Kimura’s Disease Masquerading as Soft Tissue Sarcoma: a Case Report
CASE REPORT
Hong Kong J Radiol 2023 Jun;26(2):e5-9 | Epub 16 May 2023
Kimura’s Disease Masquerading as Soft Tissue Sarcoma: a Case Report
WM Yu1, WI Sit2, PY Chu1, KS Tse3
1 Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong SAR, China
2 Department of Radiology, Tung Wah Group of Hospitals, Hong Kong SAR, China
3 Department of Diagnostic and Interventional Radiology, Hong Kong Sanatorium & Hospital, Hong Kong SAR, China
Correspondence: Dr WM Yu, Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong SAR, China. Email: ywm821@ha.org.hk
Submitted: 18 Apr 2022; Accepted: 5 Aug 2022.
Contributors: WMY designed the study. All authors acquired and analysed the data. WMY drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Research Ethics Committee (Kowloon Central/Kowloon East Cluster) of Hospital Authority, Hong Kong (Ref No.: KC/KE-19-0169-ER-4). Written informed consent for all treatments, procedures and publication was obtained from the patients.
INTRODUCTION
Kimura’s disease is a rare idiopathic chronic inflammatory disease that predominantly affects young Asian males
with the head and neck region most affected.[1] [2] Disease
manifestation as upper extremity soft tissue masses is
extremely rare[3] with only sporadic cases reported. As
Kimura’s disease is a benign entity, it is important not
to misdiagnose these soft tissue masses as sarcomas. We
present two pathologically confirmed cases of Kimura’s
disease affecting the elbows with focus on the ultrasound
and magnetic resonance imaging (MRI) features.
CASE REPORTS
Case 1
An 18-year-old man with good past health presented
with a 6-month history of bilateral painless soft tissue
elbow swellings and increased right neck swelling. He
had no constitutional symptoms, fever or itchiness.
Physical examination revealed a firm subcutaneous non-tender
mass over the medial aspect of each elbow up to
5 × 5 cm in size with no overlying skin changes or neurovascular deficit. Enlarged right neck lymph nodes
were also palpable. Laboratory tests revealed elevated
eosinophil count of 3.8 × 109/L (reference range,
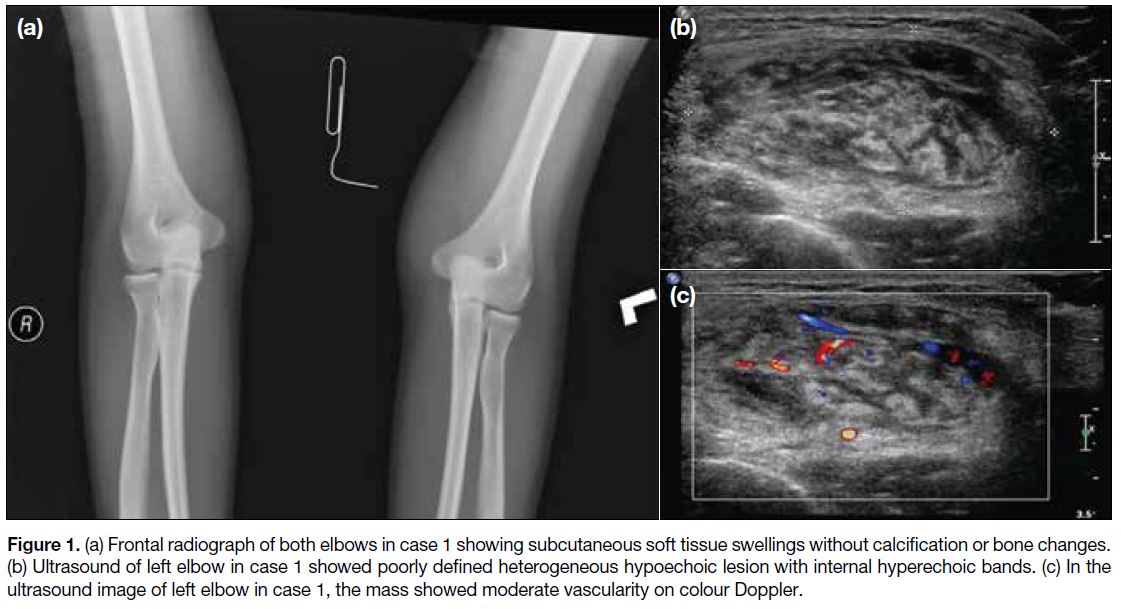
0-0.6 × 109/L). Radiograph (Figure 1a) demonstrated
soft tissue swelling at the medial aspect of both elbows
with no internal calcification or underlying bone change.
Ultrasound (Figure 1b and c) showed a poorly defined
heterogeneous hypoechoic mass in the subcutaneous
layer of the left elbow with curvilinear hyperechoic
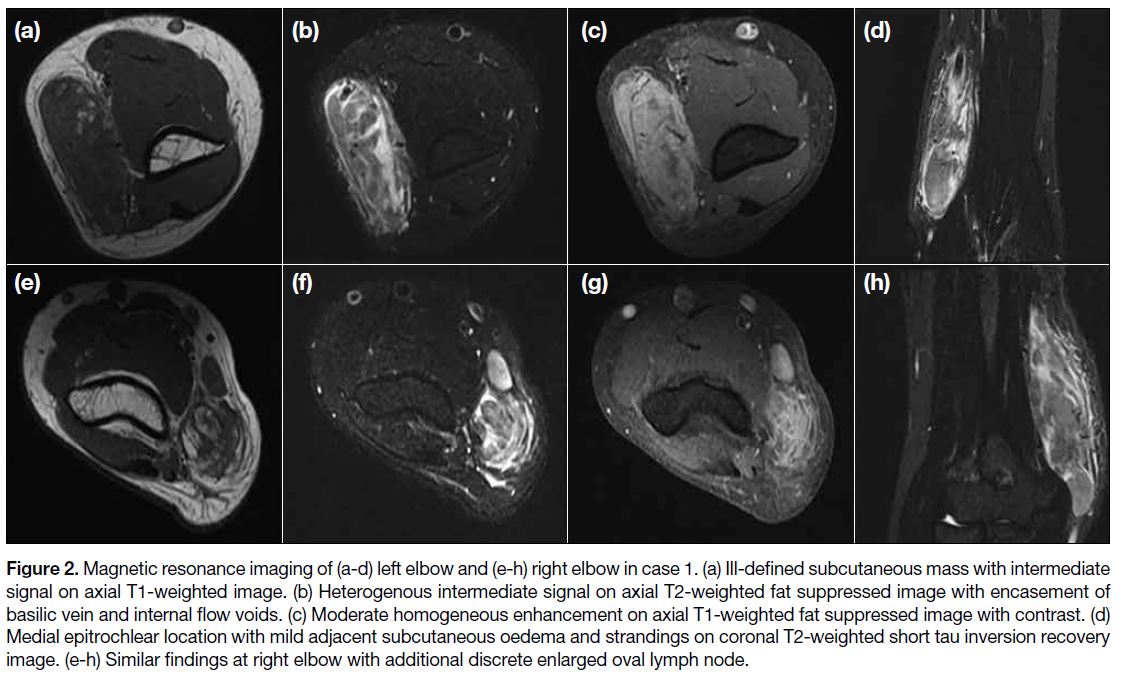
bands and moderate increased vascularity. MRI (Figure 2) showed ill-defined subcutaneous lesions at the medial
epitrochlear region of both elbows with extension to
mid humeri level. The lesions had an intermediate T1-weighted (T1W) signal, heterogeneous high signal on
T2-weighted (T2W) imaging relative to skeletal muscle
with moderate homogeneous enhancement and some
intralesional tubular flow voids. A few enlarged discrete
lymph nodes were identified and bilateral basilic veins
were encased by the lesions although patency was
maintained. There was no evidence of tumour necrosis
or cystic degeneration. Mild adjacent subcutaneous T2W hyperintense strandings, oedema, and enhancement
were also noted. No signs of muscular invasion or bone
marrow oedema were evident. Ultrasound-guided core
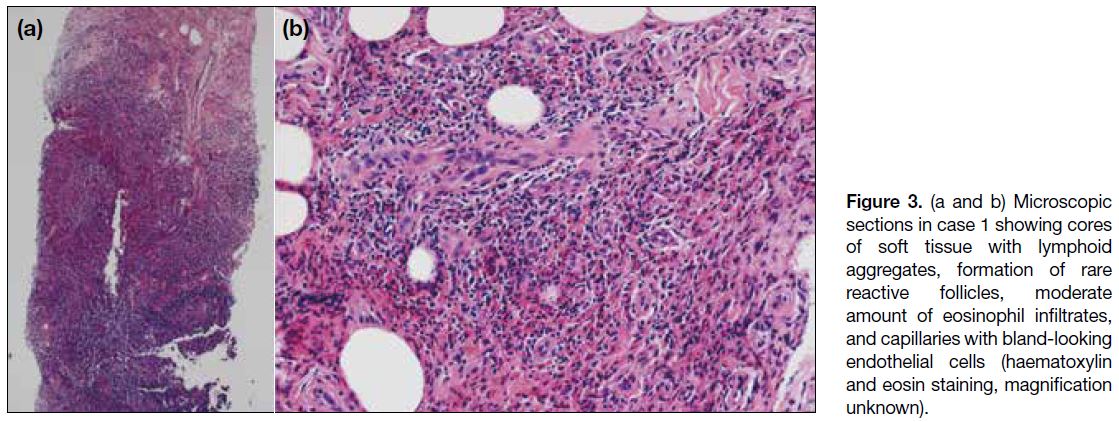
biopsy of both elbow masses revealed a large amount
of lymphoid aggregates with eosinophilic infiltrates on a background of stromal fibrosis. Follicular hyperplasia,
formation of rare reactive follicles, proliferation of
endothelial venules, and perivenular sclerosis were
seen. No malignant cells, granuloma or Reed–Sternberg
cells were detected. Histopathology results (Figure 3) were suggestive of Kimura’s disease and the patient
subsequently underwent local excision of the left elbow
mass. Excisional biopsy result of the cervical lymph
nodes was also in keeping with Kimura’s disease.
Figure 1. (a) Frontal radiograph of both elbows in case 1 showing subcutaneous soft tissue swellings without calcification or bone changes.
(b) Ultrasound of left elbow in case 1 showed poorly defined heterogeneous hypoechoic lesion with internal hyperechoic bands. (c) In the
ultrasound image of left elbow in case 1, the mass showed moderate vascularity on colour Doppler.
Figure 2. Magnetic resonance imaging of (a-d) left elbow and (e-h) right elbow in case 1. (a) Ill-defined subcutaneous mass with intermediate
signal on axial T1-weighted image. (b) Heterogenous intermediate signal on axial T2-weighted fat suppressed image with encasement of
basilic vein and internal flow voids. (c) Moderate homogeneous enhancement on axial T1-weighted fat suppressed image with contrast. (d)
Medial epitrochlear location with mild adjacent subcutaneous oedema and strandings on coronal T2-weighted short tau inversion recovery
image. (e-h) Similar findings at right elbow with additional discrete enlarged oval lymph node.
Figure 3. (a and b) Microscopic sections in case 1 showing cores of soft tissue with lymphoid aggregates, formation of rare reactive follicles, moderate amount of eosinophil infiltrates, and capillaries with bland-looking endothelial cells (haematoxylin and eosin staining, magnification unknown).
Case 2
A 23-year-old man with good past health presented with
a 1-month history of a firm painless mass over his left
arm. He also complained of incidental left groin swelling.
There was no history of trauma. Physical examination
confirmed a 4 × 4 cm soft tissue mobile mass at left
posterior arm with no skin changes and a 3 × 4 cm non-pulsatile
mass of similar consistency at the left groin with
lack of cough impulse. An elevated eosinophil count of
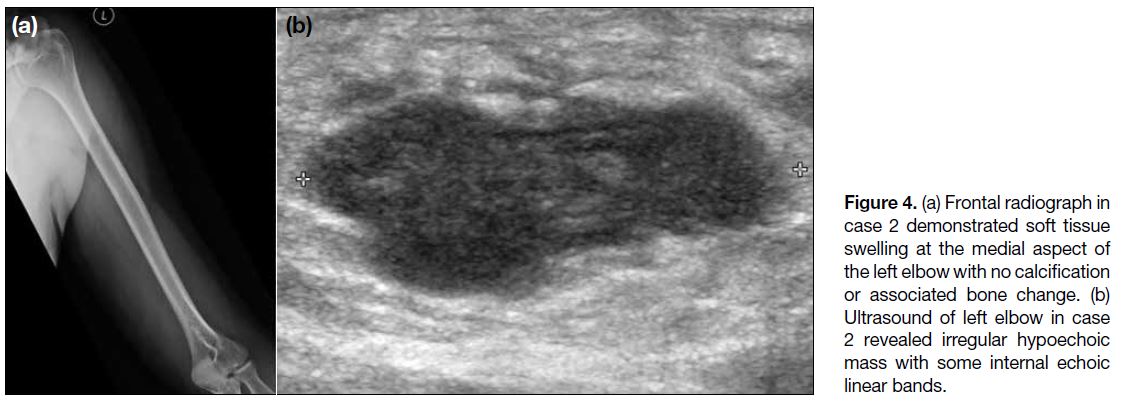
3.4 × 109/L (reference range, 0-0.6 × 109/L) was noted. Radiograph (Figure 4a) showed soft tissue swelling at
the medial aspect of his elbow with no calcification or
adjacent periosteal reaction. Ultrasound (Figure 4b)
revealed an irregular hypoechoic subcutaneous mass over the left elbow with a few internal hyperechoic
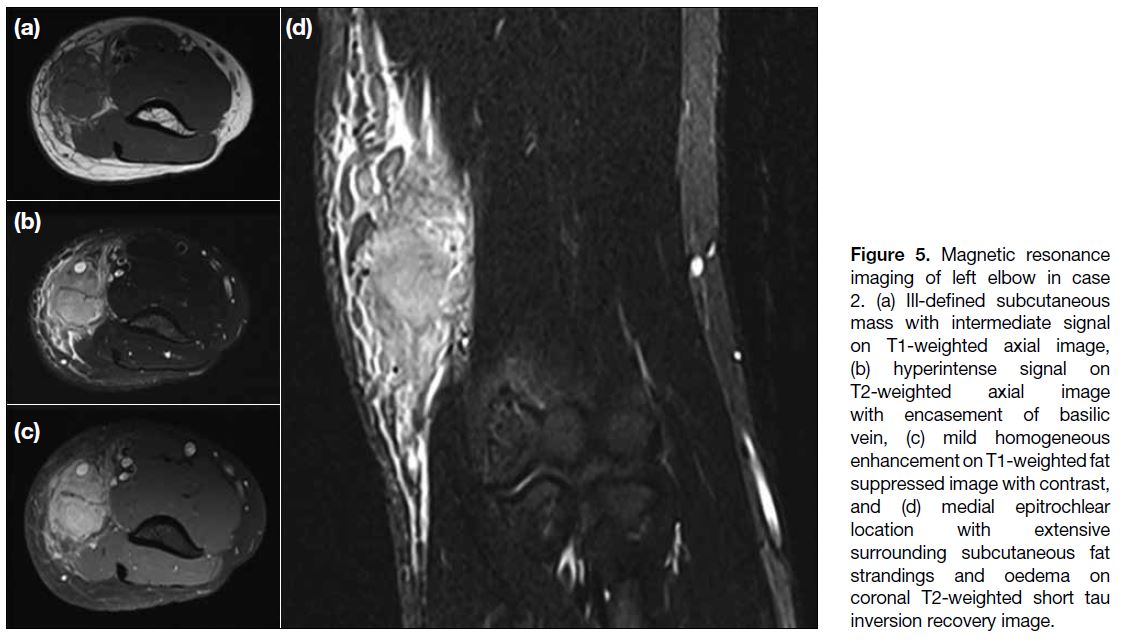
bands. MRI (Figure 5) showed an ill-defined T1W
isointense, T2W hyperintense subcutaneous mass with
homogeneous enhancement at the medial epitrochlear
region of the left elbow extending up to mid humerus
level. Marked adjacent subcutaneous oedema and
fluid together with interlobular septal thickening and
enhancement were seen. The basilic vein was encased
with preserved flow. Nearby musculature showed normal
signal and there was no bone marrow oedema. MRI of
the left groin demonstrated a cluster of enlarged lymph
nodes with marked subcutaneous strandings and oedema.
Ultrasound-guided biopsy of the elbow mass revealed
multiple reactive lymphoid follicles with germinal centre
formation, heavily infiltrated by eosinophils and bland-looking
lymphocytes. Occasional focal eosinophilic
microabscesses were seen and vasculature was slightly
prominent. Pathological features were suggestive of
Kimura’s disease. Core biopsy specimen taken from the left groin lymph node revealed similar findings. The patient was subsequently prescribed a course of oral steroid.
Figure 4. (a) Frontal radiograph in case 2 demonstrated soft tissue swelling at the medial aspect of the left elbow with no calcification or associated bone change. (b) Ultrasound of left elbow in case 2 revealed irregular hypoechoic mass with some internal echoic linear bands.
Figure 5. Magnetic resonance
imaging of left elbow in case 2. (a) Ill-defined subcutaneous mass with intermediate signal on T1-weighted axial image, (b) hyperintense signal on T2-weighted axial image with encasement of basilic vein, (c) mild homogeneous enhancement on T1-weighted fat suppressed image with contrast, and (d) medial epitrochlear location with extensive surrounding subcutaneous fat strandings and oedema on coronal T2-weighted short tau inversion recovery image.
DISCUSSION
Kimura’s disease is a rare chronic lymphoproliferative
disorder[1] first described by Kim and Szeto in 1937[4]
and later by Kimura et al in 1948[5]. It typically affects
Asians in their second or third decade of life[6] with a
higher incidence among males (male-to-female ratio
3:1). Typical presentation is painless subcutaneous
masses in the head and neck, particularly in the parotid
and submandibular regions. Other sites of involvement
include the oral cavity, axilla, groin, extremities, and
trunk. Symptoms have an insidious onset, may be
vague or asymptomatic and fluctuate for several years.[7]
Aetiology is unknown but there is speculation of an
underlying abnormal immune response.
Although imaging findings of Kimura’s disease in the
head and neck region have been previously reported
to be nonspecific and variable, consistent imaging
features are reported in Kimura’s disease of the upper
extremity.[7] [8] [9] To date, the largest published study of
imaging features of Kimura’s disease in the upper
extremity is by Choi et al[9] of nine cases. The authors
reported partial-to-poorly defined subcutaneous soft tissue masses at the medial epitrochlear region adjacent
to medial neurovascular bundles in all cases. The signal
intensity on T1W images was similar to or slightly
higher than that of muscle, while on T2W images, it was
markedly elevated compared to muscle. Enhancement
was at least moderately homogeneous. A variable
degree of surrounding subcutaneous fat strandings and
oedema as well as serpentine flow voids within the
masses were also reported. The masses usually caused
mass effect on surrounding muscles and encased
neurovascular structures without abnormal signal change
in the adjacent muscle, neurovascular bundles, bones
or joints.[9] Radiographs revealed only nonspecific soft
tissue thickening at the medial epitrochlear region with
no periosteal reaction or erosion in the adjacent bone.[9]
Shin et al[10] reported that ultrasound features of Kimura’s
disease in the upper extremities were of partially
marginated subcutaneous masses with the presence of
curvilinear hyperechoic bands and/or dots intermingled
within the hypoechoic components. Moderate to severe
vascular signals were observed in some of the hyperechoic
bands and/or dots on colour Doppler ultrasound. The
imaging findings and medial epitrochlear location in our
two cases were compatible with previous studies.
On histopathological examination, Kimura’s disease
characteristically demonstrates lymphoid follicular hyperplasia with prominent germinal centres, dense
eosinophilic infiltrates on a background of abundant
lymphoid and plasma cell infiltrates, eosinophilic
microabscesses, increased postcapillary venules and
perivenular sclerosis.[9] The vascular proliferation likely
accounts for the moderate enhancement and flow voids in
MRI, and the characteristic subcutaneous strandings and
oedema were histologically proven to be proliferation of
lymphoid follicles at subcutis.[9]
In both of our cases, there was concurrent involvement
of either cervical or groin lymph nodes. Bilateral
elbow involvement was also demonstrated in one case.
To the best of our knowledge, there have been only
four reported cases of bilateral upper limb Kimura’s
disease.[5] [7] [8] [10] These suggest that Kimura’s disease is
a systemic disease rather than a locoregional disorder.
Other reasons include frequent elevated level of serum
immunoglobulin E, peripheral blood eosinophilia,
generalised lymphadenopathy, and occasional renal
involvement.[11]
Differential diagnoses include tuberculous
lymphadenopathy, cat-scratch disease, angiolymphoid
hyperplasia with eosinophilia (ALHE), nodular fasciitis,
lymphoma, metastatic disease, and soft tissue sarcomas.[12]
Tuberculous involvement of lymph nodes would give
a matted appearance with central necrosis. Cat-scratch
disease similarly affects the medial epitrochlear region
but there would also be a contact history, painful
swelling, necrotic nodes, positive serology, and more
extensive subcutaneous oedema and strandings. It is
difficult to differentiate ALHE from Kimura’s disease
on imaging but when examined through histopathology,
ALHE does not show elements of fibrosis or sclerosis
that are evident universally in Kimura’s disease. Nodular
fasciitis presents with rapidly growing painful nodules
showing broad fascial contact but Kimura’s disease is
usually painless. Lymphoma often demonstrates bilateral
enlargement of well-defined nodes without changes to
subcutaneous fat. Metastatic lymph nodes classically
show loss of central fatty hilum, internal necrosis,
and capsular vascularity. Soft tissue sarcoma usually
manifests as a large circumscribed heterogeneous mass
with necrosis and some may show calcification. Kimura’s
disease tends to have poor margins, lack of necrosis, and
absence of calcification.
Management of Kimura’s disease is controversial and
options range from medical therapy with steroids or cytotoxic drugs to radiotherapy or surgical excision.[13] Surgery is the treatment of choice for a single, well-defined
lesion and has the benefit of pathological
confirmation. Conservative management is reserved for
those with recurrent disease and systemic involvement.
Although Kimura’s disease has an excellent prognosis
with no risk of malignant transformation, the recurrence
rate is around 25% to 75% after surgery.[7]
In conclusion, the diagnosis of Kimura’s disease requires pathological confirmation and upper limb manifestation
is rare. Nonetheless presence of the characteristic
imaging appearance along with peripheral eosinophilia
in a young Asian male should always raise a suspicion
of Kimura’s disease. The systemic nature of this entity
warrants a meticulous physical examination and imaging
to look for generalised lymphadenopathy and bilateral
involvement.
REFERENCES
1. Kase Y, Ikeda T, Yamane M, Ichimura K, Iinuma Y. Kimura’s disease: report of 4 cases with a review of 130 reported cases. Otolaryngol Head Neck Surg. 1990;63:413-8.
2. Li TJ, Chen XM, Wang SZ, Fan MW, Semba I, Kitano M. Kimura’s disease: a clinicopathologic study of 54 Chinese patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:549-55. Crossref
3. Lam AC, Au Yeung RK, Lau VW. A rare disease in an atypical location—Kimura’s disease of the upper extremity. Skeletal Radiol. 2015;44:1833-7. Crossref
4. Kim HT, Szeto C. Eosinophilic hyperplastic lymphogranuloma. Comparison with Mikulicz’s disease. Chin Med J. 1937;23:699-700.
5. Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissue. Trans Soc Pathol Jpn. 1948;37:179-80.
6. Ginsberg LE, McBride JA. Kimura’s disease. AJR Am J Roentgenol. 1998;171:1508. Crossref
7. Park JS, Jin W, Ryu KN, Won KY. Bilateral asymmetric superficial soft tissue masses with extensive involvement of both upper extremities: demonstration of Kimura’s disease by US and MRI
(2008: 12b). Eur Radiol. 2009;19:781-6. Crossref
8. Huang GS, Lee HS, Chiu YC, Yu CC, Chen CY. Kimura’s disease of the elbows. Skeletal Radiol. 2005;34:555-8. Crossref
9. Choi JA, Lee GK, Kong KY, Hong SH, Suh JS, Ahn JM, et al. Imaging findings of Kimura’s disease in the soft tissue of the upper extremity. AJR Am J Roentgenol. 2005;184:193-9. Crossref
10. Shin GW, Lee SJ, Choo HJ, Park YM, Jeong HW, Lee SM, et al. Ultrasonographic findings of Kimura’s disease presenting in the upper extremities. Jpn J Radiol. 2014;32:692-9. Crossref
11. Wang TF, Liu SH, Kao CH, Chu SC, Kao RH, Li CC. Kimura’s disease with generalized lymphadenopathy demonstrated by positron emission tomography scan. Intern Med. 2006;45:775-8. Crossref
12. Chokkappan K, Al-Riyami AM, Krishnan V, Min VL. Unusual cause of swelling in the upper limb: Kimura disease. Oman Med J. 2015;30:372-7. Crossref
13. Takeishi M, Makino Y, Nishioka H, Miyawaki T, Kurihara K. Kimura disease: diagnostic imaging findings and surgical treatment. J Craniofac Surg. 2007;18:1062-7. Crossref