Magnetic Resonance Imaging–Guided Cryotherapy for Precision Tumour Ablation
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2023 Sep;26(3):168-73 | Epub 2 Aug 2023
Magnetic Resonance Imaging–Guided Cryotherapy for Precision Tumour Ablation
JB Chiang, WL Poon, PCH Kwok, HS Fung
Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr JB Chiang, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: jbchian@gmail.com
Submitted: 10 Jun 2020; Accepted: 26 Aug 2020.
Contributors: JBC designed the study, acquired the data, analysed the data, and drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon Central/Kowloon East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: KC/KE-20-0045/ER-4). Informed consent for treatment and procedures of this retrospective study was waived by the Committee.
Abstract
Introduction
Percutaneous ablation has played an increasingly prominent role in both palliative and curative treatment of solid tumours, allowing minimally invasive tumour destruction and pain control. Percutaneous ablation is frequently performed under ultrasound or computed tomography guidance, both of which are imperfect in delineating the ablation zone. Magnetic resonance imaging (MRI) guidance provides superior soft tissue contrast, real-time radiation-free imaging, and accurate visualisation of the ablation zone. This study aimed to describe the technique, assess its safety and benefits.
Methods
We retrospectively reviewed the clinical management of patients who had undergone MRI-guided ablation from 1 May 2019 to 31 January 2020 and collected patient data including tumour characteristics, procedure details, and follow-up imaging results.
Results
A total of 14 cases were analysed (10 renal cell carcinomas, 1 hepatocellular carcinoma, 1 adrenal metastasis, 1 external iliac lymph node metastasis, and 1 chest wall fibromatosis). All cases were technically successful with ice ball coverage of the tumour in line with operative intent. Three minor adverse events (two cases of frostbite and one perinephric haematoma) occurred. One patient declined follow-up imaging. Eleven patients showed no residual or recurrence; the patient with chest wall fibromatosis showed shrinkage of the lesion.
Conclusion
MRI guidance is safe and allows accurate tumour visualisation, real-time needle puncture for cryoprobe and hydrodissection needle insertion, and precise delineation of the ablation zone in many procedures.
Key Words: Ablation techniques; Carcinoma, renal cell; Cryosurgery; Magnetic resonance imaging, cine; Magnetic resonance imaging, interventional
中文摘要
磁共振引導精確腫瘤消融冷凍療法
蔣碧茜、潘偉麟、郭昶熹、馮漢盛
簡介
經皮消融可實現微創腫瘤消除和控制疼痛,在實體腫瘤的姑息性和根治性治療中正發揮愈趨重要的作用。經皮消融通常在超聲或電腦斷層引導下進行,兩者在劃定消融區方面都有局限性。磁共振引導具有明確優勢,它的軟組織對比度好,可實時無輻射成像,並可準確描繪消融區。本研究旨在描述該技術,評估其安全性和效益。
方法
我們對在2019年5月1日至2020年1月31日期間接受磁共振引導消融的患者臨床管理進行回顧性分析,並收集患者資料,包括腫瘤特徵、手術細節和隨訪影像檢查結果。
結果
本研究共分析了14例(腎細胞癌10例,肝細胞癌1例,腎上腺轉移1例,髂外淋巴結轉移1例及胸壁纖維瘤病1例)。所有病例均技術成功,冰球覆蓋腫瘤符合手術計劃。共發生3宗輕微副反應事件(2例凍傷及1例腎周血腫)。一名患者拒絕影像學隨訪。11例患者無殘留腫瘤或復發。胸壁纖維瘤病患者表現病灶縮小。
結論
磁共振引導安全,並且可以在許多手術中實現準確的腫瘤可視化、實時插入冷凍探針和水分離針,及精確描繪消融區域。
INTRODUCTION
Percutaneous tumour ablation is important in the
treatment of both benign and malignant diseases,
allowing minimally invasive treatment for tumour
destruction and pain control. Percutaneous tumour
ablation is frequently performed under ultrasound (US)
or computed tomography (CT) guidance, both of which
have significant limitations. For example, US provides
limited visualisation of deep structures and is readily
deflected by overlying gas and bone. CT, on the other
hand, emits radiation, rendering real-time needle insertion
unfavourable. Additionally, CT has limited soft tissue
spatial resolution, making it difficult if not impossible
to visualise the tumour and important surrounding
structures. Most importantly, both techniques of imaging
guidance do not allow accurate visualisation of ablation
zones.[1] Magnetic resonance imaging (MRI) guidance
has swiftly achieved global prominence as it provides
superior soft tissue contrast, radiation-free real-time
imaging during needle insertion, and, most importantly,
clear visualisation of the ablation zone. Superior soft
tissue spatial resolution is particularly important as it
allows accurate visualisation of tumour and important
adjacent structures,[1] [2] which is otherwise difficult under
other imaging techniques. With these qualities, MRI
guidance allows safer, more precise, and radiation-free
imaging guidance of tumour ablation. Recently, the technique has been available in Hong Kong. The aim of
this retrospective single-institution study is to describe
the technique and evaluate its safety while highlighting
its use and benefits.
METHODS
Population
From 1 May 2019 to 31 January 2020, 14 patients (9
males, 5 females) underwent MRI-guided cryoablation
in our institution. Thirteen procedures were performed
on tumours with curative intent, and one as a staged
procedure to treat chest wall fibromatosis. Patients were
referred for local ablative treatment either because the
patient was not a suitable candidate for surgery or because
the lesions could not be treated by surgery (e.g., desmoid
tumours, bone metastases or lymph node metastases).
Patient and lesion characteristics are summarised in the
Table.
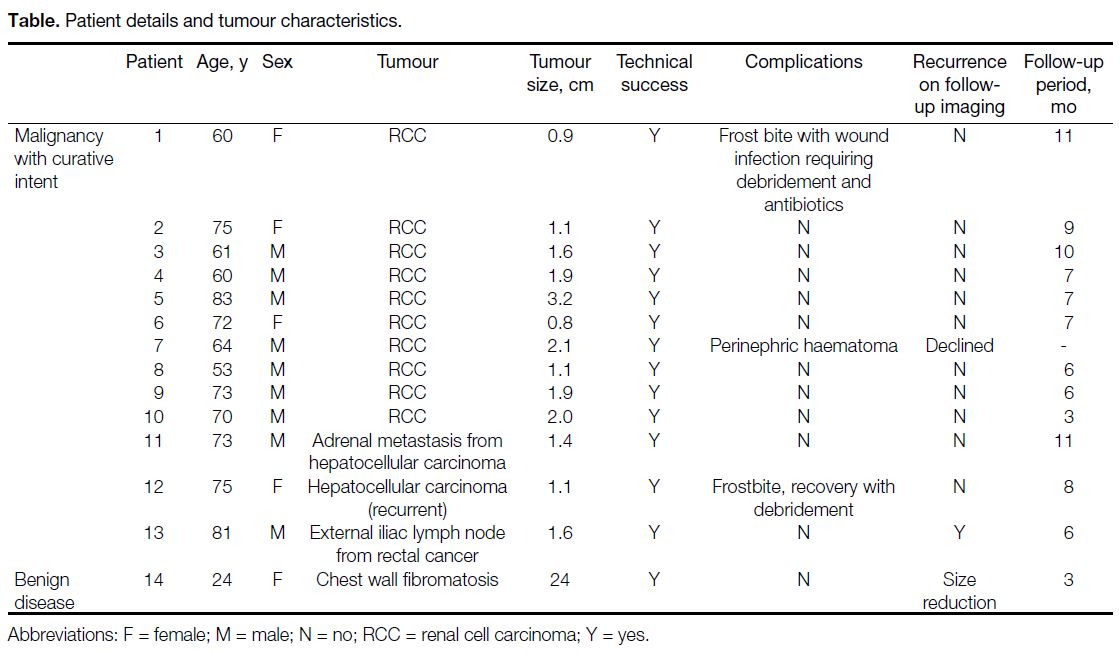
Table. Patient details and tumour characteristics
Magnetic Resonance Imaging–Guided Cryoablation
All interventions were performed in an MRI suite
dedicated to interventional procedures (Aera 1.5T;
Siemens Medical Systems, Erlangen, Germany) under
strict aseptic technique. Procedures were performed
under local lidocaine anaesthesia (n = 13) or general
anaesthesia (n = 1). Cryotherapy was performed with a cryoablation system (BTG Visual-ICE; Boston
Scientific, Marlborough [MA], United States) with one
or more cryoprobes (IceRod MRI or IceSeed MRI; Galil
Medical, Arden Hills [MN], United States) depending on
lesion characteristics, with manipulation of cryoprobes
to create different shapes and sizes of ablation zones,
often using the synergistic effects of multiple probes.
Cryoprobe insertion was performed under real-time
magnetic resonance fluoroscopic guidance using
a prototype interactive balanced steady-state free
precession sequence implemented for interactive realtime
tip tracking with interactive real-time tip tracking
module (BEAT IRTTT; Siemens Medical Systems,
Erlangen, Germany), which allows active adjustment
of scan plane orientation depending on the needs of
the operator. Occasionally, and particularly for ice ball
monitoring, multiplanar T2-weighted periodically rotated
overlapping parallel lines with enhanced reconstruction
(PROPELLER [periodically rotated overlapping
parallel lines with enhanced reconstruction]) or half-Fourier acquisition single-shot turbo spin-echo (HASTE)
sequences were acquired.
Tumour treatment was performed by ensuring that
the ablation zone included the entire tumour with an
additional safety margin of 5 to 10 mm. Treatment of the
chest wall fibromatosis was performed in this case as a staged procedure with partial coverage of the lesion for
size reduction.
Data Collection
Patient information on the Clinical Management System
of Hospital Authority from 1 May 2019 to 31 January
2020 was collected, which included patient age and
sex; target tumour type, location, size, and proximity to
important critical structures; procedure details including
mode of anaesthesia and need for hydrodissection;
complications; and duration of follow-up period. The size
of the target lesion is defined as the maximum diameter
on preprocedural MRI on the day of the procedure.
Complications were graded using the National Cancer
Instituteʼs Common Terminology Criteria for Adverse
Events (CTCAE) version 5.0. Patient outcome was
evaluated by postoperative imaging, on clinic visits,
and electronic medical records.
RESULTS
In total, 14 procedures were performed (10 renal cell
carcinomas, 1 hepatocellular carcinoma, 1 adrenal
metastasis, 1 external iliac lymph node metastasis,
and 1 chest wall fibromatosis) from 1 May 2019 to
31 January 2020 (Table). Mean age ± standard deviation
of the patients was 66 ± 14.3 years. Most procedures
(n = 13) were performed with curative intent; for these cases, mean tumour diameter was 1.6 ± 5.8 cm. One non-curative
procedure performed was a staged cryoablation
of chest wall fibromatosis measuring 24 cm, performed
for lesion size control.
All procedures performed with curative intent were
technically successful with adequate coverage of the
tumour observed during intraoperative MRI. For the
patient with cryoablation of chest wall fibromatosis
performed as a staged procedure, technical success was
achieved with coverage of the intended region of the
tumour. MRI-guided hydrodissection was performed in
two patients (14.3%) due to close proximity to the colon
(n = 1) and external iliac vessels (n = 1). A total of 85.7%
(12 out of 14) patients had two freeze/thaw cycles,
whereas 14.3% (2 out of 14) required three freeze/thaw cycles.
Three minor complications occurred. Two (14.3%)
developed CTCAE Grade 3 complications, both
developing a small area of frostbite at the needle insertion site, one of which developed before activation
of the freeze cycle. Both patients recovered after a minor
debridement. One patient (7%) developed a minor
perinephric haematoma (CTCAE Grade 1), which
recovered without intervention. No major complications
were observed.
The mean follow-up period was 7.2 ± 3.03 months.
One patient was lost to follow-up and excluded from
analysis. Of the patients with malignant disease (n = 12,
mean follow-up period = 7.6 months), tumour coverage
with no imaging evidence of residual or recurrence
was seen in 91.7% (n = 11). One patient with ablation
of an external iliac lymph node showed suspicious
fluorodeoxyglucose avidity in the iliac fossa on follow-up
positron emission tomography–CT, undetermined
but possibly residual disease. In the case of chest wall
fibromatosis, lesion size reduction was seen in the first
month after cryoablation. In this case, the disease that was
not cryoablated also showed interval shrinkage and is
softer on palpation (Figure 1), attributed to abscopal effect. No other clinical complications, such as pain, were observed in any of the patients.
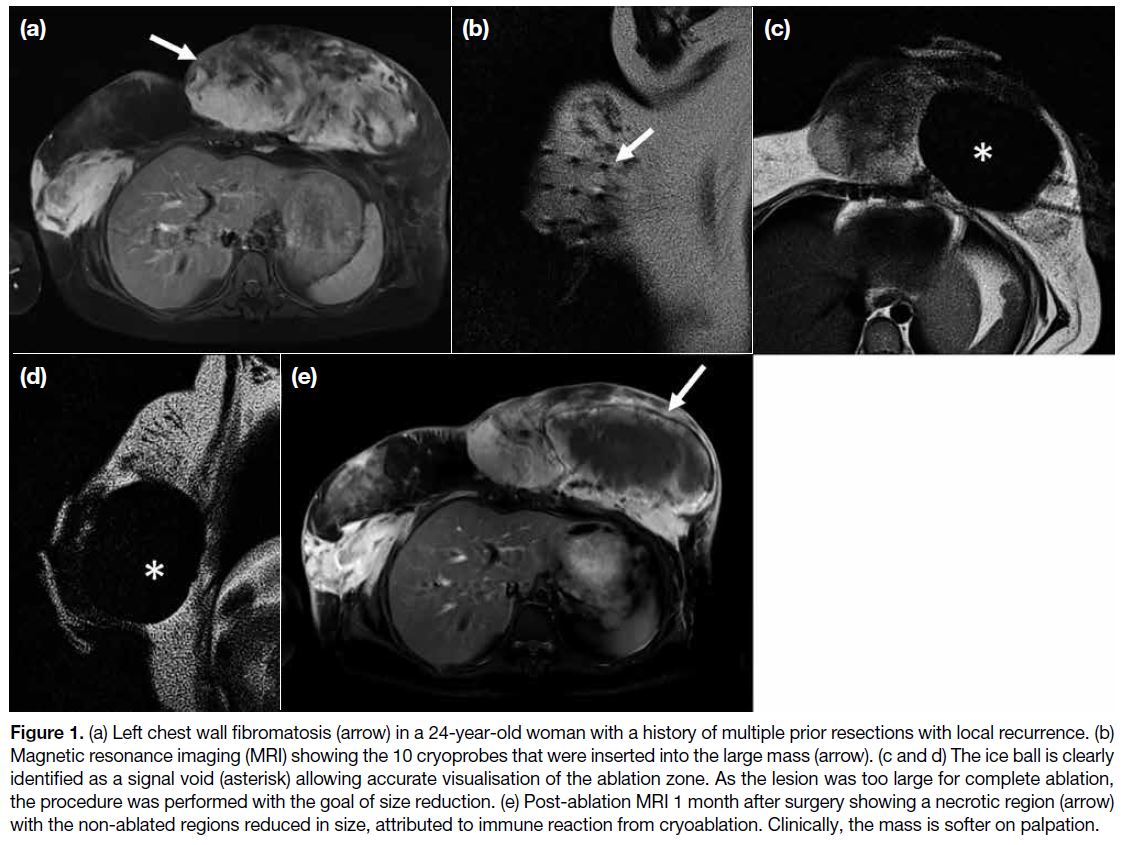
Figure 1. (a) Left chest wall fibromatosis (arrow) in a 24-year-old woman with a history of multiple prior resections with local recurrence. (b)
Magnetic resonance imaging (MRI) showing the 10 cryoprobes that were inserted into the large mass (arrow). (c and d) The ice ball is clearly
identified as a signal void (asterisk) allowing accurate visualisation of the ablation zone. As the lesion was too large for complete ablation,
the procedure was performed with the goal of size reduction. (e) Post-ablation MRI 1 month after surgery showing a necrotic region (arrow)
with the non-ablated regions reduced in size, attributed to immune reaction from cryoablation. Clinically, the mass is softer on palpation.
DISCUSSION
Cryotherapy is a thermal ablative technique that
causes tumour destruction by inducing cell damage
by freezing and thawing. Current systems use argon
and helium gases delivered via cryoprobes that induce
freezing and thawing based on the Joule-Thomson effect
(temperature change as an effect of rapid expansion of
certain gases).[3] Cryoablation has advantages over other
ablation modalities such as radiofrequency ablation due
to its intrinsic analgesic effects and potential treatment
of large tumours.[4] Additionally, cryotherapy allows
visualisation of the ice ball, particularly under MRI,
which improves predictability of tumour coverage and
prevents non-target ablation.
MRI is particularly useful in conjunction with
cryotherapy as it allows accurate visualisation of ice ball as a signal void[1] (Figure 2) as well as continuous
radiation-free multiplanar ice ball monitoring,[4] allowing
accurate assessment of ablation zone, which ensures
tumour coverage while avoiding non-target ablation.
Due to MRI’s superior soft-tissue differentiation, MRI
allows better tumour visualisation and hence improved
accuracy with needle positioning. Many lesions may only
be seen on MRI, such as non-exophytic renal masses or
hepatic dome lesions,[5] where MRI greatly improves the
accuracy of needle positioning.
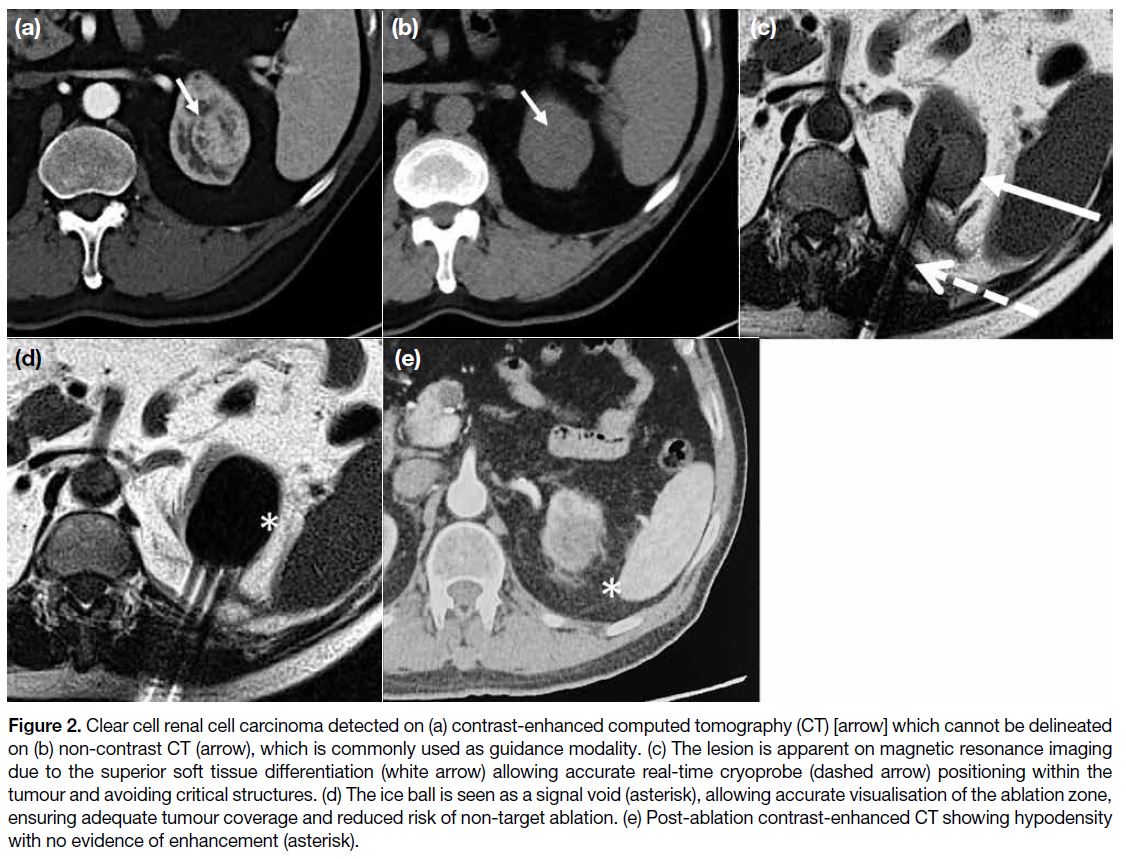
Figure 2. Clear cell renal cell carcinoma detected on (a) contrast-enhanced computed tomography (CT) [arrow] which cannot be delineated on (b) non-contrast CT (arrow), which is commonly used as guidance modality. (c) The lesion is apparent on magnetic resonance imaging due to the superior soft tissue differentiation (white arrow) allowing accurate real-time cryoprobe (dashed arrow) positioning within the
tumour and avoiding critical structures. (d) The ice ball is seen as a signal void (asterisk), allowing accurate visualisation of the ablation zone, ensuring adequate tumour coverage and reduced risk of non-target ablation. (e) Post-ablation contrast-enhanced CT showing hypodensity
with no evidence of enhancement (asterisk).
MRI-guided intervention may be performed in an open- or
closed-bore system.[1] Open-bore systems allow easier
access to the patient and enhanced operator flexibility
but are limited by lower magnetic field strength
(0.2-1 T).[1] Closed-bore systems are more desirable
as they provide higher magnetic field strength, which
improves imaging rate and quality,[1] [2] [3] although at the
expense on operator comfort and flexibility. Such
systems have specific requirements to allow for interventional use, such as a large bore width (70 cm)
and short bore length (125-150 cm).[1] This is necessary
to allow the interventional radiologist to stretch into the
bore to work at the isocentre, allowing real-time freehand
manipulation. Additional body and surface coils may be
used to increase contrast resolution of images[6] but of all
the procedures described, the spine or body coil within
the magnet may suffice.
MRI-guided cryoablation has been described in the
literature as a treatment option for a variety of tumours.
Ahrar et al[2] described percutaneous cryotherapy of small
renal tumours in 18 patients in a 1.5-T MRI. The mean
follow-up period was 16.7 months, with rates of overall
survival, disease-specific survival, and metastasis-free
survival of 88.9%, 100%, and 100%, respectively.
Similarly, MRI-guided liver cryotherapy was described
to be safe and feasible. Mala et al[7] described percutaneous
cryoablation of six patients with liver metastases from
colorectal carcinoma using an open 0.5-T MRI, which
they concluded allowed good visualisation of tumour
for cryoprobe positioning in order to puncture the
tumour. Shimizu et al[8] treated 16 tumours with MRI-guided
cryoablation on a 0.3-T open-bore magnet with
1- and 3-year overall survival rates of 93.8% and 79.3%,
respectively. The complete ablation rate was reported as
80.8% at 3 years.
As the indications for ablation have expanded in both
malignant and benign tumours and with palliative and
curative intent, MRI guidance may be the preferred
imaging technique in certain lesions. MRI guidance
has proven beneficial for established ablation targets
including renal,[1] [2] [9] prostate,[1] [10] and soft tissue[1] tumours
such as desmoids. Although less established, we find
MRI guidance highly useful in liver ablation, especially
in recurrent tumours and lesions located in the hepatic
dome, as described by Wang et al.[5]
Limitations
This paper describes our early experience of MRI-guided
cryoablation with multiple shortcomings including a small sample size, short follow-up periods, and
heterogeneity of treated lesions. Existing studies have
mainly been retrospective analyses of cohorts of MRI or
CT guidance in heterogeneous patient groups; controlled
trials with larger sample sizes and comparison with other
methods of imaging guidance will be useful.
CONCLUSION
MRI guidance is a safe imaging technique that allows
accurate tumour and non-target organ visualisation,
real-time needle puncture, and precise delineation of the
ablation zone.
REFERENCES
1. Cazzato RL, Garnon J, Shaygi B, Tsoumakidou G, Caudrelier J, Koch G, et al. How to perform a routine cryoablation under MRI guidance. Top Magn Reson Imaging. 2018;27:33-8. Crossref
2. Ahrar K, Ahrar JU, Javadi S, Pan L, Milton DR, Wood CG, et al. Real-time magnetic resonance imaging–guided cryoablation of small renal tumors at 1.5 T. Invest Radiol. 2013;48:437-44. Crossref
3. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-69. Crossref
4. Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, et al. Percutaneous image–guided cryoablation:
current applications and results in the oncologic field. Med Oncol. 2016;33:140. Crossref
5. Wang L, Liu M, Liu L, Zheng Y, Xu Y, He X, et al. MR-guided percutaneous biopsy of focal hepatic dome lesions with free-hand combined with MR fluoroscopy using 1.0-T open high-field
scanner. Anticancer Res. 2017;37:4635-41. Crossref
6. Ahrar JU, Stafford RJ, Alzubaidi S, Ahrar K. Magnetic resonance imaging–guided biopsy in the musculoskeletal system using a cylindrical 1.5-T magnetic resonance imaging unit. Top Magn
Reson Imaging. 2011;22:189-96. Crossref
7. Mala T, Edwin B, Samset E, Gladhaug I, Hol PK, Fosse E. Magnetic-resonance–guided percutaneous cryoablation of hepatic
tumours. Eur J Surg. 2001;167:610-7. Crossref
8. Shimizu T, Sakuhara Y, Abo D, Hasegawa Y, Kodama Y, Endo H. Outcome of MR-guided percutaneous cryoablation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:816-23. Crossref
9. Mogami T, Harada J, Kishimoto K, Sumida S. Percutaneous MR-guided cryoablation for malignancies, with a focus on renal cell carcinoma. Int J Clin Oncol. 2007;12:79-84. Crossref
10. King AJ, Dudderidge T, Darekar A, Schimitz K, Heard R, Everitt C. Establishing MRI-guided prostate intervention at a UK centre. Br J Radiol. 2019;92:20180918. Crossref
11. Moynagh MR, Kurup AN, Callstrom MR. Thermal ablation of bone metastases. Semin Intervent Radiol. 2018;35:299-308. Crossref