頸部探查而成為用於大多數單一副甲狀腺腺瘤患者的標準技術。術前影像學檢查對於準確定位副甲狀腺腺瘤至關重要。副甲狀腺四維電腦斷層掃描已成為術前檢查中解決問題的有用工具。本文回顧基本成像原理、技術及圖像解釋和報告的實用技巧,以上均對術前定位至關重要。
INTRODUCTION
Primary hyperparathyroidism is a relatively common
disorder with an average population prevalence of
0.3%.[1] [2] Long-term primary hyperparathyroidism with
the accompanying hypercalcaemia may cause skeletal
(pathological fractures), renal (nephrolithiasis, polyuria,
and polydipsia), and gastrointestinal (peptic ulceration
and pancreatitis) abnormalities. With more widespread
use of biochemical screening in developed countries, the
paradigm has shifted to an asymptomatic presentation,
with or without elevated calcium levels.[3] [4] [5] [6] [7]
Surgical treatment remains the only curative option,
with a shift in approach for the majority of patients with
a solitary adenoma from bilateral neck exploration to
minimally invasive parathyroidectomy (MIP),[8] offering
similar outcomes with reduction in complications and
shorter postoperative recovery.[8] [9] Preoperative imaging
localisation of the parathyroid glands is essential to a
successful MIP. Traditionally achieved by ultrasound
and/or technetium-99m (99mTc) sestamibi scintigraphy,
in recent years four-dimensional computed tomography
(4DCT) has emerged as a robust alternative modality,
offering more accurate localisation.[10] [11] This article on
4DCT reviews the technique, practical guidelines, and
tips on what to look for; diagnostic performance; and
advantages/disadvantages compared with other imaging
modalities.
INDICATIONS FOR PARATHYROID IMAGING
International guidelines[12] [13] [14] have suggested that
preoperative imaging for parathyroid localisation
followed by parathyroidectomy is indicated when
there is primary hyperparathyroidism together with
any of the following conditions: (1) symptomatic
hyperparathyroidism; (2) significantly elevated calcium
levels (>1 mg/dL or 0.25 mmol/L above upper reference
limit); (3) evidence of end-organ involvement, including
renal (nephrocalcinosis, hypercalciuria, and impaired
renal function) or skeletal (osteoporosis, fragility
fractures as a manifestation of osteoporosis or vertebral
insufficiency fractures); and (4) recurrent or persistent
hyperparathyroidism after initial surgery.[15]
In addition, preoperative imaging followed by
parathyroidectomy may also be considered when there
is biochemical hyperparathyroidism in any of the
following circumstances: (1) presence of neurocognitive
or neuropsychiatric symptoms attributable to
hyperparathyroidism[16]; (2) aged <50 years old, in the absence of symptoms or evidence of end-organ
involvement; and (3) inability or unwillingness to comply
with follow-up protocols after the initial diagnosis of
primary hyperparathyroidism.
IMAGING TECHNIQUE
4DCT refers to evaluation of the lesions acquired in
three dimensions[17] and their enhancement characteristics
over time.[18] Various protocols with different timed
phases have been established in the literature,[18] although
it most commonly consists of an arterial phase at 25 to
30 seconds after contrast injection, together with venous
phase at 45 to 60 seconds and/or a delayed phase at 80 to
90 seconds. A very delayed phase at approximately 120
seconds has also been adopted in some centres.[19] The
addition of a non-contrast phase is also controversial; a
previous study has demonstrated that 22% of parathyroid
lesions might have been missed if a pre-contrast scan had
been excluded,[20] although another retrospective study[21]
showed that a two-phase protocol has only slightly
reduced accuracy which has to be balanced with the
radiation exposure of an additional phase. The use of
dual energy acquisition to generate virtual non-contrast
images for parathyroid adenomas have also been
reported,[22] [23] although in our experience the virtual non-contrast
images based on subtraction of iodine signal also
diminished the hyperattenuation of the normal thyroid
parenchyma, rendering more difficult visualisation of the
parathyroid glands in the perithyroidal region.
Optimisation of the imaging protocol is of foremost
importance in achieving accurate parathyroid
localisation. The range of scanning has to cover all
potential sites of ectopic parathyroid glands, from angle
of the mandible to the upper mediastinum at the level
of the aortic arch, although the pre-contrast scan can
be limited to the thyroid bed (from hyoid bone to the
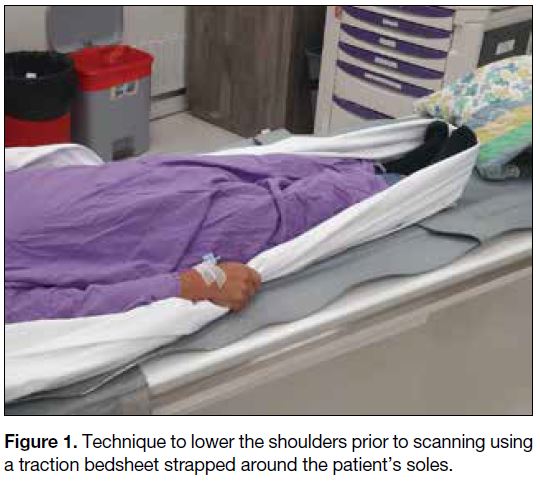
inferior margin of clavicular head).[17] Prior to scanning,
a technique to move the shoulders down is also applied
by asking the patient to pull tightly onto a traction
bedsheet across his/her soles (Figure 1) so as to reduce
the beam hardening artefacts over the lower pole of the
thyroid, the level where inferior parathyroid adenomas
are most commonly located (Figure 2).[17] A rapid rate
of contrast injection (at least 4 mL/s) is also important
to better demonstrate the differences in enhancement
characteristics. Contrast injection should be performed
via a right arm vein to minimise streak artefacts over
the midline upper mediastinum and lower neck. In our
centre, we perform a non-contrast scan through the
thyroid bed, followed by an arterial phase at 25 seconds and a delayed phase at 80 seconds in a caudocranial
direction from the aortic arch through the mandibular
angle. Scanning parameters using SOMATOM Drive
system (Siemens Healthineers, Munich, Germany) are
as follows: 100kV, 300 mA (pre-contrast) and 400 mA
(arterial and delayed), pitch 0.8, and 0.6-mm section
thickness with multiplanar reconstruction.
Figure 1. Technique to lower the shoulders prior to scanning using a traction bedsheet strapped around the patient’s soles.
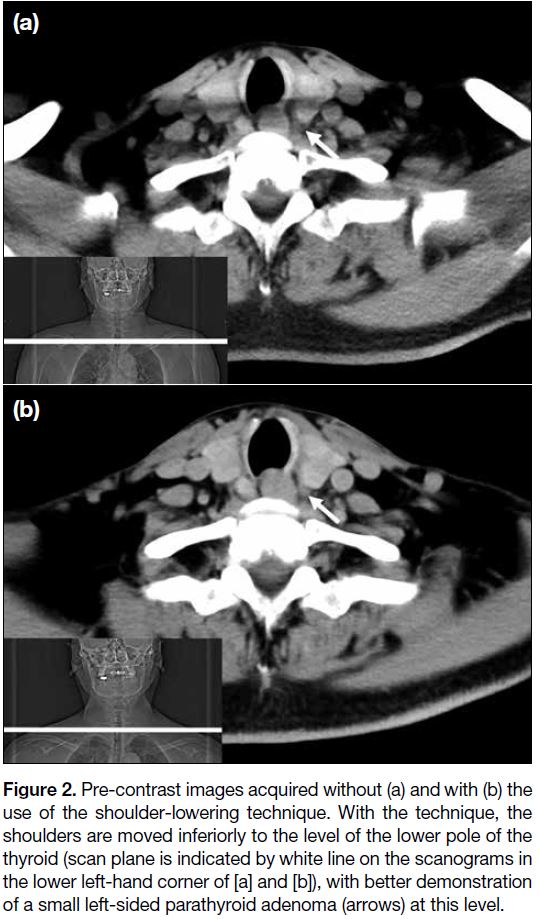
Figure 2. Pre-contrast images acquired without (a) and with (b) the
use of the shoulder-lowering technique. With the technique, the
shoulders are moved inferiorly to the level of the lower pole of the
thyroid (scan plane is indicated by white line on the scanograms in
the lower left-hand corner of [a] and [b]), with better demonstration
of a small left-sided parathyroid adenoma (arrows) at this level.
IMAGE INTERPRETATION AND REPORTING
A structured template for reporting is useful to ensure that all important areas for management are included.
How Many Lesions Are There?
Most cases of primary hyperparathyroidism (85%-90%)
are the result of a solitary hyperfunctioning adenoma,
with multiglandular disease (6%), double adenoma (4%),
and carcinoma (1%) being less common. Conversely,
most if not all second hyperparathyroidism from renal
failure is due to multiglandular hyperplasia.
Practical tip
The number of enlarged, hyperfunctioning parathyroid
glands is an important factor for surgical planning.
Patients with solitary lesions are eligible for MIP while
patients with >1 lesion may require a unilateral or
bilateral neck exploration.
Position of Parathyroid Glands
Approximately 80% of patients have four normal glands
in two symmetrical pairs. The superior glands originate
from the fourth pharyngeal pouch and descend caudally, orthotopically located posterior to the upper two-thirds
of the thyroid lobe (most at the equator of the gland) and
posterior to the recurrent laryngeal nerve. The inferior
parathyroid glands originate from the third pharyngeal
pouch, joining the thymus with a longer route of descent
and are usually found more ventrally around the inferior
thyroid poles and anterior to the recurrent laryngeal
nerve.[24] [25] Supernumerary glands have been reported in
approximately 15% of patients, while in 3% of cases
only three glands can be found.[26]
Ectopic parathyroid glands have a prevalence of 14% to 16% in patients with hyperparathyroidism and up to 43%
in anatomical studies.[27] Superior ectopic glands are most
commonly found in the retro-oesophageal region and
trachea-oesophageal groove.[28] [29] These may result from
over-descent in 10% to 20% of cases, mimicking, or even more caudal to, an orthotopic inferior parathyroid.[30]
Inferior ectopic parathyroid glands have a more variable
location due to the longer descent, most commonly
found in the thymic region and upper mediastinum with
less common sites including undescended glands above
the superior thyroid pole or within the carotid sheath.[28] [29]
Intrathyroid parathyroid glands have also been reported
in 1% to 3% of patients[31] [32] and are often difficult to
detect by preoperative imaging or intra-operatively.
Practical tips
Firstly, a detailed description of location in relation to
level and adjacent structures is required, such as hyoid,
larynx, trachea, oesophagus, thyroid gland, carotid
sheath, suprasternal notch, and mediastinal vessels.
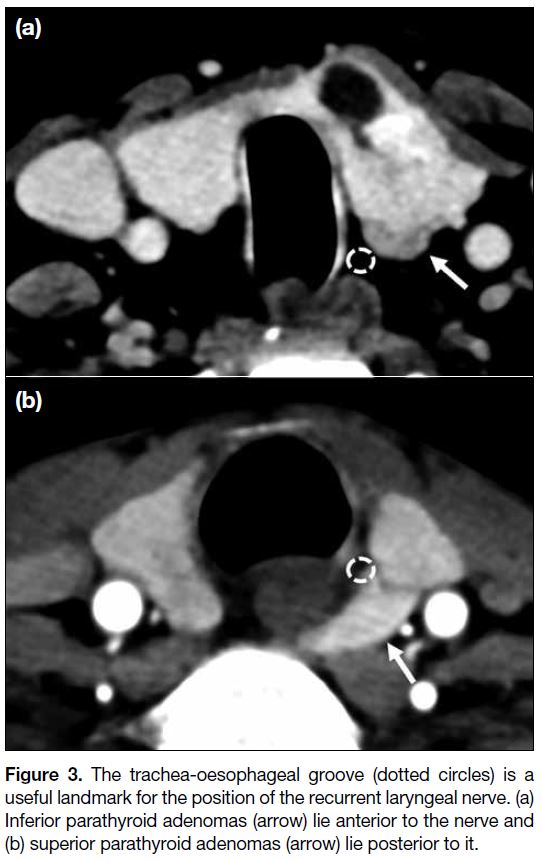
The relationship of an enlarged parathyroid gland to
the trachea-oesophageal groove (expected location of
recurrent laryngeal nerve) is also important, as superior
parathyroid glands are posterior and inferior parathyroid
glands are anterior to the groove (Figure 3). Secondly,
check all areas from the angle of the mandible down
to and including to the superior mediastinum. Lastly,
the presence of an intervening fat plane is useful in
distinguishing a parathyroid adenoma from a thyroid
nodule, although this can be absent in small parathyroid
adenomas within or abutting the thyroid capsule. It
is therefore important to check all scanned phases,
particularly the pre-contrast phase where parathyroid
adenomas are relatively hypodense to the thyroid
parenchyma.
Figure 3. The trachea-oesophageal groove (dotted circles) is a
useful landmark for the position of the recurrent laryngeal nerve. (a)
Inferior parathyroid adenomas (arrow) lie anterior to the nerve and
(b) superior parathyroid adenomas (arrow) lie posterior to it.
How to Call a Lesion as an Abnormal Parathyroid Gland?
Size and shape
The normal parathyroid gland is up to 2 mm in thickness and 5 to 10 mm in length[33]; hence, a gland is usually only
visualised when enlarged and abnormal. Enlarged glands
may be oval, round, pyramidal or lobulated and tend to
be larger in parathyroid adenomas than multiple gland
hyperplasia.
Enhancement pattern
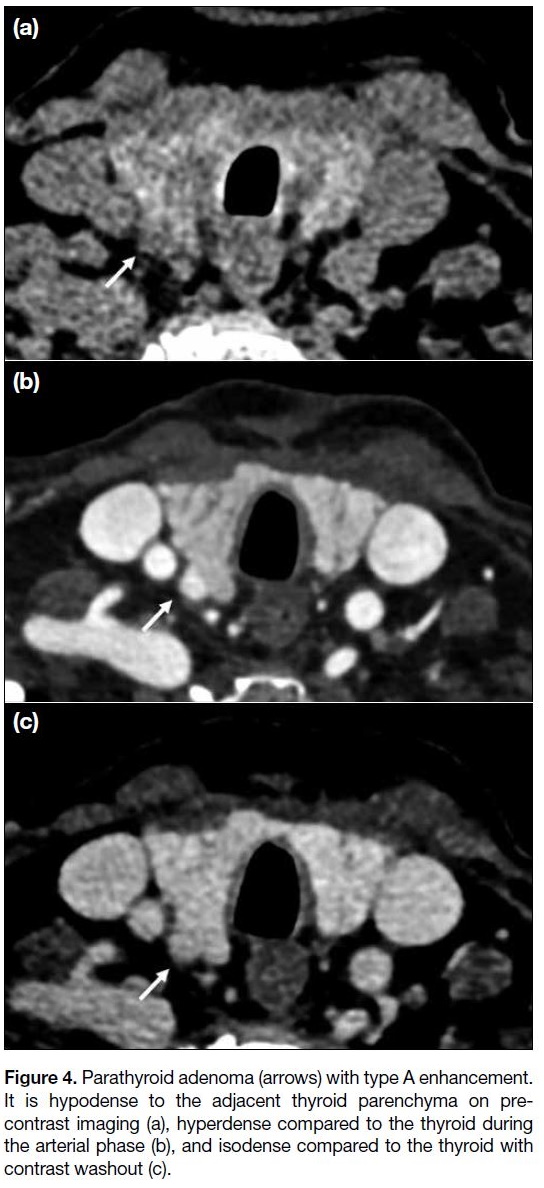
The typical adenoma enhancement pattern would be
hypodense to the thyroid on pre-contrast, hyperenhancing
relative to the thyroid on arterial phase, and hypoenhancing
on delayed phase. However, these imaging features are
not consistently present in all parathyroid adenomas, as
a recent study using dynamic CT[18] has demonstrated that
hyperfunctioning parathyroid tissue only shows a short
period of hyperenhancement relative to the thyroid,
with vast heterogeneity in the presence and timing of hyperenhancement amongst different adenomas. Three
types of variations in enhancement have been previously
described (Figures 4, 5, and 6).[20]
Figure 4. Parathyroid adenoma (arrows) with type A enhancement.
It is hypodense to the adjacent thyroid parenchyma on pre-contrast
imaging (a), hyperdense compared to the thyroid during
the arterial phase (b), and isodense compared to the thyroid with
contrast washout (c).
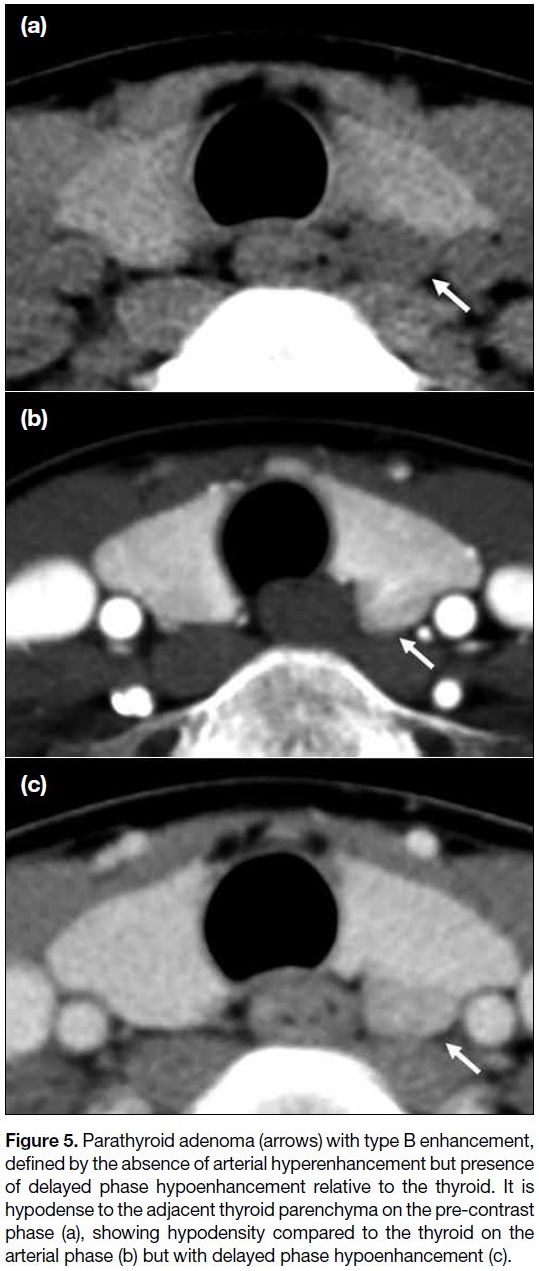
Figure 5. Parathyroid adenoma (arrows) with type B enhancement,
defined by the absence of arterial hyperenhancement but presence
of delayed phase hypoenhancement relative to the thyroid. It is
hypodense to the adjacent thyroid parenchyma on the pre-contrast
phase (a), showing hypodensity compared to the thyroid on the
arterial phase (b) but with delayed phase hypoenhancement (c).
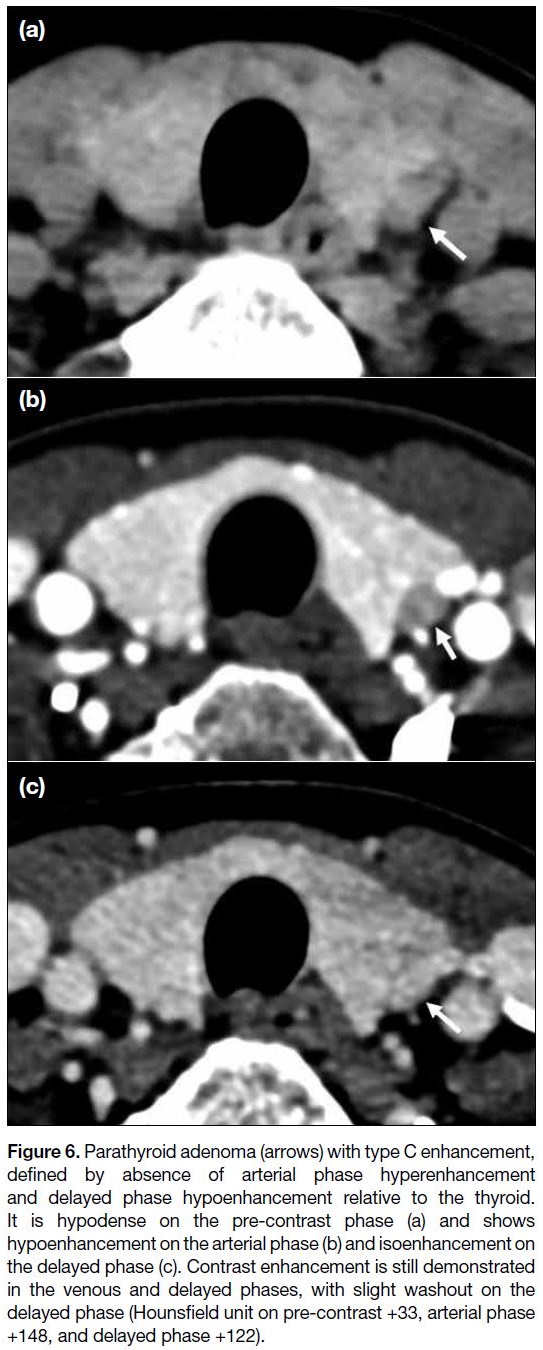
Figure 6. Parathyroid adenoma (arrows) with type C enhancement,
defined by absence of arterial phase hyperenhancement
and delayed phase hypoenhancement relative to the thyroid.
It is hypodense on the pre-contrast phase (a) and shows
hypoenhancement on the arterial phase (b) and isoenhancement on
the delayed phase (c). Contrast enhancement is still demonstrated
in the venous and delayed phases, with slight washout on the
delayed phase (Hounsfield unit on pre-contrast +33, arterial phase
+148, and delayed phase +122).
Ancillary features
The presence of a fat plane between the nodule and
thyroid is used to discriminate a parathyroid adenoma
from a thyroid nodule.
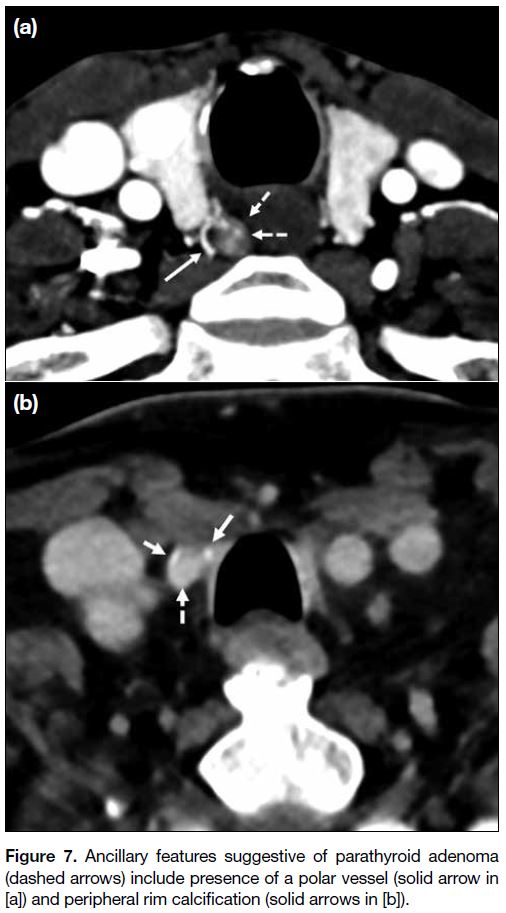
The presence of a polar vessel[34] entering the superior
or inferior pole of the parathyroid is a useful ancillary
feature to support the diagnosis of parathyroid adenoma
(Figure 7a). Rim or central calcification (Figure 7b),
internal cystic changes, or necrosis have been described
as variant appearances of parathyroid adenomas, which
can be present in up to 10% of cases.[35] [36] Acute necrosis
in a parathyroid adenoma has been associated with
spontaneous reduction in hormone levels.[37] Accurate
differentiation of parathyroid carcinoma from adenoma
is difficult,[38] although the presence of a large (>3 cm) parathyroid gland, irregular borders and peritumoural infiltration into adjacent structures aids in distinguishing carcinoma from adenoma.[39] [40]
Figure 7. Ancillary features suggestive of parathyroid adenoma
(dashed arrows) include presence of a polar vessel (solid arrow in
[a]) and peripheral rim calcification (solid arrows in [b]).
Practical tips
Firstly, small lymph nodes may be mistaken for
parathyroid adenomas, although the presence of
progressive enhancement on delayed phase and the
presence of a fatty hilum are useful distinguishing features. Secondly, for indeterminate lesions it
is important to review previous ultrasound and
scintigraphy, as lesions concordant with previous
imaging findings afford much more diagnostic
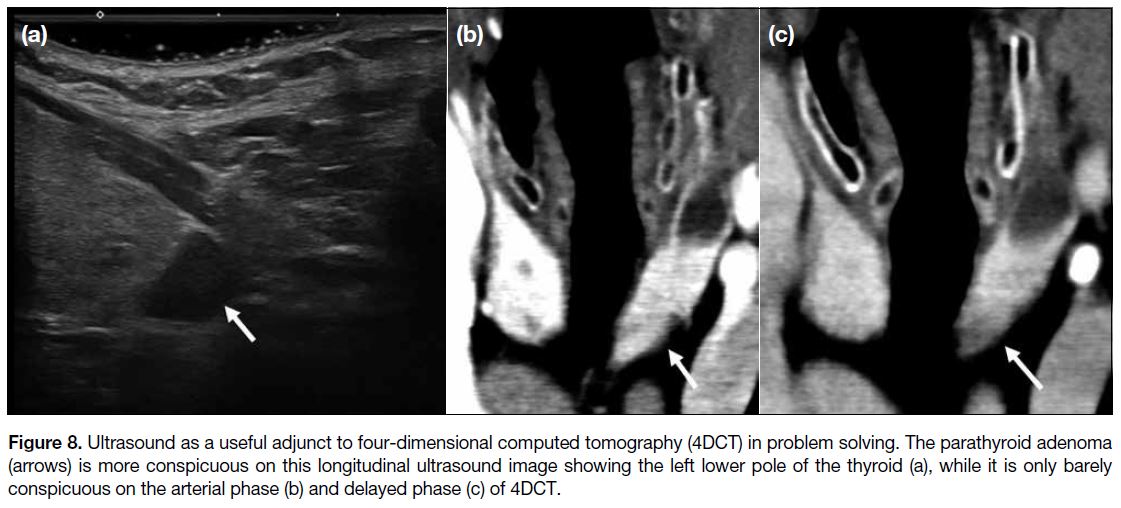
confidence. Repeating the ultrasound, especially in cases where a previous study was negative or discordant
from 4DCT, is an important problem-solving tool and
increases diagnostic confidence without incurring
additional radiation exposure (Figure 8).
Figure 8. Ultrasound as a useful adjunct to four-dimensional computed tomography (4DCT) in problem solving. The parathyroid adenoma
(arrows) is more conspicuous on this longitudinal ultrasound image showing the left lower pole of the thyroid (a), while it is only barely
conspicuous on the arterial phase (b) and delayed phase (c) of 4DCT.
Other Important Findings which may Affect Surgical Planning
The presence of concomitant thyroid nodules should
be reported, which requires further evaluation with
ultrasound and/or fine-needle aspiration cytology, as
any suspicious thyroid nodule would necessitate a
hemi- or total thyroidectomy in the same operation.
This is also useful in investigating the possibility of a
rare intrathyroid parathyroid adenoma, especially in
patients where no other candidate lesion is identified. In
addition, the presence of anomalies of the major arteries,
such as an aberrant right subclavian artery, which can
be associated with a non-recurrent laryngeal nerve,[41]
should be highlighted as this constitutes potential surgical hazard. Evidence of any previous head and neck
surgery and altered anatomy should also be identified for
accurate operative planning.
Performance of Four-Dimensional Computed Tomography and Comparison with Other Imaging Modalities
Ultrasound, nuclear scintigraphy, and 4DCT are widely
accepted modalities for parathyroid localisation.[42]
While 4DCT has been adopted in some centres as first-line
imaging,[43] the choice and sequence of imaging
investigations remain variable across centres depending
on availability and expertise.[44] [45] One of the main
advantages of 4DCT is the ability to clearly demonstrate
to the surgeon the location of a parathyroid adenoma and
its relationship to adjacent structures. Multiple studies
have proven 4DCT as a robust technique in parathyroid
localisation,[10] [19] [44] [46] with the latest meta-analysis showing
a sensitivity of 81% and positive predictive value of
91%.[47] The 4DCT has been shown to be superior to
99mTc sestamibi and ultrasound for localisation in several
studies,[10] [11] [48] although some other studies showed no
significant difference in diagnostic accuracy.[49] [50] In our
centre, a combination of parathyroid ultrasound and
99mTc sestamibi scintigraphy, which are more readily
available, still serves as a first-line diagnostic approach
while 4DCT is reserved for patients with negative or
discordant localisation. Repeated parathyroid ultrasound
in the same session as 4DCT can often enhance diagnostic
confidence.
The 4DCT technique has also been proven useful in
hyperparathyroid patients with previously negative
ultrasound and/or scintigraphy.[51] Limited studies have
also explored its application in patients with secondary
hyperparathyroidism,[52] in whom bilateral neck
exploration remains mandatory but which can identify
ectopic or supernumerary glands to improve the rate of
surgical success.[44]
The issue of radiation exposure is also of concern.
A previous study by Mahajan et al[53] has shown that
the effective dose of 4DCT (10.4 mSv) is higher than
99mTc sestamibi scintigraphy (7.8 mSv). Although more
recent studies have demonstrated a lower effective dose
of 4DCT (4.5-8.9 mSv),[43] the organ dose to the thyroid
bed from 4DCT remains significantly higher. The risk of
attributed malignancy to radiation exposure from 4DCT
should be balanced with the benefit of reducing surgical
morbidity, by converting to a less complex MIP and
lower chance of reoperation. This is especially helpful
in younger patients where radiation exposure bears a
more significant long-term cumulative effect. Despite
a younger age of presentation of hyperparathyroidism
in the Chinese population,[54] the majority of patients
are still >50 years of age and the benefits of proceeding
with 4DCT examination is mostly justified. In our
institution, a combination of ultrasound and 99mTc
sestamibi scintigraphy remains the initial investigation,
with patients referred for 4DCT if the initial workup is
inconclusive.
Finally, it is important to emphasise that all modalities have their advantages and disadvantages. Therefore,
a multimodality approach and careful review of all
previous parathyroid imaging studies are the keys to
increase diagnostic confidence.
SUMMARY
4DCT of the parathyroids offers a promising alternative for preoperative localisation of parathyroid adenoma,
not only as a problem-solving tool but with potential for
becoming the modality of choice in the initial workup. A
good understanding of underlying anatomy and imaging
principles, optimisation of CT protocol, correlation
with other imaging modalities, and structured reporting
tailored to answer specific question by surgeons are all
important in delivering accurate preoperative localisation
for better surgical outcomes.
REFERENCES
1. Adami S, Marcocci C, Gatti D. Epidemiology of primary hyperparathyroidism in Europe. J Bone Miner Res. 2002;17 Suppl 2:N18-23.
2. Christensson T, Hellström K, Wengle B, Alveryd A, Wikland B. Prevalence of hypercalcaemia in a health screening in Stockholm. Acta Med Scand. 1976;200:131-7. Crossref
3. Pradeep PV, Jayashree B, Mishra A, Mishra SK. Systematic review of primary hyperparathyroidism in India: the past, present, and the future trends. Int J Endocrinol. 2011;2011:921814. Crossref
4. Liu JM, Cusano NE, Silva BC, Zhao L, He XY, Tao B, et al. Primary hyperparathyroidism: a tale of two cities revisited—New York and Shanghai. Bone Res. 2013;1:162-9. Crossref
5. Insogna KL. Primary hyperparathyroidism. N Engl J Med. 2018;379:1050-9. Crossref
6. Dawood NB, Yan KL, Shieh A, Livhits MJ, Yeh MW, Leung AM. Normocalcaemic primary hyperparathyroidism: an update on diagnostic and management challenges. Clin Endocrinol (Oxf).
2020;93:519-27. Crossref
7. Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. 2018;14:115-25. Crossref
8. Laird AM, Libutti SK. Minimally invasive parathyroidectomy versus bilateral neck exploration for primary hyperparathyroidism. Surg Oncol Clin N Am. 2016;25:103-18. Crossref
9. Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585-91. Crossref
10. Yeh R, Tay YD, Tabacco G, Dercle L, Kuo JH, Bandeira L, et al. Diagnostic performance of 4D CT and sestamibi SPECT/CT in localizing parathyroid adenomas in primary hyperparathyroidism. Radiology. 2019;291:469-76. Crossref
11. Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis
of preoperative localization techniques for patients with
primary hyperparathyroidism. Ann Surg Oncol. 2012;19:577-83. Crossref
12. National Institute for Health and Care Excellence. Hyperparathyroidism
(primary): diagnosis, assessment and initial treatment. London:
National Institute for Health and Care Excellence; 2019.
13. Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al.
The American Association of Endocrine Surgeons Guidelines for
Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151:959-68. Crossref
14. Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R,
Marcocci C, et al. Guidelines for the management of asymptomatic
primary hyperparathyroidism: summary statement from the Fourth
International Workshop. J Clin Endocrinol Metab. 2014;99:3561-9. Crossref
15. Stack BC Jr, Tolley NS, Bartel TB, Bilezikian JP, Bodenner D,
Camacho P, et al. AHNS Series: Do you know your guidelines?
Optimizing outcomes in reoperative parathyroid surgery: definitive
multidisciplinary joint consensus guidelines of the American Head
and Neck Society and the British Association of Endocrine and
Thyroid Surgeons. Head Neck. 2018;40:1617-29. Crossref
16. Walker MD, McMahon DJ, Inabnet WB, Lazar RM, Brown I, Vardy S, et al. Neuropsychological features in primary hyperparathyroidism: a prospective study. J Clin Endocrinol
Metab. 2009;94:1951-8. Crossref
17. Hoang JK, Sung WK, Bahl M, Phillips CD. How to perform parathyroid 4D CT: tips and traps for technique and interpretation. Radiology. 2014;270:15-24. Crossref
18. Raeymaeckers S, De Brucker Y, Vanderhasselt T, Buls N, De Mey J. Detection of parathyroid adenomas with multiphase 4DCT: towards a true four-dimensional technique. BMC Med Imaging. 2021;21:64. Crossref
19. Starker LF, Mahajan A, Björklund P, Sze G, Udelsman R, Carling T. 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol. 2011;18:1723-8. Crossref
20. Bahl M, Sepahdari AR, Sosa JA, Hoang JK. Parathyroid adenomas and hyperplasia on four-dimensional CT scans: three patterns of enhancement relative to the thyroid gland justify a three-phase protocol. Radiology. 2015;277:454-62. Crossref
21. Griffith B, Chaudhary H, Mahmood G, Carlin AM, Peterson E,
Singer M, et al. Accuracy of 2-phase parathyroid CT for the
preoperative localization of parathyroid adenomas in primary
hyperparathyroidism. AJNR Am J Neuroradiol. 2015;36:2373-9. Crossref
22. Leiva-Salinas C, Flors L, Durst CR, Hou Q, Patrie JT, Wintermark M, et al. Detection of parathyroid adenomas using a monophasic dual-energy computed tomography acquisition: diagnostic performance and potential radiation dose reduction. Neuroradiology. 2016;58:1135-41. Crossref
23. Roskies M, Liu X, Hier MP, Payne RJ, Mlynarek A, Forest V, et al.
3-phase dual-energy CT scan as a feasible salvage imaging modality
for the identification of non-localizing parathyroid adenomas: a
prospective study. J Otolaryngol Head Neck Surg. 2015;44:44. Crossref
24. Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat. 2012;25:19-31. Crossref
25. Kunstman JW, Kirsch JD, Mahajan A, Udelsman R. Clinical review: parathyroid localization and implications for clinical management. J Clin Endocrinol Metab. 2013;98:902-12. Crossref
26. Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14-21.
27. Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp Clin Endocrinol Diabetes. 2012;120:604-10. Crossref
28. Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg. 2006;191:418-23. Crossref
29. Roy M, Mazeh H, Chen H, Sippel RS. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg. 2013;37:102-6. Crossref
30. Duke WS, Vernon HM, Terris DJ. Reoperative parathyroidectomy: overly descended superior adenoma. Otolaryngol Head Neck Surg. 2016;154:268-71. Crossref
31. Goodman A, Politz D, Lopez J, Norman J. Intrathyroid parathyroid adenoma: incidence and location—the case against thyroid lobectomy. Otolaryngol Head Neck Surg. 2011;144:867-71. Crossref
32. McIntyre RC Jr, Eisenach JH, Pearlman NW, Ridgeway CE,
Liechty RD. Intrathyroidal parathyroid glands can be a cause of
failed cervical exploration for hyperparathyroidism. Am J Surg.
1997;174:750-3; discussion 753-4. Crossref
33. Grimelius L, Bondeson L. Histopathological diagnosis of parathyroid diseases. Pathol Res Pract. 1995;191s:353-65. Crossref
34. Bahl M, Muzaffar M, Vij G, Sosa JA, Choudhury KR, Hoang JK. Prevalence of the polar vessel sign in parathyroid adenomas on the arterial phase of 4D CT. AJNR Am J Neuroradiol. 2014;35:578-81. Crossref
35. Chandramohan A, Sathyakumar K, John RA, Manipadam MT, Abraham D, Paul TV, et al. Atypical ultrasound features of parathyroid tumours may bear a relationship to their clinical and biochemical presentation. Insights Imaging. 2014;5:103-11. Crossref
36. Ahuja AT, Wong KT, Ching AS, Fung MK, Lau JY, Yuen EH, et al. Imaging for primary hyperparathyroidism—what beginners should know. Clin Radiol. 2004;59:967-76. Crossref
37. Chan WB, Chow CC, King AD, Yeung VT, Li JK, So WY, et al. Spontaneous necrosis of parathyroid adenoma: biochemical and imaging follow-up for two years. Postgrad Med J. 2000;76:96-8. Crossref
38. Al-Kurd A, Mekel M, Mazeh H. Parathyroid carcinoma. Surg Oncol. 2014;23:107-14. Crossref
39. Ferraro V, Sgaramella LI, Di Meo G, Prete FP, Logoluso F, Minerva F, et al. Current concepts in parathyroid carcinoma: a single centre experience. BMC Endocr Disord. 2019;19(Suppl
1):46. Crossref
40. Takumi K, Fukukura Y, Hakamada H, Nagano H, Kumagae Y, Arima H, et al. CT features of parathyroid carcinomas: comparison with benign parathyroid lesions. Jpn J Radiol. 2019;37:380-9. Crossref
41. Henry BM, Sanna S, Graves MJ, Vikse J, Sanna B, Tomaszewska IM, et al. The non-recurrent laryngeal nerve: a meta-analysis and clinical considerations. PeerJ. 2017;5:e3012. Crossref
42. Bunch PM, Randolph GW, Brooks JA, George V, Cannon J, Kelly HR. Parathyroid 4D CT: what the surgeon wants to know. Radiographics. 2020;40:1383-94. Crossref
43. Leong D, Ng K, Boeddinghaus R, Lisewski D. Three-phase four-dimensional
computed tomography as a first-line investigation in
primary hyperparathyroidism. ANZ J Surg. 2021;91:1798-803. Crossref
44. Expert Panel on Neurological Imaging; Zander D, Bunch PM, Policeni B, Juliano AF, Carneiro-Pla D, et al. ACR Appropriateness Criteria® Parathyroid Adenoma. J Am Coll Radiol. 2021; 18(11S):S406-22. Crossref
45. Bunch PM, Kelly HR. Preoperative imaging techniques in primary
hyperparathyroidism: a review. JAMA Otolaryngol Head Neck
Surg. 2018;144:929-37. Crossref
46. Brown SJ, Lee JC, Christie J, Maher R, Sidhu SB, Sywak MS,
et al. Four-dimensional computed tomography for parathyroid
localization: a new imaging modality. ANZ J Surg. 2015;85:483-7. Crossref
47. Sun L, Yao J, Hao P, Yang Y, Liu Z, Peng R. Diagnostic role of four-dimensional
computed tomography for preoperative parathyroid
localization in patients with primary hyperparathyroidism:
a systematic review and meta-analysis. Diagnostics (Basel).
2021;11:664. Crossref
48. Suh YJ, Choi JY, Kim SJ, Chun IK, Yun TJ, Lee KE, et al. Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism.
Otolaryngol Head Neck Surg. 2015;152:438-43. Crossref
49. Wan QC, Li JF, Tang LL, Lv J, Xie LJ, Li JP, et al. Comparing
the diagnostic accuracy of 4D CT and 99mTc-MIBI SPECT/CT for
localizing hyperfunctioning parathyroid glands: a systematic review
and meta-analysis. Nucl Med Commun. 2021;42:225-33. Crossref
50. Krakauer M, Wieslander B, Myschetzky PS, Lundstrøm A, Bacher T, Sørensen CH, et al. A prospective comparative study of parathyroid dual-phase scintigraphy, dual-isotope
subtraction scintigraphy, 4D-CT, and ultrasonography in primary
hyperparathyroidism. Clin Nucl Med. 2016;41:93-100. Crossref
51. Beland MD, Mayo-Smith WW, Grand DJ, Machan JT, Monchik JM.
Dynamic MDCT for localization of occult parathyroid adenomas
in 26 patients with primary hyperparathyroidism. AJR Am J
Roentgenol. 2011;196:61-5. Crossref
52. Hiramitsu T, Tomosugi T, Okada M, Futamura K, Tsujita M, Goto N, et al. Pre-operative localisation of the parathyroid glands in secondary hyperparathyroidism: a retrospective cohort study. Sci Rep. 2019;9:14634. Crossref
53. Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative
localization of parathyroid tumors in primary hyperparathyroidism.
World J Surg. 2012;36:1335-9. Crossref
54. Zhao L, Liu JM, He XY, Zhao HY, Sun LH, Tao B, et al. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: data from 2000 to 2010 in a single clinical center. J Clin Endocrinol Metab. 2013;98:721-8. Crossref