Transradial Access for Neurointervention: a Case Series from a Tertiary Centre in Hong Kong
ORIGINAL ARTICLE
Hong Kong J Radiol 2023 Jun;26(2):84-90 | Epub 9 Jun 2023
Transradial Access for Neurointervention: a Case Series from a Tertiary Centre in Hong Kong
KH Fung1, NR Mahboobani1, JC Ng1, KWS Ko1, VWT Chan1, KW Shek1, NL Chan2, JK Sham2, CSK Chau3, JWT Lo3, TL Poon2, KF Fok2, WL Poon1
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Neurosurgery, Queen Elizabeth Hospital, Hong Kong SAR, China
3 Department of Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr KH Fung, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: fkh666@ha.org.hk
Submitted: 29 Nov 2021; Accepted: 13 May 2022.
Contributors: KHF, NRM and WLP designed the study. All authors acquired the data. KHF analysed the data and drafted the manuscript. NRM critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present research are included in this published article (and its supplementary information files).
Ethics Approval: This research was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) of Hospital Authority, Hong Kong (Ref No.: KC/KE-21-0225/ER-1). Patient consent was waived by the Committee due to the retrospective nature of the research.
Abstract
Introduction
Despite several retrospective studies showing the safety and efficacy of transradial access (TRA) for a variety of neurointerventions, the evidence in Asian populations is limited. The smaller size of the radial artery in Asians could cause technical difficulty in access as well as access site complications. This study aimed to assess the feasibility and safety of TRA for neurointervention in an Asian population.
Methods
We performed a retrospective review of neurointerventions performed with TRA in our hospital between January 2018 and June 2021. Technical success was defined as TRA with insertion of the sheath and completion of the intervention without crossover to conventional transfemoral access (TFA). The primary endpoint was the in-hospital stay plus the 30-day incidence of access site haematoma requiring surgical treatment or transfusion, symptomatic radial artery occlusion, hand ischaemia, arteriovenous fistula, pseudoaneurysm, and wound infection. The secondary endpoints were procedure-related complications including intra-operative vessel injury, cerebral thromboembolism, and haemorrhagic complications.
Results
A total of 45 patients underwent neurointerventions (transcatheter embolisation of aneurysms/arteriovenous malformations/tumours, and extracranial carotid stenting) via TRA. The technical success rate was 93.3%. There were no significant access site complications. The overall procedure-related complication rate was 11.1%.
Conclusion
In an Asian population, neurointervention via TRA is feasible, with a low crossover rate and low incidence of access site complications. In this case series, there was no increase in the procedure-related complication rate when compared with TFA.
Key Words: Aneurysm; Arteriovenous malformations; Carotid stenosis; Radial artery
中文摘要
神經介入的橈動脈入路:來自香港一所三級醫療中心的病例系列
馮景謙、馬承志、吳昆倫、高偉琛、陳煒達、石家偉、陳諾麟、沈雋、鄒韶君、勞慧婷、潘德立、霍錦福、潘偉麟
引言
儘管多項回顧性研究表明經橈動脈入路用於各種神經介入的安全性和有效性,但亞洲人群的證據有限。亞洲人的橈動脈較小可能會導致導管插入困難以及插入部位併發症。本研究旨在評估經橈動脈入路在亞洲人群中進行神經介入的可行性和安全性。
方法
我們對2018年1月至2021年6月期間在本院使用經橈動脈入路進行的神經介入進行了回顧性分析。技術成功的定義為經橈動脈入路插入鞘管並完成介入而無需採用傳統經股動脈通路。主要終點是住院時間加上需要手術治療或輸血的穿刺部位血腫、有症狀的橈動脈閉塞、手部缺血、動靜脈瘺、假性動脈瘤和傷口感染的30天發生率。次要終點是手術相關併發症,包括術中血管損傷、腦血栓栓塞和出血併發症。
結果
共有45名患者通過經橈動脈入路接受了神經介入(動脈瘤/動靜脈畸形/腫瘤的經導管栓塞,以及顱外頸動脈支架置入術)。技術成功率為93.3%。沒有明顯的插入部位併發症。總體手術相關併發症發生率為11.1%。
結論
在亞洲人群中通過經橈動脈入路進行神經介入是可行的,需採用經橈動脈入路的手術率低,穿刺部位併發症發生率亦低。在本病例系列中,與經股動脈通路相比,手術相關併發症的發生率沒有增加。
INTRODUCTION
Transradial access (TRA) has evolved as the standard
approach for cardiac interventions. Compared to
conventional transfemoral access (TFA), TRA has a
demonstrated lower rate of access site complications,
improved postprocedural quality of life, and reduced
hospital costs in large-scale randomised trials.[1] [2] [3] [4] [5] [6] [7] [8] [9] At
first, TRA was not widely used in neurointervention
due to technical challenges in puncturing and obtaining
access for a large-bore sheath in the small radial artery.
In recent years, TRA has been gaining popularity for
neurointerventions due to two major advantages. First,
the superficial location and compressibility of the
radial artery can reduce access site bleeding and related
complications, especially when large-bore vascular
access is needed together with the need to administer
dual antiplatelet treatment. Second, TRA has anatomical
and technical advantage in patients with type III and
bovine arch morphology.[10]
There are reports from Western countries demonstrating
low rates of access site complications and crossover to
TFA in TRA neurointerventions.[11] [12] [13] However, there are limited reports on TRA for neurointervention in Asian
populations. There are differences in the size of the radial
arteries between patients of various ethnicities. The mean
internal diameter of the radial artery has been reported to
be 3.64 ± 0.74 mm in the Western population[14] compared
to 2.63 ± 0.35 mm in the Asian population.[15] The smaller
radial artery diameter in Asians could potentially affect
arterial accessibility of and also the rate of access site
complications.
The aim of our study was to assess our experience
with TRA in 45 neurointerventions in a tertiary
neurointervention centre with a predominant Asian
patient population.
METHODS
This was a retrospective study performed in a tertiary
neurointervention centre in Hong Kong. Our patient
population is primarily Asian and predominantly
Chinese. We reviewed consecutive neurointerventional
cases performed with TRA in Queen Elizabeth
Hospital between January 2018 and June 2021. The
neurointerventions performed include carotid stenting, transcatheter embolisation (TCE) of intracranial
aneurysms, stenting of intracranial arteries, TCE of
arteriovenous malformations, and tumour TCE.
The decision to perform neurointervention using TRA was made prior to the procedure in cases with factors
reported to favour TRA, which include but are not limited
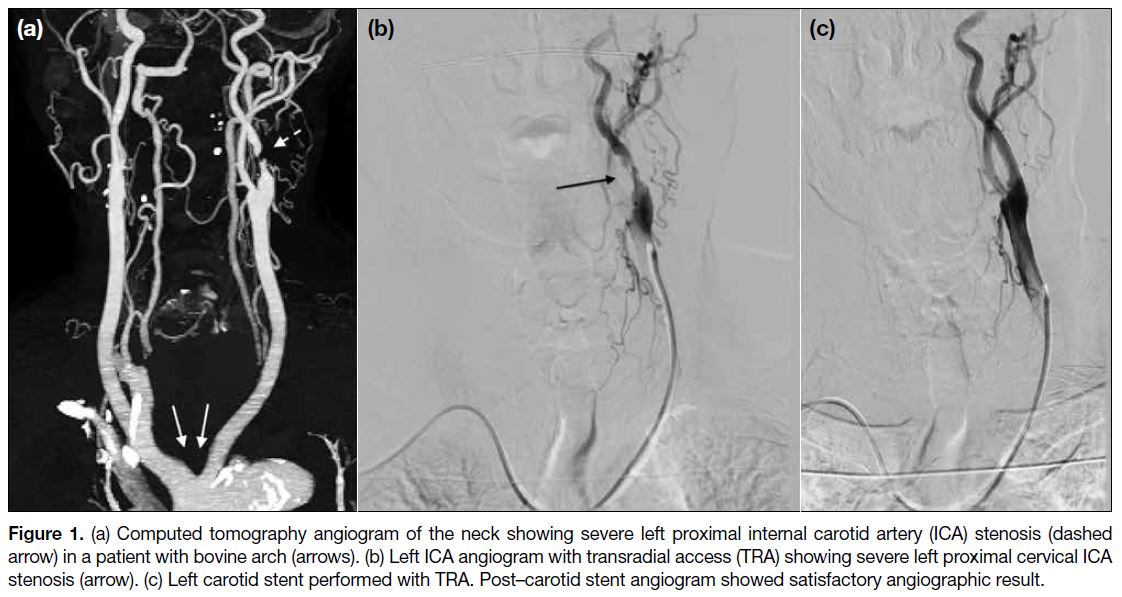
to type II/III aortic arch, bovine arch (Figure 1), posterior
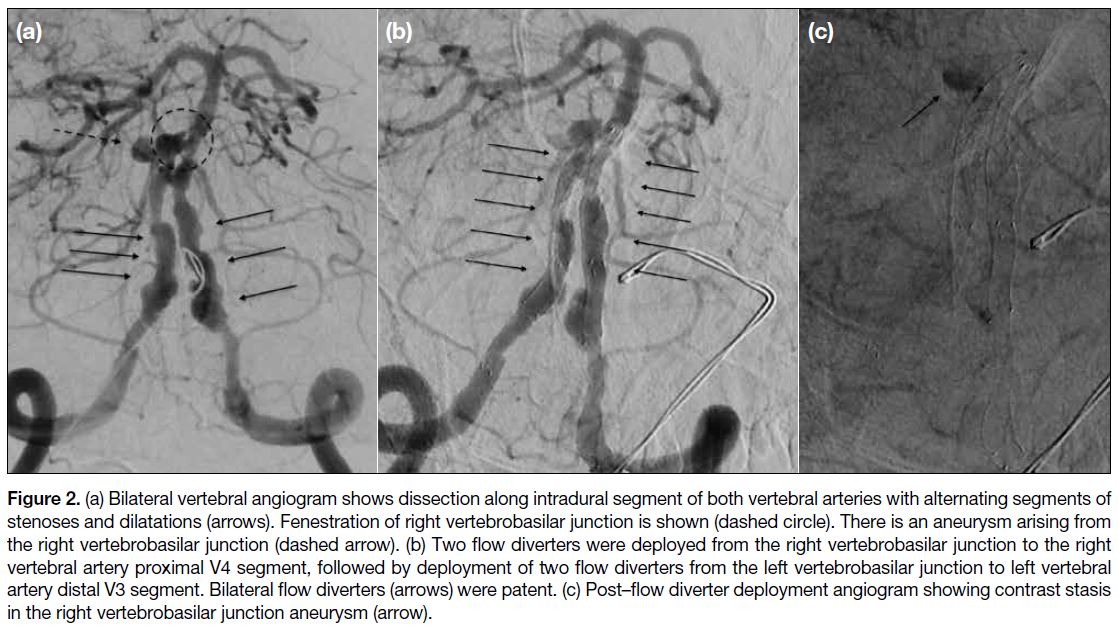
circulation vascular lesions (Figure 2), high bleeding risk
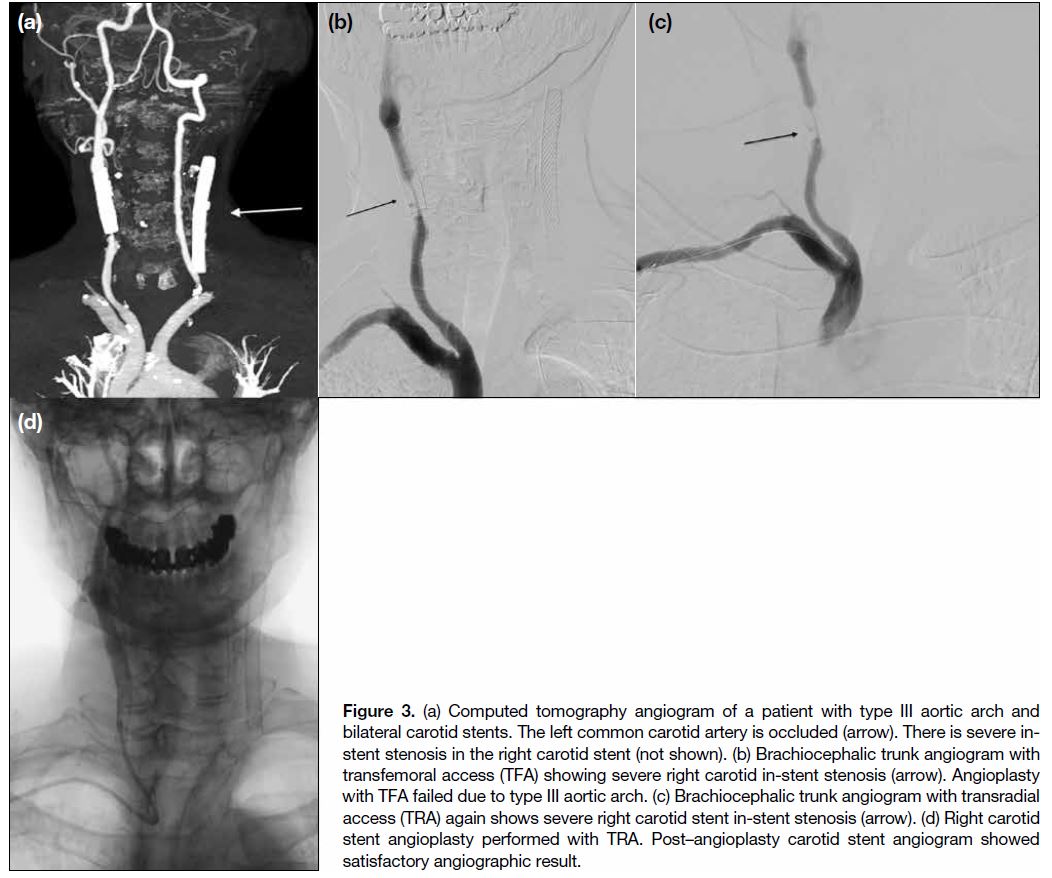
due to use of dual antiplatelet therapy, obesity, and failed TFA (Figure 3). All cases meeting the inclusion criteria
were included in this study except there was one case
excluded as the patient was observed with Barbeau type
D waveform. The list of factors was based on medical
knowledge and neurointervention experience, and the
decision was made by neurointervention operators.
Figure 1. (a) Computed tomography angiogram of the neck showing severe left proximal internal carotid artery (ICA) stenosis (dashed arrow) in a patient with bovine arch (arrows). (b) Left ICA angiogram with transradial access (TRA) showing severe left proximal cervical ICA stenosis (arrow). (c) Left carotid stent performed with TRA. Post–carotid stent angiogram showed satisfactory angiographic result.
Figure 2. (a) Bilateral vertebral angiogram shows dissection along intradural segment of both vertebral arteries with alternating segments of stenoses and dilatations (arrows). Fenestration of right vertebrobasilar junction is shown (dashed circle). There is an aneurysm arising from the right vertebrobasilar junction (dashed arrow). (b) Two flow diverters were deployed from the right vertebrobasilar junction to the right vertebral artery proximal V4 segment, followed by deployment of two flow diverters from the left vertebrobasilar junction to left vertebral artery distal V3 segment. Bilateral flow diverters (arrows) were patent. (c) Post–flow diverter deployment angiogram showing contrast stasis in the right vertebrobasilar junction aneurysm (arrow).
Figure 3. (a) Computed tomography angiogram of a patient with type III aortic arch and bilateral carotid stents. The left common carotid artery is occluded (arrow). There is severe in-stent stenosis in the right carotid stent (not shown). (b) Brachiocephalic trunk angiogram with transfemoral access (TFA) showing severe right carotid in-stent stenosis (arrow). Angioplasty with TFA failed due to type III aortic arch. (c) Brachiocephalic trunk angiogram with transradial access (TRA) again shows severe right carotid stent in-stent stenosis (arrow). (d) Right carotid stent angioplasty performed with TRA. Post–angioplasty carotid stent angiogram showed satisfactory angiographic result.
Endovascular Procedure
Our standard approach was to perform the Barbeau
test prior to radial artery puncture. For Barbeau types
A, B and C, the neurointervention would proceed with
TRA; for Barbeau type D, neurointervention would be
performed with TFA.
The puncture site of the radial artery was either at the
wrist (2 to 3 cm proximal to palmar wrist crease) or the distal radial artery (at the anatomical snuffbox). The
choice of access site was based on the calibre of the
radial artery measured with ultrasound at the respective
sites and also operators’ preference. The choice of right
or left radial artery depended on the location of the target
lesion. For example, for right vertebral artery or right
internal carotid artery lesion, right transradial approach
was used; for left vertebral artery lesion, left transradial
approach was used.
TRA was achieved with a single-wall puncture under
ultrasound guidance, followed by insertion of a 6-F sheath
(Radifocus Introducer II Transradial Kit; Terumo, Tokyo,
Japan). An antispasmodic cocktail (2.5 mg of verapamil
and 200 μg of nitroglycerine) was administered via the
radial sheath; this became our standard practice and was administered in the last 38 cases in this series with close
monitoring of blood pressure. Haemodilution (aspirating
a substantial amount [a few mm] of blood into syringe)
and slow injection of the antispasmodic cocktail were
adopted to mitigate the burning sensation associated
with the cocktail and to avoid a sudden drop in blood
pressure. A bolus of heparin (50 units/kg) and heparin
infusion (600 units/h) were administered intravenously.
The supra-aortic vessels were catheterised by advancing
a guide catheter (Benchmark 071; Penumbra, Alameda
[CA], US; Neuron 053, Penumbra, Alameda [CA], US;
or Mach 1; Boston Scientific, Natick [MA], US), over
a standard hydrophilic angled 0.035-inch guidewire
(Terumo, Tokyo, Japan), with or without the aid of a
5-Fr diagnostic catheters such as a Simmons 2–shaped
catheter (Terumo, Tokyo, Japan), Torcon NB Advantage
Catheter (Cook Medical, Bloomington [IN], US) or JB2
catheter (Cordis, Miami [FL], US). The guide catheter
could be preloaded with the diagnostic catheter or
exchanged for a diagnostic catheter over a guidewire.
Upon completion of the procedure, the radial artery
puncture site was closed with application of a haemostatic
bandage (Stepty P; Nichiban, Tokyo, Japan) for 4 hours.
Patients were then examined for access site haematoma
and for distal perfusion. All patients were reviewed for
access site complications during the hospital stay and
underwent follow-up in the outpatient clinic.
Outcome
Technical success was defined as TRA with insertion
of the sheath and completion of neurointervention
without crossover to conventional TFA for intervention.
The primary endpoint was the in-hospital stay plus 30-
day incidence of significant access site complications
including access site haematoma requiring surgical
treatment or transfusion, symptomatic radial artery
occlusion, hand ischaemia, arteriovenous fistula,
pseudoaneurysm, or wound infection. The secondary
endpoints were procedure-related complications
including intraoperative vessel injury, and cerebral
thromboembolic and haemorrhagic complications.
RESULTS
Between January 2018 and June 2021, 45
neurointerventions were performed with TRA in our
institution. Patient demographics, neurointervention
performed, target lesion, rationale for TRA, and
location of radial artery puncture are listed in the online supplementary Table.
All 45 patients were Asian and 43 of them (95.6%)
were Chinese. There were 17 cases (37.8%) of TCE of
aneurysm(s) in the anterior circulation, 16 cases (35.6%)
of TCE of aneurysm(s) in the posterior circulation
(Figure 2), 10 cases (22.2%) of carotid stenting
(Figures 1 and 3), one case (2.2%) of embolisation
of a meningioma, and one case (2.2%) of TCE of an
arteriovenous malformation in the posterior fossa.
We performed 46 radial artery punctures in the 45
neurointerventions. There were 34 punctures (73.9%) at
wrist level and 12 punctures (26.1%) at the anatomical
snuffbox.
The overall rate of technical success of TRA was 93.3%,
with no instances of failure in obtaining radial access.
There was no case of radial artery vasospasm nor radial
loop requiring crossover to TFA. There were three
cases with crossover (6.7%) to TFA due to severe acute
angulation between the right subclavian artery and the
right common carotid artery.
For the primary safety endpoints, there was no
significant access site haematoma, symptomatic radial
artery occlusion, hand ischaemia, arteriovenous fistula,
pseudoaneurysm, or wound infection during in-hospital
stay and 30 days thereafter.
For secondary endpoints, five patients (11.1%) had
procedure-related complications. There were two cases
of intra-operative aneurysm rupture, two cases of
thromboembolism (one case resolved with intra-arterial
eptifibatide injection with no clinical sequelae; the other
case suffered a middle cerebral artery territory infarct
noted on postoperative day 2), and one case of intraoperative
in-stent stenosis.
DISCUSSION
There is increasing utilisation of TRA in diagnostic
and interventional cerebral angiography, with good
clinical outcomes. It is becoming the preferred choice
of access by patients.[16] [17] There are published case
series demonstrating feasibility and safety of TRA
in a variety of neurointerventions, such as aneurysm
TCE,[11] flow diverting stent placement[12] and mechanical
thrombectomy,[13] which were all performed in Western
countries. There is no corresponding literature in Asian
populations.
Our case series is the first which consists of Asian
(100%) and predominantly Chinese patients (95.6%). It demonstrates a high success rate in performing
neurointerventions with TRA, which is similar to
published case series with Caucasian patients, despite the
smaller radial artery diameter in Asians when compared
to Caucasians.[14] [15] The crossover rate in our case series
was similar compared to other published case series.
In a systemic review of TRA in neurointerventions
which consisted of 21 studies (n = 1342 patients),[10] the
crossover rate was 4.77%. Radial artery spasm is one of
the potential difficulties in performing neurointervention
with TRA. It was only rarely encountered in this case
series. The antispasmodic cocktail was very effective in
preventing and treating radial artery spasm. The fact that
we performed all neurointerventions apart from carotid
stenting with general anaesthesia was a protective
factor. Another potential difficulty in performing
neurointervention with TRA was radial loops. Radial
loops were only rarely encountered in this case series.
The radial loop is a rare vascular anomaly with a reported
frequency of 2.3% in one large multicentre case series.[18]
In the few cases with radial loop which we encountered in
this case series, the loop was reduced with advancement
of the catheter with the aid of a guidewire.
TRA also demonstrated safety among our patient group
with no significant access site complications observed in
our case series. In a systematic review,[10] the major access
site complication rate was reported to be 0.15%.
The overall procedure-related complication rate in
our case series was 11.1% (5 out of 45 cases). All five
complicated cases were TCE of intracranial aneurysms.
In subgroup analysis, the complication rate of TCE of
intracranial aneurysms with TRA was 15.2%, which is
within the reported range in the literature.[19] [20] [21] [22] The overall
TRA procedure–related complication rate was similar to
that with TFA in our centre (10%-20%).
Limitations
Our study has a few limitations. First, it was a single-centre study which limits its generalisability. However,
the neurointerventions in this series were performed
by 11 operators with variable lengths of experience in
neurointervention from <1 year to >20 years. This could
suggest that TRA can be performed by operators with
different levels of experience.
Second, this study has a small sample size.
Neurointervention with TRA was increasingly performed
in our centre because operators were gaining experience and confidence in TRA. According to cardiac literature
and studies regarding diagnostic cerebral angiography
with TRA, there is a 30- to 50-case learning curve,[23] [24]
and we expect our crossover and procedure-related
complication rate will improve with our increasing case
volume of TRA.
CONCLUSION
This case series is believed to be the first one to
demonstrate that TRA is feasible and safe to perform
for a variety of neurointerventions in Asian patients,
who have relatively smaller radial artery calibres when
compared to Caucasian patients. The crossover rate was
low and there was a high success rate of 93.3% with TRA.
There were no significant access site complications in
this case series. There was no increase in the procedurerelated
complication rate with TRA when compared with
TFA in our centre.
REFERENCES
1. Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus
femoral access for coronary angiography or intervention and the
impact on major bleeding and ischemic events: a systematic review
and meta-analysis of randomized trials. Am Heart J. 2009;157:132-40. Crossref
2. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349-56. Crossref
3. Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der
Wieken R. A randomized comparison of percutaneous transluminal
coronary angioplasty by the radial, brachial and femoral approaches:
the access study. J Am Coll Cardiol. 1997;29:1269-75. Crossref
4. Mamas MA, Tosh J, Hulme W, Hoskins N, Bungey G, Ludman P, et al. Health economic analysis of access site practice in England during changes in practice: insights from the British Cardiovascular Interventional Society. Circ Cardiovasc Qual Outcomes.
2018;11:e004482. Crossref
5. Mann JT 3rd, Cubeddu MG, Schneider JE, Arrowood M. Right
radial access for PTCA: a prospective study demonstrates reduced
complications and hospital charges. J Invasive Cardiol. 1996;8
Suppl D:40D-44D.
6. Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi M, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of
a multicentre, randomised controlled trial. Lancet. 2018;392:835-48. Crossref
7. Sciahbasi A, Pristipino C, Ambrosio G, Sperduti I, Scabbia EV,
Greco C, et al. Arterial access-site–related outcomes of patients
undergoing invasive coronary procedures for acute coronary
syndromes (from the ComPaRison of Early Invasive and
Conservative Treatment in Patients With Non–ST-ElevatiOn Acute
Coronary Syndromes [PRESTO-ACS] Vascular Substudy). Am J
Cardiol. 2009;103:796-800. Crossref
8. Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T,
et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised
multicentre trial. Lancet. 2015;385:2465-76. Crossref
9. Kok MM, Weernink MG, von Birgelen C, Fens A, van der Heijden LC, van Til JA. Patient preference for radial versus femoral vascular access for elective coronary procedures: the PREVAS study. Catheter Cardiovasc Interv. 2018;91:17-24. Crossref
10. Joshi KC, Beer-Furlan A, Crowley RW, Chen M, Munich SA. Transradial approach for neurointerventions: a systematic review of the literature. J Neurointerv Surg. 2020;12:886-92. Crossref
11. Chivot C, Bouzerar R, Yzet T. Transitioning to transradial access for cerebral aneurysm embolization. AJNR Am J Neuroradiol. 2019;40:1947-53. Crossref
12. Li Y, Chen SH, Spiotta AM, Jabbour P, Levitt MR, Kan P, et al. Lower complication rates associated with transradial versus transfemoral flow diverting stent placement. J Neurointerv Surg. 2021;13:91-5. Crossref
13. Phillips TJ, Crockett MT, Selkirk GD, Kabra R, Chiu AH, Singh T, et al. Transradial versus transfemoral access for anterior circulation mechanical thrombectomy: analysis of 375 consecutive cases. Stroke Vasc Neurol. 2021;6:207-13. Crossref
14. Bertrand B, Sene Y, Huygue O, Monségu J. Doppler ultrasound imaging of the radial artery after catheterization [in French]. Ann Cardiol Angeiol (Paris). 2003;52:135-8. Crossref
15. Yoo BS, Lee SH, Ko JY, Lee BK, Kim SN, Lee MO, et al. Procedural outcomes of repeated transradial coronary procedure. Catheter Cardiovasc Interv. 2003;58:301-4. Crossref
16. Snelling BM, Sur S, Shah SS, Khandelwal P, Caplan J, Haniff R, et al. Transradial cerebral angiography: techniques and outcomes. J Neurointerv Surg. 2018;10:874-81. Crossref
17. Stone JG, Zussman BM, Tonetti DA, Brown M, Desai SM, Gross BA, et al. Transradial versus transfemoral approaches for diagnostic cerebral angiography: a prospective, single-center,
non-inferiority comparative effectiveness study. J Neurointerv
Surg. 2020;12:993-8. Crossref
18. Lo TS, Nolan J, Fountzopoulos E, Behan M, Butler R, Hetherington SL, et al. Radial artery anomaly and its influence on transradial coronary procedural outcome. Heart. 2009;95:410-5. Crossref
19. Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268-80. Crossref
20. Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470-6. Crossref
21. Lozier AP, Connolly ES Jr, Lavine SD, Solomon RA. Guglielmi detachable coil embolization of posterior circulation aneurysms: a systematic review of the literature. Stroke. 2002;33:2509-18. Crossref
22. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98:959-66. Crossref
23. Hess CN, Peterson ED, Neely ML, Dai D, Hillegass WB, Krucoff MW, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a
study from the National Cardiovascular Data Registry. Circulation.
2014;129:2277-86. Crossref
24. Zussman BM, Tonetti DA, Stone J, Brown M, Desai SM, Gross BA, et al. Maturing institutional experience with the
transradial approach for diagnostic cerebral arteriography:
overcoming the learning curve. J Neurointerv Surg. 2019;11:1235-8. Crossref