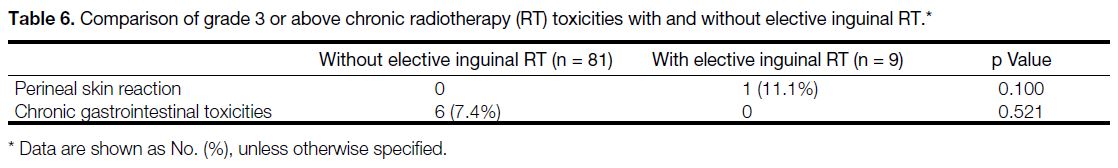Effect of Elective Inguinal Irradiation in Low Rectal Cancer with Anal Canal Invasion
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2023 Sep;26(3):174-84 | Epub 7 Sep 2023
Effect of Elective Inguinal Irradiation in Low Rectal Cancer with Anal Canal Invasion
HS Wong, WYL Choi, KT Yuen
Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr HS Wong, Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China. Email: whs871@ha.org.hk
Submitted: 26 Oct 2022; Accepted: 12 Dec 2022.
Contributors: HSW designed the study, acquired and analysed the data, and drafted the manuscript. WYLC and KTY critically revised the
manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong [Ref
No.: KW/EX-22-035 (171-04)]. A waiver of patient consent was obtained from the Committee due to the retrospective nature of the study.
Declaration: This manuscript was posted on Research Square as a registered online preprint (https://doi.org/10.21203/rs.3.rs-1914914/v1).
Abstract
Introduction
We investigated whether omitting elective inguinal irradiation during neoadjuvant or adjuvant
radiation/chemoradiation therapy is feasible for patients with low rectal cancer with anal canal invasion (ACI) and
nonpalpable inguinal lymph nodes (ILNs) at presentation.
Methods
Ninety low rectal cancer patients with ACI who underwent neoadjuvant or adjuvant radiation/chemoradiation therapy with or without elective inguinal radiotherapy (RT) between 2011 and 2021 were recruited.
None had palpable ILN. The failure pattern, ILN recurrence rate, survival data, and prognostic factors were analysed.
Results
Among 81 patients omitting elective inguinal RT, the 3-year ILN failure rate was 4.9%. Meanwhile, there
was no inguinal failure with elective RT. One case of isolated ILN failure was successfully salvaged by surgery. In
multivariate Cox regression analysis, positive pathological lymph node(s) after neoadjuvant treatment predicted a
worse locoregional recurrence-free survival (odds ratio [OR] = 9.066; p ≤ 0.001), distant metastasis recurrence-free
survival (OR = 6.426; p = 0.002), and overall survival (OR = 11.750; p ≤ 0.001). Chemotherapy concurrent with RT
was associated with better locoregional recurrence-free survival (OR = 33.338; p = 0.001) and overall survival (OR
= 13.917; p = 0.006). Grade ≥3 acute and chronic toxicities occurred in 33.3% and 19.8%, respectively, of patients
with elective inguinal irradiation, compared with 11.1% and 7.4%, respectively, in patients who did not receive it.
Conclusion
Omission of elective inguinal irradiation resulted in a low inguinal failure rate and similar survival
outcomes for low rectal cancer patients with ACI. Additionally, it might spare patients from unnecessary acute and chronic RT toxicities.
Key Words: Chemoradiotherapy; Chemoradiotherapy, adjuvant; Neoadjuvant therapy; Rectal neoplasms
中文摘要
伴肛門侵犯的低位直腸癌患者進行預防性腹股溝照射的影響
王曉生、蔡源霖、袁錦堂
引言
我們探討為臨床上出現肛門侵犯及觸摸不到腹股溝淋巴結的低位直腸癌患者進行前輔助放療或輔助放療/放化療時不接受預防性腹股溝照射是否可行。
方法
本研究招募了90名出現肛門侵犯的低位直腸癌患者,他們在2011至2021年間曾進行前輔助放療或輔助放療/放化療,部分有接受預防性腹股溝放療,部分則沒有。全部患者均沒有觸摸到的腹股溝淋巴結。本研究分析了失敗模式、腹股溝淋巴結復發率、存活數據及預後因素。
結果
在81名沒有接受預防性腹股溝放療的患者中,三年腹股溝淋巴結失敗率為4.9%。同時,預防性放療並沒有腹股溝失敗的情況。一例個別的腹股溝淋巴結失敗成功通過手術挽救。多變量Cox迴歸分析顯示,前輔助放療後的陽性病理性淋巴結預測較差的局部無復發存活(勝算比 = 9.066;p ≤ 0.001)、無遠端轉移復發存活(勝算比 = 6.426;p = 0.002)及整體存活(勝算比 = 11.750;p ≤ 0.001)。放療期間同時進行化療與較佳的局部無復發存活(勝算比 = 33.338;p = 0.001)及整體存活(勝算比 = 13.917;p = 0.006)相關。在接受預防性腹股溝照射的患者中,分別有33.3%及19.8%出現≥3級急性及慢性毒性;沒有接受該照射的患者出現上述兩種毒性的比例則分別為11.1%及7.4%。
結論
沒有接受預防性腹股溝照射的伴肛門侵犯的低位直腸癌患者,其腹股溝失敗率低,與有接受該照射的患者相比,存活結果相近,而且可能避免出現不必要的急性及慢性放療毒性。
INTRODUCTION
Neoadjuvant chemoradiotherapy (CRT) reduces the risk
of a positive circumferential margin and local recurrence
in patients with low rectal cancer.[1] Prospective
randomised trials have demonstrated significantly lower
locoregional recurrence rates with adjuvant CRT when
compared with observation or either modality alone in
stage II/III rectal cancer.[2]
The clinical target volume (CTV) during radiation/chemoradiation therapy must cover areas with potential
metastatic risk while avoiding organs at risk to avoid
radiation-related complications. In low rectal cancer
with anal canal invasion (ACI), tumour can spread to
inguinal lymph nodes (ILNs) through the perirectal
and pudendal lymphatics, as well as the lymphatics
draining the infradentate and perianal skin. An advanced
rectal primary tumour can cause proximal lymphatic
obstruction and retrograde lymph node metastasis.[3]
The European Society for Medical Oncology Clinical
Practice Guidelines proposed in 2010 recommends prophylactic irradiation of medial ILNs if the rectal
tumour extends below the dentate line.[4] Radiation
of ILNs in cases where tumour extends into the anal
sphincter has been advocated by the 2016 international
consensus guidelines on CTV delineation.[5] According
to the 2020 American Society for Radiation Oncology
Clinical Practice Guidelines, ILNs and external iliac
nodes should be conditionally included in the CTV for
patients with rectal malignancies with ACI.[6] However,
the contouring atlas of the Radiation Therapy Oncology
Group has no consensus on the subject.[7]
In three retrospective trials,[8] [9] [10] the ILN failure rates in
rectal cancer patients with ACI who received neoadjuvant
or adjuvant radiation/chemoradiation therapy without
elective inguinal irradiation were not high enough (3-year
failure rate: 3.7%[8]; 5-year actuarial rate: 3.5%-4%[9] [10]) to
justify inguinal irradiation as a standard procedure.
The treatment policy at our institution for low rectal
cancer with ACI and clinically negative ILN at presentation has been based on the practice of the
attending oncologists. We looked at the feasibility of
omitting elective inguinal irradiation for patients with
low rectal cancer with ACI and clinically negative ILN.
METHODS
Data Collection
From 2011 to 2021, the clinical data of 110 patients with
low rectal cancer with ACI who received neoadjuvant
or adjuvant radiation/chemoradiation therapy in our
tertiary oncology centre were collected from the
institutional database and retrospectively reviewed. The
inclusion criteria were: (1) histologically confirmed
locally advanced rectal adenocarcinoma without distant
metastasis (based on the Eighth Edition of the American
Joint Committee on Cancer Staging Manual); (2)
tumours with ACI, defined as the tumour’s lower edge
being within 3 cm of the anal verge (or being located at
or below the dentate line) on digital rectal examination,
colonoscopy or magnetic resonance imaging; and (3)
an Eastern Cooperative Oncology Group performance
status score of 0 to 2.
The exclusion criteria were: (1) inguinal metastasis
on presentation by clinical and imaging studies;
(2) occurrence of distant failure before surgery; (3)
ineligibility for radical surgery as determined by clinical
and imaging studies; (4) local excision; (5) incomplete
radiation/chemoradiation therapy; (6) in the setting
of recurrence indicated for radiation/chemoradiation
therapy; and (7) second malignancies within 5 years.
Missing data were dealt with by listwise deletion.
Patients lost to follow-up were censored and their life
expectancy was counted till the last follow-up date.
Pretreatment Workup
Pretreatment workup for clinical staging included digital
rectal examination, complete blood count, liver and
renal function tests, serum carcinoembryonic antigen,
colonoscopy, chest radiography, computed tomography
(CT) of the thorax, abdomen and pelvis with or without
transrectal ultrasonography, and pelvic magnetic
resonance imaging. Fluorine-18 fluorodeoxyglucose
positron emission tomography/CT (PET/CT) was
performed at the physician’s discretion and patient
accessibility.
Chemoradiotherapy Treatment
The patients received either long-course or short-course
radiotherapy (RT). Long-course RT was administered to the entire pelvis at a dose of 45 Gy in 25 daily fractions,
followed by a 5.4-Gy boost in three daily fractions over
5.5 weeks. Short-course RT was delivered to the whole
pelvis at a dose of 25 Gy in 5 daily fractions over 1
week. All patients underwent CT simulation for three-dimensional
conformal planning, with a comfortably
full bladder and an empty rectum. In patients declining
elective inguinal irradiation, a three-field treatment
plan was adopted using a posterior-anterior field and
lateral opposing beams. With patients electing inguinal
irradiation, a pair of anterior-posterior opposing fields
was used. The prescription dose was set at the 100%
isodose line. The initial radiation field encompassed the
gross tumour volume (GTV) (preoperative radiation/chemoradiation therapy) or tumour bed (postoperative
CRT), and the regional lymphatics including the
mesorectal, internal iliac, presacral, and distal common
iliac lymphatics plus or minus ILN. The superior
boundary was the L5-S1 junction; the inferior border
was set 3 cm caudal to the GTV or tumour bed and the
anterior border was placed 3 cm anterior to the sacral
promontory, while the posterior border was placed 1 cm
posterior to the sacrum. The GTV or tumour bed were
included in the boost field, with 3-cm margins in all
directions.
Chemotherapy was administered concurrently with
long-course RT using bolus 5-fluorouracil (FU)
[500 mg/m2 intravenous bolus; Days 1-3 and Days
29-31].[11] As there has been evidence for better treatment
outcomes with continuous oral capecitabine,[12] [13]
continuous oral capecitabine (825 mg/m2 twice per
day) was used as a concomitant chemotherapeutic agent
since April 2021. If patients were deemed unsuitable for
chemotherapy, long-course RT alone was an alternative.
Either abdominal-perineal resection or low anterior
resection with complete mesorectal excision was
performed. Typically, the interval between preoperative
CRT and surgery was 8 weeks, and that between surgery
and postoperative CRT was 10 weeks. Four months
of adjuvant chemotherapy was administered using six
cycles of capecitabine and oxaliplatin, eight cycles of
modified leucovorin/fluorouracil/oxaliplatin, or six
cycles of capecitabine depending on patients’ tolerance.
Study Endpoints
The 3-year inguinal failure rate, locoregional recurrence-free survival (LRFS), distant metastasis recurrence-free
survival (DMRFS), overall survival (OS), and failure
pattern were analysed. LRFS, DMRFS, and OS risk
factors were also investigated. LRFS was measured from the start of treatment to locoregional relapse, death from
any causes, or last follow-up. DMRFS was measured
from the start of treatment to distant relapse, death from
any causes, or last follow-up. OS was calculated from
the date of the first treatment to the date of death or the
last follow-up.
Follow-up
The patients were evaluated for symptoms, physical
examination findings, and blood tests including
carcinoembryonic antigen in outpatient clinics on a
regular basis. A thorax, abdomen, and pelvic CT or
PET/CT would be arranged if there was clinical suspicion
of disease recurrence. Colonoscopies were performed 1
year after surgery and every 3 years thereafter.
Statistical Analysis
The 3-year LRFS, DMRFS, and OS rates were presented
using the Kaplan-Meier method. Fisher’s exact tests
were used to explore the difference between categorical
variables, while Mann–Whitney U tests were used to
explore the difference between continuous variables.
Clinicopathologic variables were entered into a Cox
proportional hazard regression multivariable regression
model and analysed for effects on LRFS, DMRFS and
OS. All analyses were performed using SPSS (Windows
version 21.0; IBM Corp, Armonk [NY], United States). A
p value of <0.05 was considered statistically significant.
Research Reporting Guidelines
The STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) checklist for
observational cohort studies was implemented in the
preparation of the manuscript.
RESULTS
Patient Characteristics
This study enrolled 90 eligible individuals from a
larger primary cohort of 110 patients. The full course of
radiation/chemoradiation therapy was completed by all
patients. The study excluded five patients who refused
or were ineligible for surgery, six patients who had local
excision only, one patient with upfront distant metastasis,
four patients who developed distant metastasis after
neoadjuvant radiation/chemoradiation, two patients
with upfront inguinal metastasis, and two patients with
recurrent rectal cancer.
The median duration of follow-up was 45 months (range,
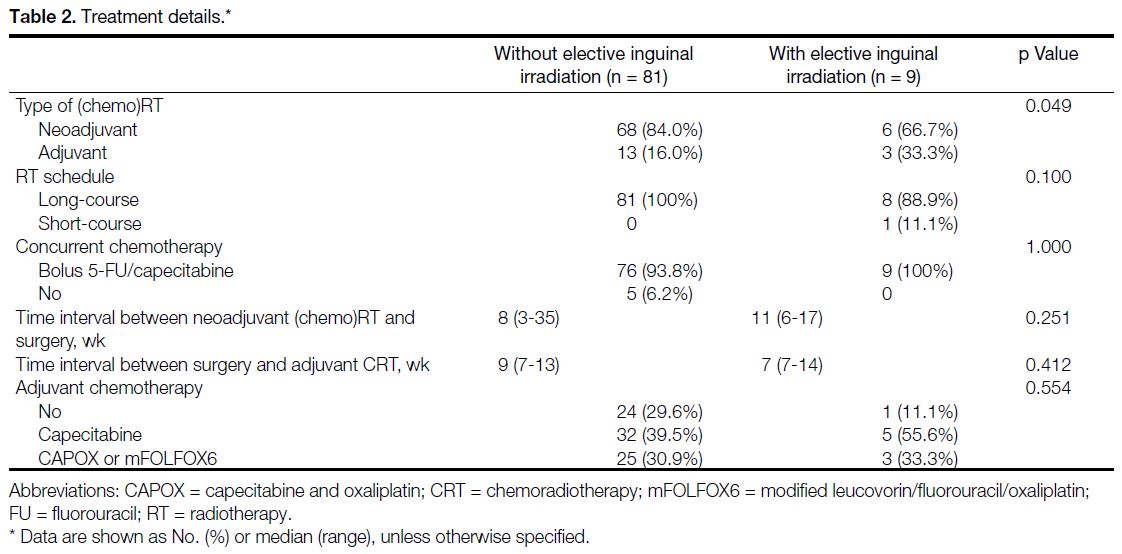
2-118). Tables 1 and 2 list the clinical data, pathological data, and treatment characteristics of the patients.
Table 1. Baseline clinical and pathological characteristics of patients with and without elective inguinal irradiation.
Table 2. Treatment details.
Failure Rates and Patterns
Patients who did not receive elective inguinal radiation
(n = 81) had a 3-year ILN failure rate of 4.9% (n = 4).
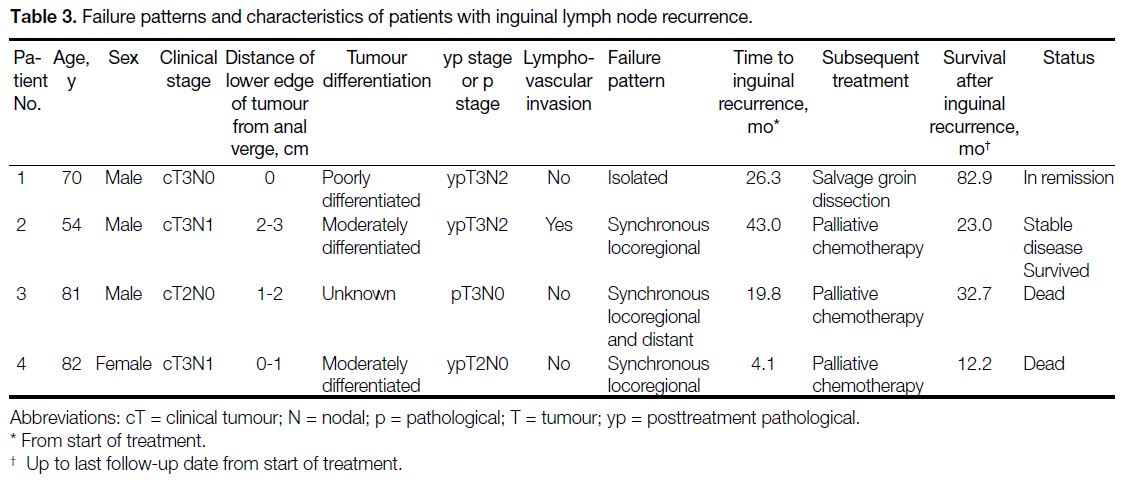
Patients who received elective inguinal radiation (n = 9) did not experience any inguinal failure. Of the four
patients with ILN failure, only one of them had isolated
ILN failure, while the other three had synchronous
locoregional recurrence and/or distant failure. In other
words, omitting inguinal irradiation resulted in only
one case (1.2%) of isolated inguinal nodal failure.
Salvage surgery was successfully performed for this
patient, who achieved disease remission and survived.
Palliative chemotherapy was administered to patients
with synchronous locoregional recurrence and/or distant
failure, two of whom died due to disease progression.
Failure patterns and characteristics of patients with ILN
recurrence are listed in Table 3.
Table 3. Failure patterns and characteristics of patients with inguinal lymph node recurrence.
Survival Outcomes and Prognostic Factors
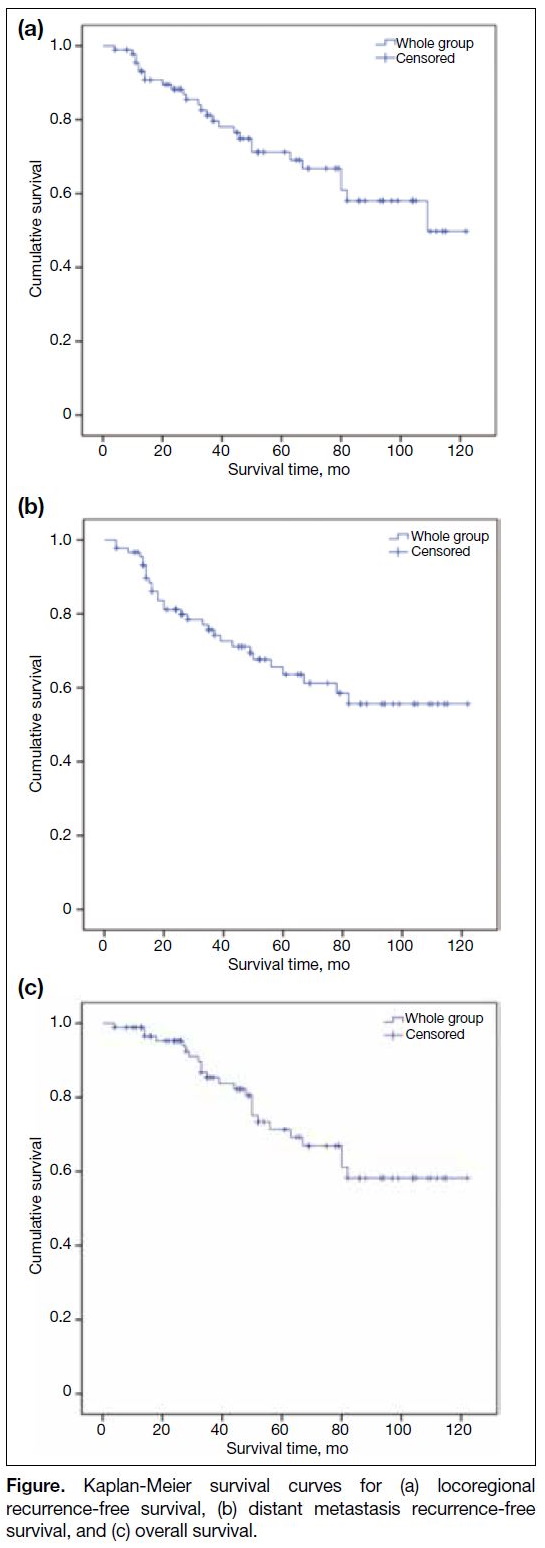
The Figure illustrates the Kaplan-Meier curves, depicting 3-year LRFS, DMRFS, and OS of 81.1%, 77.0%, and 86.8%, respectively.
Figure. Kaplan-Meier survival curves for (a) locoregional recurrence-free survival, (b) distant metastasis recurrence-free survival, and (c) overall survival.
In multivariable Cox regression analysis, positive
pathological lymph node after neoadjuvant treatment
predicted worse LRFS (odds ratio [OR] = 9.066, 95%
confidence interval [CI] = 3.291-24.972; p < 0.001),
DMRFS (OR = 6.426, 95% CI = 1.944-21.244; p = 0.002) and OS (OR = 11.750, 95% CI = 3.583-38.526; p < 0.001). Positive tumour resection margin correlated
with worse LRFS (OR = 27.296, 95% CI = 5.592-133.241; p < 0.001) and OS (OR = 49.982, 95% CI = 4.561-547.759; p = 0.001). Chemotherapy concurrent
with RT was associated with better LRFS (OR = 33.338,
95% CI = 4.525-245.633; p = 0.001) and OS (OR = 13.917, 95% CI = 2.095-92.437; p = 0.006). Meanwhile,
elective inguinal RT was not associated with statistical
differences in LRFS, DMRFS or OS. Details of simple
and multivariable analyses are shown in Table 4.
Table 4. Simple and multivariable Cox regression models for locoregional recurrence-free survival (LRFS), distant metastasis recurrence-free survival (DMRFS), and overall survival (OS).
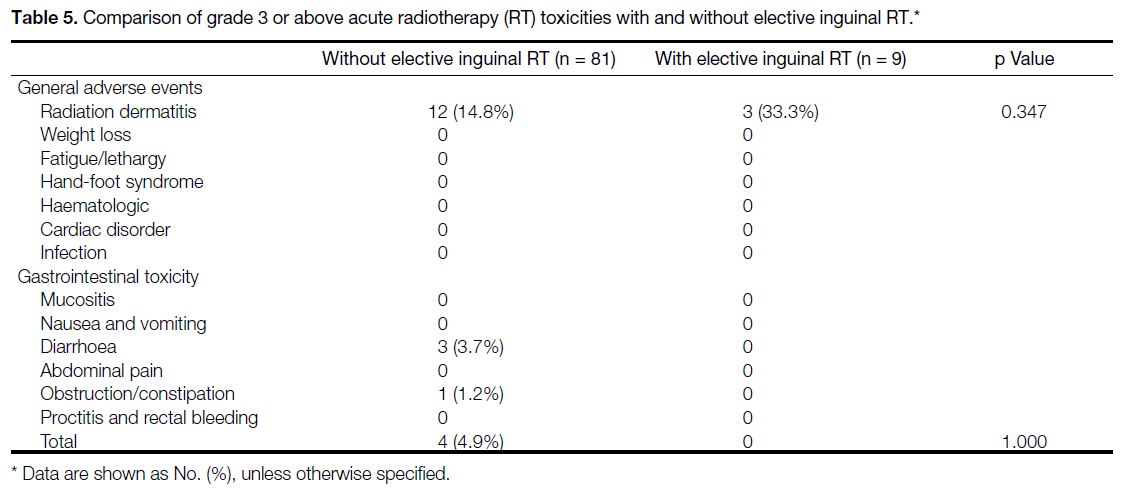
Treatment Toxicities
Grade ≥3 acute toxicity occurred in 16 out of 81 of
patients (19.8%) who did not receive inguinal radiation
and 3 out of 9 patients (33.3%) who underwent inguinal
RT. Inguinal irradiation caused 3 out of 9 patients (33.3%)
to develop grade ≥3 perineal dermatitis, compared to 12
out of 81 patients (14.8%) who did not have inguinal
irradiation. The above difference, however, did not reach
statistical significance. Table 5 shows the acute toxicities
profile (Common Terminology Criteria for Adverse
Events Grade ≥3).
Table 5. Comparison of grade 3 or above acute radiotherapy (RT) toxicities with and without elective inguinal RT.
In terms of chronic toxicity, 1 out of 9 patients (11.1%)
who had elective inguinal irradiation developed a
protracted gap wound after excision of a perineal
recurrence, while there were no recorded chronic perineal
skin toxicities in patients who did not receive inguinal
irradiation. Among the 81 patients who did not receive
elective inguinal irradiation, five (6.2%) experienced
intestinal obstruction and one (1.2%) developed
rectovaginal fistula. No chronic gastrointestinal toxicities
have been reported in patients with elective inguinal
irradiation, though the abovementioned differences were not statistically significant. Table 6 shows the chronic
toxicities profile (the Radiation Therapy Oncology
Group and the European Organisation for Research and
Treatment of Cancer Grade ≥3).
Table 6. Comparison of grade 3 or above chronic radiotherapy (RT) toxicities with and without elective inguinal RT.
DISCUSSION
For rectal cancer, determining optimal radiation
targets based on their location and mode of spread is
a challenge. Despite the theoretical risk that tumour
cells in low rectal cancer with ACI could spread to the
ILN region, there has been no consensus on whether to include the inguinal nodal region in CTV for this patient subgroup. More clinical evidence is needed to optimise the CTV for these patients in order to reduce irradiation
of normal tissue.
The low ILN failure rate (4.9%) in our study, which
mirrored the findings of other retrospective studies,[8] [9] [10]
showed that most patients with low rectal cancer
with ACI would not benefit from elective inguinal
irradiation during neoadjuvant or adjuvant (chemo)RT. Some experts, however, still recommend elective
ILN irradiation based on acceptable morbidities.[14] In
our study, the acute toxicity associated with inguinal
irradiation cannot be neglected. There were more
acute grade 3 perineal dermatitis among patients who
received elective inguinal irradiation (33.3% vs. 14.8%),
though none required a treatment break. Meanwhile,
the reported chronic complications of elective inguinal
irradiation appeared relatively minor in our study. Only
1 out of 9 patients (11.1%) who had elective inguinal
irradiation developed a protracted gap wound after
perineal recurrence.
Measures were developed to identify patients who were
at a higher risk of developing inguinal nodal metastasis.
Firstly, Song et al[8] created a nomogram to predict the
probability of ILN failures according to tumour location,
histological grade, and presence of perineural invasion.
It can be used as a guide to select patients for elective
inguinal irradiation at high risk of ILN failure, but the
presence of perineural invasion may not be known until
postoperatively. Shiratori et al[15] have also noted that
dentate line involvement and ILNs > 8 mm may predict
the development of inguinal nodal metastasis. PET/CT
has been suggested to detect abnormal inguinal uptake
for inguinal nodal region irradiation. Although up to
17% of patients with distal rectal cancer, especially
those ultra-low tumours, had inguinal nodes showing
fluorodeoxyglucose uptake on PET/CT, the false
positivity rate was high, as nearly half of these nodes no
longer demonstrated uptake after CRT despite the fact
that the inguinal region is not included in the radiation
field. Moreover, none of these patients in that study
developed inguinal recurrence after 22 months of follow-up.[16] A review of sentinel nodes in anal cancer revealed
that 44% of all node metastases located in lymph nodes
measured <5 mm in diameter.[17] The spatial resolution of
PET/CT is limited to a few millimetres, suggesting it may
not have sufficient sensitivity and specificity to select
outpatients for inguinal irradiation.[18] The sentinel node
technique was also studied in rectal cancer with ACI.
A small prospective study of 15 patients showed no
recurrence in the groin for patients whose sentinel
lymph nodes were determined to be negative for
metastatic adenocarcinoma.[19] However, a systematic
review indicated that the sentinel lymph node procedure
showed only a fair sensitivity rate of 82% (95% CI = 60%-93%), regardless of tumour stage, localisation
or pathological technique.[20] Due to the relatively low
sensitivity, technically demanding procedures, risk of
surgical morbidity, and doubtful impact on subsequent
clinical management, this is not currently a standard practice for
low rectal cancer with ACI.
Only one patient (25%) developed isolated ILN
metastases among all the four patients with inguinal
recurrence. Salvage treatment for isolated ILN recurrence
can provide long-term ILN control in our study. As a
result, prophylactic treatment of the inguinal region
may not be necessary. The other three patients (75%)
who experienced inguinal recurrence had synchronous
locoregional and/or distant recurrences. One may
question whether early detection and treatment of occult
inguinal nodal metastases can help prevent subsequent
distant metastases. Damin et al[18] observed that despite
inguinal dissection, 75% of sentinel ILN–positive cases
developed hepatic or pulmonary metastases within 6
months of the surgery. Thus, localised treatment of the
inguinal region may not affect the final clinical outcome,
which is determined mainly by the occurrence of
metastasis to distant organs.[19] In this context, a sentinel
lymph node metastasis could represent a potential marker
for systemic dissemination of the disease.[19]
From our results, patients who had positive pathological
lymph node(s) following neoadjuvant therapy and/or a
positive resection margin had an inferior rate of 3-year
LRFS and OS, implying that more aggressive neoadjuvant
treatment is needed to shrink the tumour before surgery,
such as the addition of an induction or consolidation
chemotherapy regimen. Several recently published large-scale
randomised controlled trials consistently showed
that total neoadjuvant treatment can improve disease-free
survival, pathological complete remission rate, and
the risk of disease-related treatment failure in patients
with high-risk rectal cancer.[20] [21] [22] [23] Among them, the phase 3 STELLAR trial was the first trial to demonstrate OS
benefit, which found that short-course RT followed by
perioperative chemotherapy resulted in better 3-year OS
rates than CRT followed by postoperative chemotherapy,
with 86.5% vs. 75.1% (OR = 0.67, 95% CI = 0.46-0.97; p = 0.033).[23] Furthermore, in our study, as compared to
radiation alone, concomitant chemotherapy was linked
with a superior LRFS and OS. A Cochrane review found
that preoperative CRT improved local control (OR = 0.56, 95% CI = 0.42-0.75; p < 0.0001) in resectable stage
III rectal cancer but did not increase OS (OR = 1.01, 95%
CI = 0.85-1.20; p = 0.88).[24] The STELLAR OS benefit
may be attributable to different patient selection criteria
as our included patient population was restricted to low
rectal cancer with ACI. This high-risk group may derive
more benefit from concurrent chemotherapy. Additional
studies are encouraged to validate the OS benefit of
preoperative CRT against RT alone in resectable low
rectal cancer with ACI.
Song et al[8] also investigated the impact of excluding
irradiation of ILNs during neoadjuvant (chemo)RT in
low rectal cancer with ACI. Their 3-year ILN failure
rate was 3.7%. Our 3-year RFS rate (76.6% vs. 77.7%)
is comparable to their disease-free survival rate, but our
3-year OS rate (86.8% vs. 91.9%) outcome appeared
slightly inferior. Reasons for our relatively inferior OS
may be multifactorial. Our research population had an
older median age (67 years vs. 57 years). Our study also
covered a small number of patients with worse Eastern
Cooperative Oncology Group performance status (a score
of 2) [6.7%], whereas their study only included individuals
with a score of 0 to 1. Almost all of our patients (93.3%)
received bolus 5-FU as concurrent chemotherapy, with the
exception of one patient who received oral capecitabine,
compared to 78.1% of capecitabine patients in their
study.[8] Patients receiving prolonged 5-FU infusion had a
significantly longer time to relapse and improved survival
compared with bolus 5-FU.[11] Two randomised controlled
trials have shown that patients with rectal cancer who
received neoadjuvant or adjuvant capecitabine CRT
had non-inferior disease-free and OS when compared to
continuous 5-FU.[12] [13] Therefore, concurrent chemotherapy
with oral capecitabine should produce better outcomes
compared with bolus 5-FU. In addition, induction (7.0%)
and consolidation chemotherapy (30.8%) were used in
their research, which might further improve treatment
outcomes.[8]
Limitations
Our study had several limitations. First, it was a
retrospective study based on data from a single centre,
and this may add selection and information bias. Second,
our small sample size reduced the power of the study.
Despite a trend towards lower acute and chronic skin toxicity rates without inguinal RT, it did not reach
statistical significance. In light of the small number of
patients with inguinal RT and the retrospective nature
of the study, these results should be interpreted with
caution. Moreover, the elective inguinal RT group’s
small sample size may make it difficult to statistically
compare survival rates with those who did not receive
inguinal RT. Third, some baseline characteristics (i.e.,
baseline carcinoembryonic antigen level, clinical tumour
staging, and proportion of patients receiving neoadjuvant
vs. adjuvant radiation/chemoradiation therapy between
patients with or without elective inguinal irradiation)
were imbalanced, and this might create bias to the
interpretation of results. Lastly, as there was no uniform
follow-up imaging in our study population, survival
outcomes may have been overstated.
CONCLUSION
Omission of elective inguinal irradiation resulted in a
low inguinal failure rate and similar survival outcomes
for low rectal cancer patients with ACI. This study
demonstrated that the majority of inguinal recurrences
also had synchronous locoregional recurrence and/or distant failure, while isolated inguinal recurrences
were uncommon and could be salvaged by inguinal
dissection. These findings added to the body of evidence
supporting the omission of elective ILN irradiation
for this patient subgroup. Better-designed randomised
studies are warranted to define the role of elective
inguinal irradiation and to elucidate the best strategy for
treatment escalation.
REFERENCES
1. Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci. 2016;12:1022-31. Crossref
2. Kachnic LA. Adjuvant chemoradiation for localized rectal cancer: current trends and future directions. Gastrointest Cancer Res. 2007;1(4 Suppl 2):S64-72.
3. Lee IK. The Lymphatic Spread of the Rectal Cancer. In: Kim NK, Sugihara K, Liang JT, editors. Surgical Treatment of Colorectal Cancer: Asian Perspectives on Optimization and Standardization.
Singapore: Springer; 2018. p 47-53. Crossref
4. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22-40. Crossref
5. Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol.
2016;120:195-201. Crossref
6. Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT, et al. Radiation therapy for rectal cancer: executive
summary of an ASTRO Clinical Practice Guideline. Pract Radiat
Oncol. 2021;11:13-25. Crossref
7. Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, et al. Elective clinical target volumes for conformal
therapy in anorectal cancer: a Radiation Therapy Oncology Group
consensus panel contouring atlas. Int J Radiat Oncol Biol Phys.
2009;74:824-30. Crossref
8. Song M, Li S, Zhang Y, Geng J, Wang H, Zhu X, et al. Is elective inguinal or external iliac irradiation during neoadjuvant (chemo) radiotherapy necessary for locally advanced lower rectal cancer with anal sphincter invasion? Pract Radiat Oncol. 2022;12:125-34. Crossref
9. Taylor N, Crane C, Skibber J, Feig B, Ellis L, Vauthey JN, et al. Elective groin irradiation is not indicated for patients with adenocarcinoma of the rectum extending to the anal canal. Int J
Radiat Oncol Biol Phys. 2001;51:741-7. Crossref
10. Yeo SG, Lim HW, Kim DY, Kim TH, Kim SY, Baek JY, et al. Is elective inguinal radiotherapy necessary for locally advanced rectal adenocarcinoma invading anal canal? Radiat Oncol. 2014;9:296. Crossref
11. O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-7. Crossref
12. Allegra CJ, Yothers G, O’Connell MJ, Beart RW, Wozniak TF, Pitot HC, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III
randomized clinical trial. J Natl Cancer Inst. 2015;107:djv248. Crossref
13. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus
fluorouracil for locally advanced rectal cancer: a randomised,
multicentre, non-inferiority, phase 3 trial. Lancet Oncol.
2012;13:579-88. Crossref
14. Lee WR, McCollough WM, Mendenhall WM, Marcus RB Jr, Parsons JT, Million RR. Elective inguinal lymph node irradiation for pelvic carcinomas. The University of Florida experience.
Cancer. 1993;72:2058-65. Crossref
15. Shiratori H, Nozawa H, Kawai K, Hata K, Tanaka T, Kaneko M, et al. Risk factors and therapeutic significance of inguinal lymph node metastasis in advanced lower rectal cancer. Int J Colorectal Dis. 2020;35:655-64. Crossref
16. Perez RO, Habr-Gama A, São Julião GP, Proscurshim I, Ono CR,
Lynn P, et al. Clinical relevance of positron emission tomography/computed tomography–positive inguinal nodes in rectal cancer after
neoadjuvant chemoradiation. Colorectal Dis. 2013;15:674-82. Crossref
17. Damin DC, Rosito MA, Schwartsmann G. Sentinel lymph node in carcinoma of the anal canal: a review. Eur J Surg Oncol. 2006;32:247-52. Crossref
18. Damin DC, Tolfo GC, Rosito MA, Spiro BL, Kliemann LM. Sentinel lymph node in patients with rectal cancer invading the
anal canal. Tech Coloproctol. 2010;14:133-9. Crossref
19. van der Pas MH, Meijer S, Hoekstra OS, Riphagen II, de Vet HC, Knol DL, et al. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol.
2011;12:540-50. Crossref
20. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CA, Putter H,
Kranenbarg EM, et al. Short-course radiotherapy followed by
chemotherapy before total mesorectal excision (TME) versus
preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): a
randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. Crossref
21. Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy
for cT4 or fixed cT3 rectal cancer: results of a randomized phase
III study. Ann Oncol. 2016;27:834-42. Crossref
22. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX
and preoperative chemoradiotherapy for patients with locally
advanced rectal cancer (UNICANCER-PRODIGE 23): a
multicentre, randomised, open-label, phase 3 trial. Lancet Oncol.
2021;22:702-15. Crossref
23. Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally
advanced rectal cancer (STELLAR). J Clin Oncol. 2022;40:1681-92. Crossref
24. McCarthy K, Pearson K, Fulton R, Hewitt J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:CD008368. Crossref