Multidisciplinary Management of Ovarian, Fallopian Tube and Peritoneal Cancers with Emphasis on the Role of Cross-Sectional Imaging
PICTORIAL ESSAY
Hong Kong J Radiol 2023 Jun;26(2):142-52 | Epub 31 May 2023
Multidisciplinary Management of Ovarian, Fallopian Tube and Peritoneal Cancers with Emphasis on the Role of Cross-Sectional Imaging
OL Chan1, SC Young2, WWL Yip3, WH Chong1, KY Kwok1
1 Department of Radiology, Tuen Mun Hospital, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, Tuen Mun Hospital, Hong Kong SAR, China
3 Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr OL Chan, Department of Radiology, Tuen Mun Hospital, Hong Kong SAR, China. Email: col950@ha.org.hk
Submitted: 23 Nov 2021; Accepted: 7 Apr 2022.
Contributors: All authors designed the study. OLC acquired and analysed the data, and drafted the manuscript. SCY, WWLY, WHC and KYK critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the New Territories West Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: NTWC/REC/21066). A waiver for written informed consent of patients was granted by the Committee as this manuscript is for pictorial review only and does not involve patient treatment/procedures.
INTRODUCTION
Ovarian cancer is the eighth most common cancer
among women worldwide and the second most common
gynaecological cancer mortality after cervical cancer.[1]
In Hong Kong, ovarian cancer ranks as the sixth most
common cancer in female and the most common cause of
gynaecological cancer mortality in 2020.[2] Approximately
two-thirds of patients with ovarian cancer are reported to
present with stage III-IV disease.[3] [4] High-grade serous
carcinoma accounts for about 70% of malignant ovarian
tumours.[5]
There is recent evidence that ovarian or peritoneal cancer may have a common origin from the fimbrial end of the
fallopian tubes. The ovarian cancer staging system was
updated in 2014 to include cancer of the fallopian tubes
and peritoneum.[3]
One of the most important prognostic factors in ovarian
cancer is the volume of residual disease after surgery. Cytoreductive surgery is the mainstay of treatment and
is considered optimal if there is no or ≤1 cm of gross
residual tumour and suboptimal if the residual tumour
is >1 cm.[6] [7]
In patients who present with advanced-stage disease,
preoperative imaging to assess the abdominopelvic
disease burden can help identify those at risk of having
>1 cm gross residual disease and help avoid futile
laparotomy. These patients may be first treated with
neoadjuvant chemotherapy followed by reassessment
imaging and interval debulking surgery if optimal
tumour debulking is deemed possible.[3]
At our institution, management of patients with ovarian,
fallopian tube and peritoneal cancers is discussed by a
multidisciplinary team consisting of gynaecologists,
radiologists, and oncologists. A multidisciplinary
approach to cancer management has been shown to
improve a patient’s quality of life and prognosis.[8] [9]
This article describes the role of imaging in the
management of patients with ovarian cancer. The anatomy
of common sites of peritoneal lesions is reviewed and
features that may preclude optimal debulking surgery are
highlighted.
ROLE OF IMAGING
The role of imaging in the management of ovarian
cancer is to delineate the disease extent so that primary
treatment can be planned and indicate possible sites for
image-guided core biopsy for histological confirmation
when necessary.[4]
Previous studies compared the diagnostic accuracy of
different imaging modalities for detection of peritoneal
carcinomatosis in ovarian cancer. Computed tomography
(CT), magnetic resonance imaging, and positron
emission tomography/computed tomography (PET/CT) all demonstrated >90% accuracy when compared
with diagnostic laparoscopy.[10] Magnetic resonance
imaging has the advantage of providing better soft tissue
differentiation but is less readily available and motion
artefacts may affect image quality. PET/CT is useful
for whole-body assessment but is likewise not readily
available. CT remains the most commonly performed
pretreatment imaging since it has high accuracy and
accessibility.
Compared with diagnostic laparoscopy, CT has a high
accuracy of >90% for detection of peritoneal deposits.
It is also more accurate in detecting peritoneal disease
in upper abdominal regions when compared with
laparoscopy. Limitations of CT are nonetheless its
reported decreased sensitivity of <80% in depicting
implants <1 cm in size, and inferior accuracy compared
with laparoscopy in detecting disease in pelvic and small
intestinal mesenteric regions.[11]
At our institution, CT of the abdomen and pelvis is
performed for pretreatment staging, with the lung bases
included in the scan range. The latter enables a search
for suspicious cardiophrenic lymph nodes and pleural
effusion that will require further investigations such as
pleural tapping to look for stage IV disease.[4] Looking
for stage IV disease is essential because this group of
patients may not be candidates for surgical debulking.
In patients with inoperable disease, interval debulking
is considered after two to three cycles of systemic
chemotherapy.[3] As well as monitoring cancer antigen 125 level, CT should be used to assess treatment response and
the feasibility of interval debulking surgery. PET/CT is
often supplementary when CT findings are inconclusive.[4]
Arrangement of timely follow-up imaging and planning
for interval debulking surgery following neoadjuvant
chemotherapy can be facilitated by a multidisciplinary
meeting.
PERITONEAL ANATOMY AND FLOW
OF PERITONEAL FLUID
Knowledge of the anatomy of the peritoneum and
flow of peritoneal fluid is important when assessing
peritoneal spread of disease. The internal surfaces
of the abdominopelvic cavity are lined with parietal
peritoneum. The visceral peritoneum lines the organs
that are intraperitoneal. The potential space between
these two layers of peritoneum is the peritoneal cavity
and generally contains a small amount of fluid to allow
frictionless movement of visceral organs within the
abdominal cavity. Ascites is often detected in a disease
state and may be due to increased capillary permeability
or obstructed lymphatics resulting in overall increased
peritoneal fluid.[12]
There are multiple peritoneal folds and reflections that
compartmentalise the abdominopelvic cavity. The
transverse mesocolon divides the peritoneal cavity into
the supracolic and infracolic spaces. The supracolic
space is separated by the falciform ligament into the left
and right. The right supracolic space contains the right
subphrenic space, subhepatic space, and the lesser sac.
The left supracolic space includes the perihepatic and
left subphrenic space. The infracolic space is further
divided by the small bowel mesentery into the larger left
and smaller right spaces. The small bowel mesentery
attaches from the ligament of Trietz at the left upper
quadrant to the ileocaecal junction at the right iliac fossa.
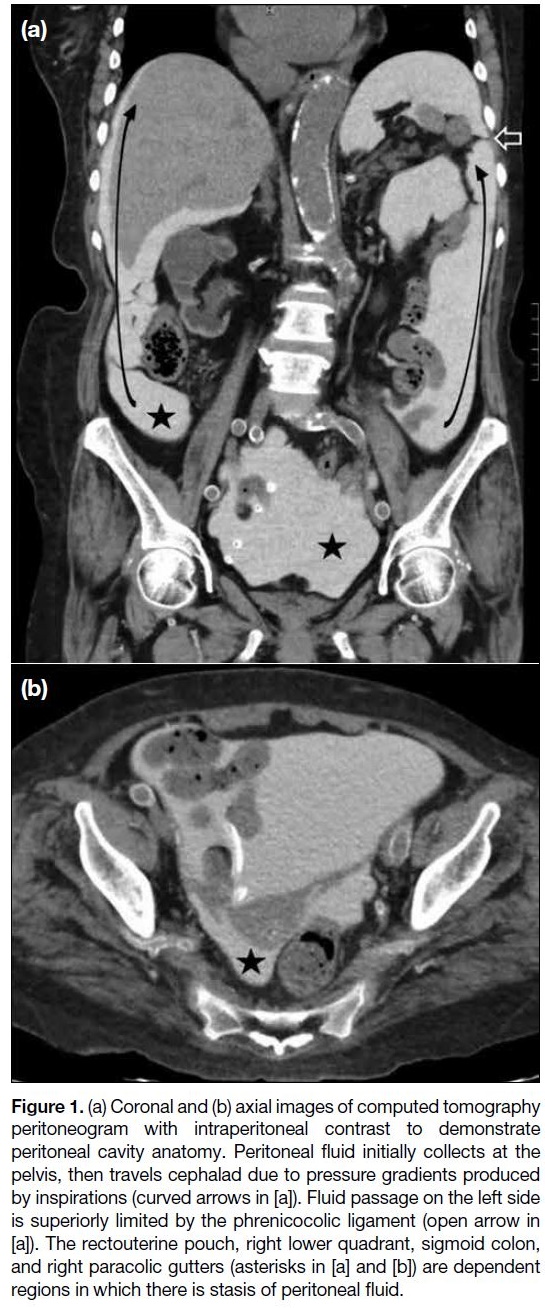
Initially, peritoneal fluid collects at a gravity-dependent site, the pouch of Douglas in woman and the rectovesical
space in men. It travels in a cephalad direction, entering
the paracolic gutters and then the supracolic spaces. On
the left side, fluid passage is superiorly limited by the
phrenicocolic ligament, hence more peritoneal fluid
flows into the right paracolic gutter (Figure 1). The
flow of peritoneal fluid is slow or arrested at dependent
regions due to gravity, and fluid stasis allows tumour
cells to be deposited. The four dependent areas include
the rectouterine pouch, right lower quadrant, sigmoid
colon, and right paracolic gutters.
Figure 1. (a) Coronal and (b) axial images of computed tomography
peritoneogram with intraperitoneal contrast to demonstrate
peritoneal cavity anatomy. Peritoneal fluid initially collects at the
pelvis, then travels cephalad due to pressure gradients produced
by inspirations (curved arrows in [a]). Fluid passage on the left side
is superiorly limited by the phrenicocolic ligament (open arrow in
[a]). The rectouterine pouch, right lower quadrant, sigmoid colon,
and right paracolic gutters (asterisks in [a] and [b]) are dependent
regions in which there is stasis of peritoneal fluid.
ASSESSMENT OF ABDOMINOPELVIC DISEASE BURDEN
The most frequent routes for dissemination of ovarian
cancer are by direct pelvic invasion and via transcoelomic peritoneal spread.[13] Less frequently, it may also
spread along the lymphatics via the utero-ovarian,
infundibulopelvic and round ligament pathways.[3] The
most common lymphatic spread is along the utero-ovarian
pathway to the para-aortic and paracaval nodes
at the level of the kidney.[7] Haematogenous spread is rare
and seldom present at initial diagnosis.
Primary Tumour
The size and location of the primary ovarian tumour
should be described. Pelvic sidewall invasion is suspected
when the distance between the tumour and the muscular
pelvic sidewall is <3 mm, or when there is encasement
of >90% of the circumference of iliac vessels.[13] Any
invasion to the adjacent organs such as the urinary
bladder or rectum should be noted since it may require
additional surgical input from other subspecialties to
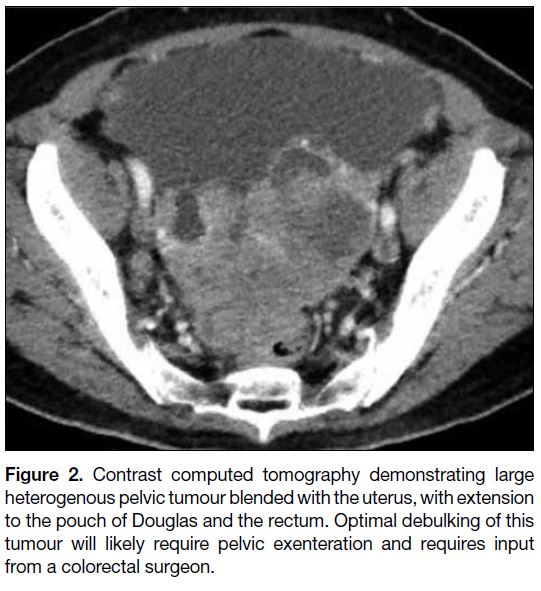
achieve optimal debulking (Figure 2).
Figure 2. Contrast computed tomography demonstrating large
heterogenous pelvic tumour blended with the uterus, with extension
to the pouch of Douglas and the rectum. Optimal debulking of this
tumour will likely require pelvic exenteration and requires input
from a colorectal surgeon.
Peritoneal Carcinomatosis
Assessment of peritoneal carcinomatosis is crucial
since it affects staging and subsequent management.
The presence of ascites raises a suspicion of peritoneal
involvement and loculated ascites usually indicates
peritoneal metastasis. Signs of early peritoneal disease
are subtle and can be easily missed. Multiplanar
reformatted CT images are helpful in the assessment
of peritoneal lesions. Coronal and sagittal reformatted images improve detection of lesions on curved surfaces
such as the diaphragm, paracolic gutters, and pelvis
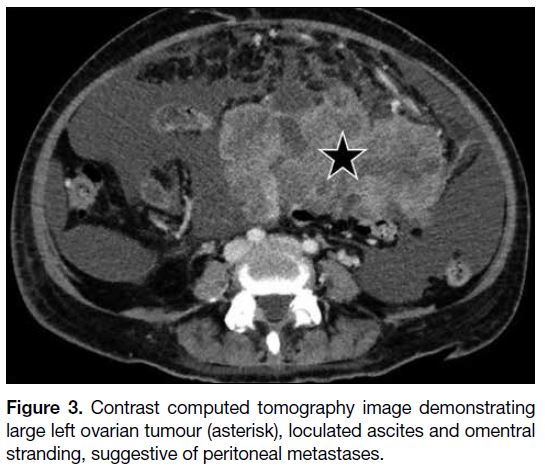
(Figure 3).[7]
Figure 3. Contrast computed tomography image demonstrating
large left ovarian tumour (asterisk), loculated ascites and omentral
stranding, suggestive of peritoneal metastases.
The operability of peritoneal lesions is related to
multiple factors such as the location of peritoneal
deposits, their morphology and multiplicity, and their
size and relationship with adjacent organs. Peritoneal
carcinomatosis can present with a wide range of
morphological appearance on CT. Subtle soft tissue
infiltration, mild thickening or nodularity of the
peritoneum may be the only findings in early peritoneal
disease. Soft tissue peritoneal implants are usually
observed in advanced peritoneal disease. These can
present as solitary or multiple nodules, or coalesce to
form plaque-like lesions and larger masses. They may
show contrast enhancement; metastases from serous
cystadenocarcinoma may be calcified.[12]
Upper Abdomen
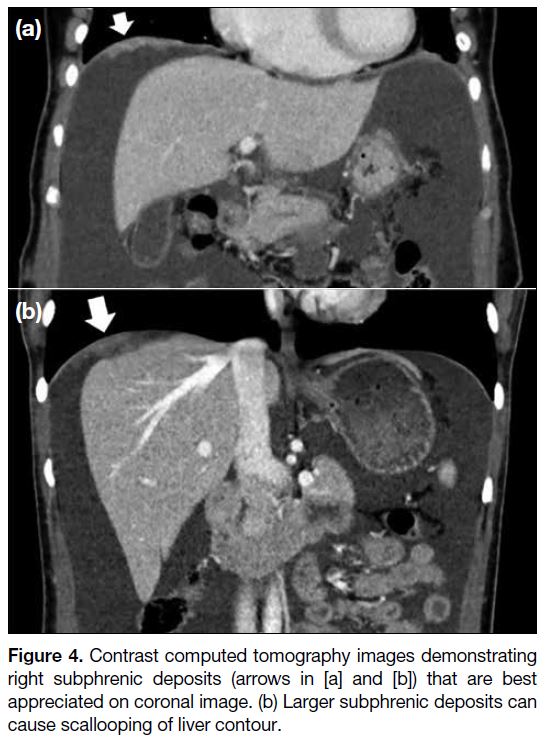
The right subphrenic region is frequently involved since
there is preferential flow of peritoneal fluid along the
right side of the abdomen. Coronal and sagittal images
enable better assessment of peritoneal deposits at the
hemidiaphragm. Nodular- or plaque-like thickening may
be observed. Larger deposits at the subphrenic region
may cause scalloping of the liver or splenic contour. On
post-contrast phase, these implants are hypoenhancing
relative to the liver or splenic parenchyma (Figure 4).
Figure 4. Contrast computed tomography images demonstrating right subphrenic deposits (arrows in [a] and [b]) that are best appreciated on coronal image. (b) Larger subphrenic deposits can cause scallooping of liver contour.
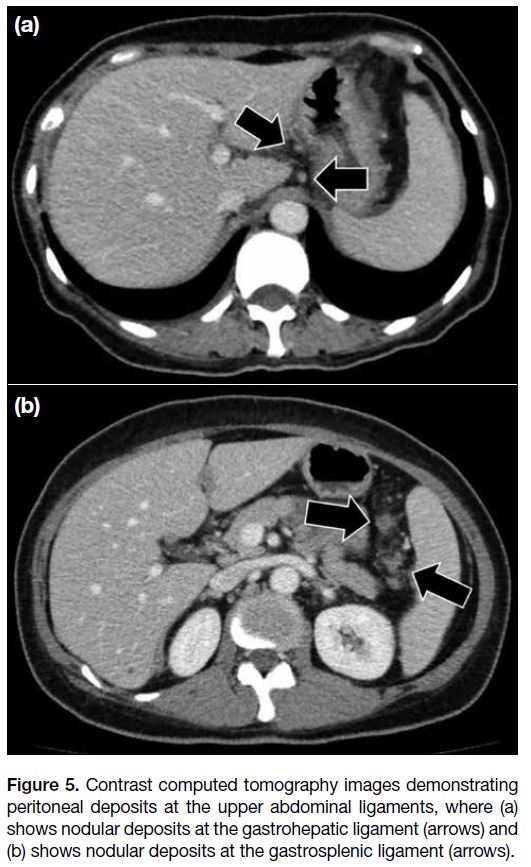
In the upper abdomen, it is also essential to look for any involvement of the peritoneal ligaments, including the
gastrohepatic and gastrosplenic ligaments (Figure 5).
Increased soft tissue stranding, thickening or nodular
deposits at these ligaments usually suggest involvement.
Figure 5. Contrast computed tomography images demonstrating
peritoneal deposits at the upper abdominal ligaments, where (a)
shows nodular deposits at the gastrohepatic ligament (arrows) and
(b) shows nodular deposits at the gastrosplenic ligament (arrows).
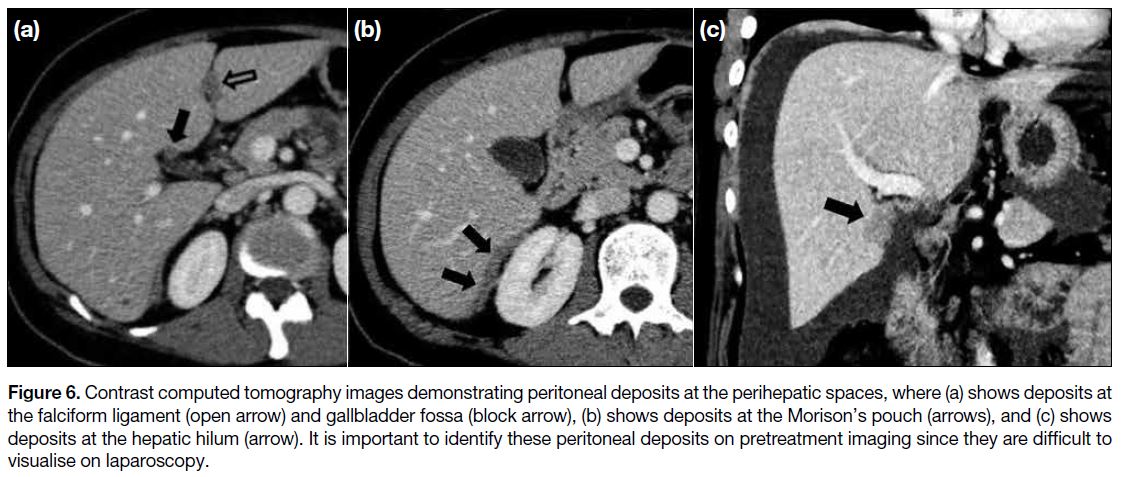
Any tumour deposits at the perihepatic spaces should be
specified, including the fissure for falciform ligament,
gallbladder fossa, porta hepatism, and lesser sac (Figure 6). Peritoneal lesions at these sites, particularly when
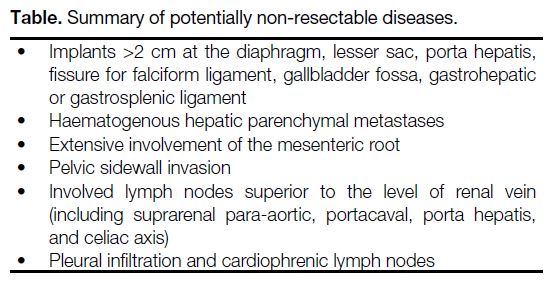
>2 cm in size, are probably non-resectable.[14] Any
subcapsular implants at the Morison’s pouch extending
to the inferior vena cava must be described because they
pose a surgical challenge due to increased bleeding risk.[7]
Figure 6. Contrast computed tomography images demonstrating peritoneal deposits at the perihepatic spaces, where (a) shows deposits at
the falciform ligament (open arrow) and gallbladder fossa (block arrow), (b) shows deposits at the Morison’s pouch (arrows), and (c) shows
deposits at the hepatic hilum (arrow). It is important to identify these peritoneal deposits on pretreatment imaging since they are difficult to
visualise on laparoscopy.
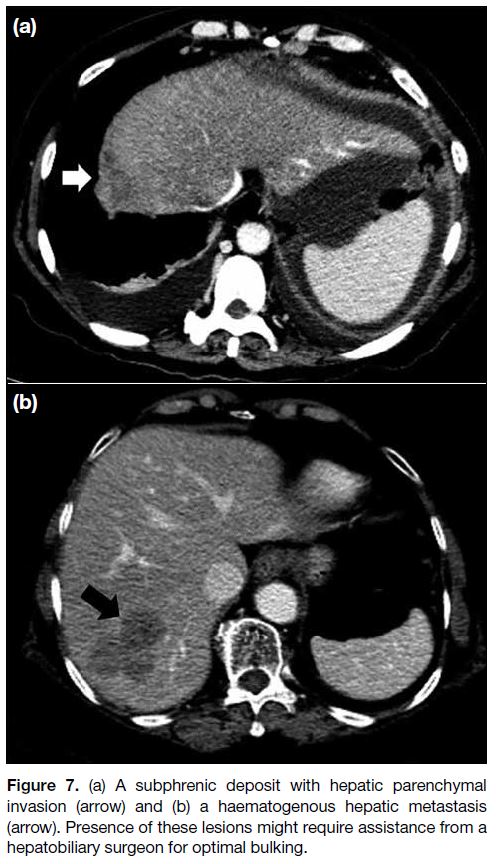
Since haematogenous metastases are extremely rare at
presentation, apparent parenchymal involvement of the
liver and spleen are more commonly caused by invasive
serosal surface implants rather than haematogenous
metastases (Figure 7). Differentiation of a surface lesion
versus parenchymal invasion lesion is particularly
important for the liver because partial hepatectomy may be required in the latter with assistance of a hepatobiliary
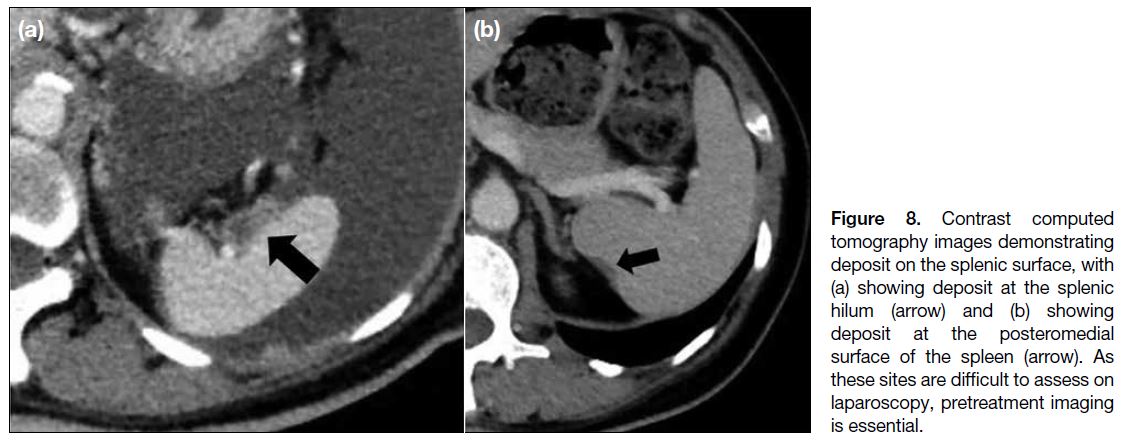
surgeon. Distinguishing between a surface lesion
and invasive parenchymal lesion at the spleen is less
important because splenectomy is more easily performed
(Figure 8).[13]
Figure 7. (a) A subphrenic deposit with hepatic parenchymal
invasion (arrow) and (b) a haematogenous hepatic metastasis
(arrow). Presence of these lesions might require assistance from a
hepatobiliary surgeon for optimal bulking.
Figure 8. Contrast computed
tomography images demonstrating deposit on the splenic surface, with (a) showing deposit at the splenic hilum (arrow) and (b) showing deposit at the posteromedial surface of the spleen (arrow). As these sites are difficult to assess on laparoscopy, pretreatment imaging is essential.
Paracolic Gutters, Omentum, and Mesentery
The bilateral paracolic gutters are also common sites of
peritoneal deposits because of peritoneal fluid stasis. It
is helpful to assess with both axial and coronal images.
Tumour deposits present as irregular thickening and
nodularity (Figure 9).
Figure 9. (a) Axial
and (b) coronal images from contrast computed tomography demonstrating deposits at the paracolic gutters (arrows).
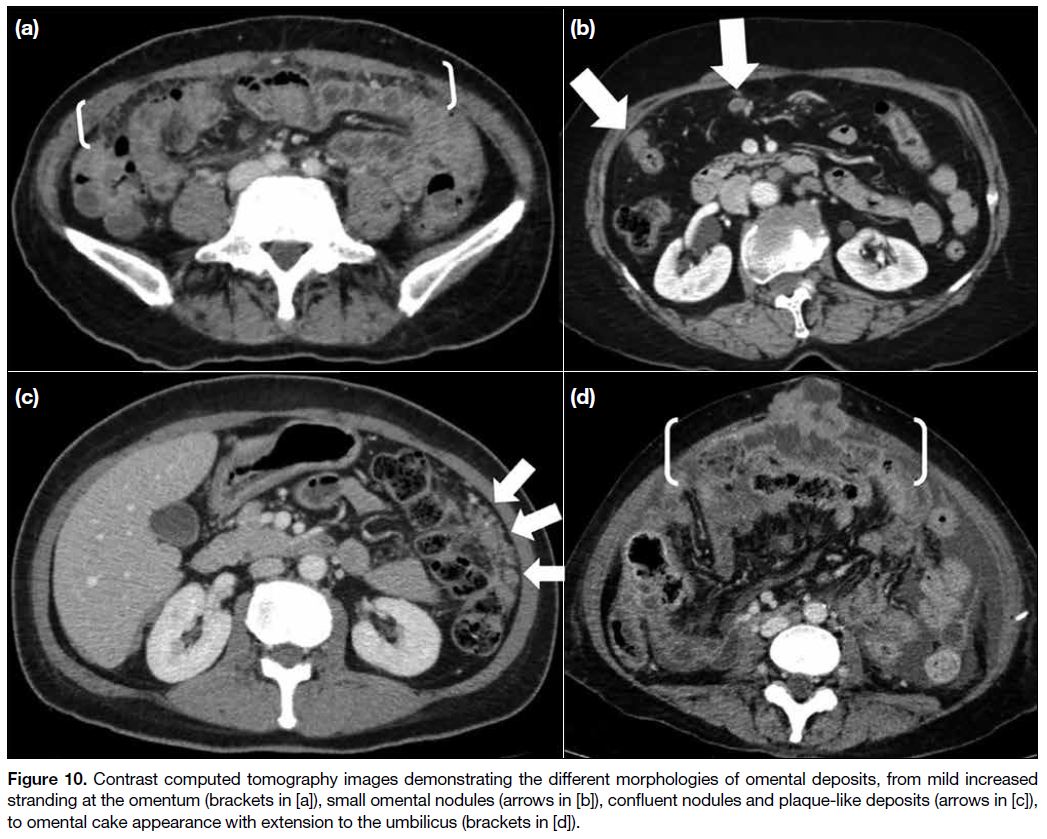
Infiltration of the omental fat can present as increased
soft tissue stranding and omental nodules of various
sizes. When these small nodules coalesce, they give
rise to larger omental plaques or mass-like lesions that
are commonly referred to as omental cakes. Greater
omental involvement usually does not preclude surgery
since omentectomy is routinely performed in debulking
surgery. Nonetheless extension of omental metastases to
the anterior abdominal wall or umbilicus may preclude
surgery (Figure 10).[4] [12]
Figure 10. Contrast computed tomography images demonstrating the different morphologies of omental deposits, from mild increased
stranding at the omentum (brackets in [a]), small omental nodules (arrows in [b]), confluent nodules and plaque-like deposits (arrows in [c]),
to omental cake appearance with extension to the umbilicus (brackets in [d]).
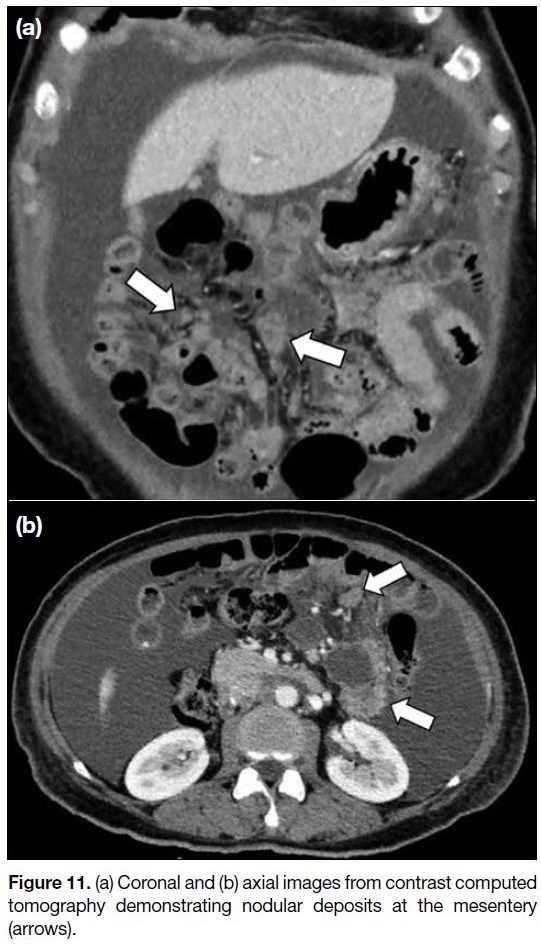
Infiltration of the mesentery can present as misty
mesentery or clustered soft-tissue nodules (Figure 11).
Peritoneal deposits at the mesentery can cause tethering
of the bowel loops and intestinal obstruction. Intestinal
obstruction is a common morbidity associated with
metastatic ovarian cancer, reported to occur in about 50% of cases.[12] Detection of segmental small bowel
obstruction and extensive tumour deposits on the small
bowel surface or at the mesenteric root is important
because these features may preclude surgery.[14] Serosal
implants at the bowel loops are difficult to detect,
particularly in the absence of complications such as
intestinal obstruction. Involvement of the small bowel
may present as segmental mural thickening and softtissue
mass involving the serosa and adjacent mesentery.
Depending on the extent of involvement, these bowel
serosal implants may be resected with the assistance of a
gastrointestinal surgeon.
Figure 11. (a) Coronal and (b) axial images from contrast computed tomography demonstrating nodular deposits at the mesentery (arrows).
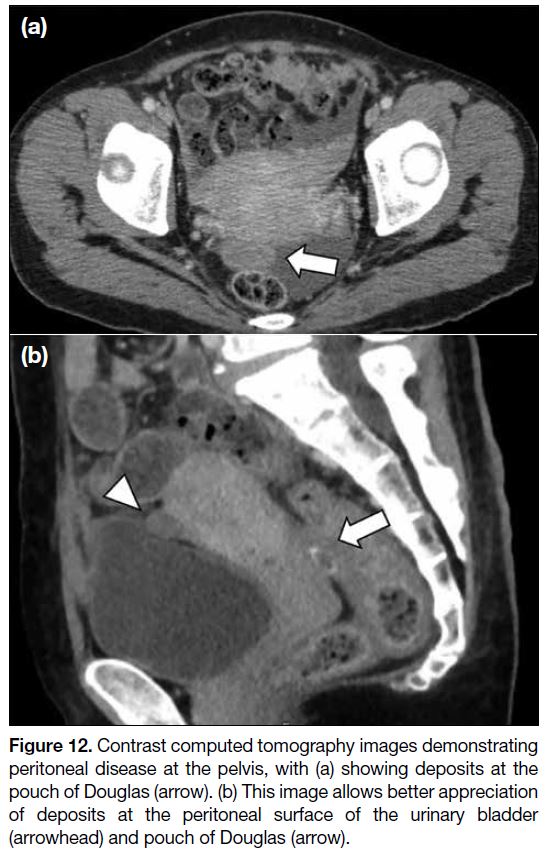
In the pelvis, common sites of deposits include the
surface of the urinary bladder, sigmoid mesocolon,
pelvic sidewall, pouch of Douglas, and surface of the
sigmoid and rectum. Lesions at the bilateral uterosacral
ligament and pelvic sidewall are better seen on axial and
coronal images. Lesions at the peritoneal surface of the
bladder, pouch of Douglas, and rectosigmoid regions are
better observed on sagittal images. Again, these deposits
can present as soft tissue thickening or a mass (Figure 12).
Figure 12. Contrast computed tomography images demonstrating peritoneal disease at the pelvis, with (a) showing deposits at the pouch of Douglas (arrow). (b) This image allows better appreciation of deposits at the peritoneal surface of the urinary bladder (arrowhead) and pouch of Douglas (arrow).
Lymphadenopathy
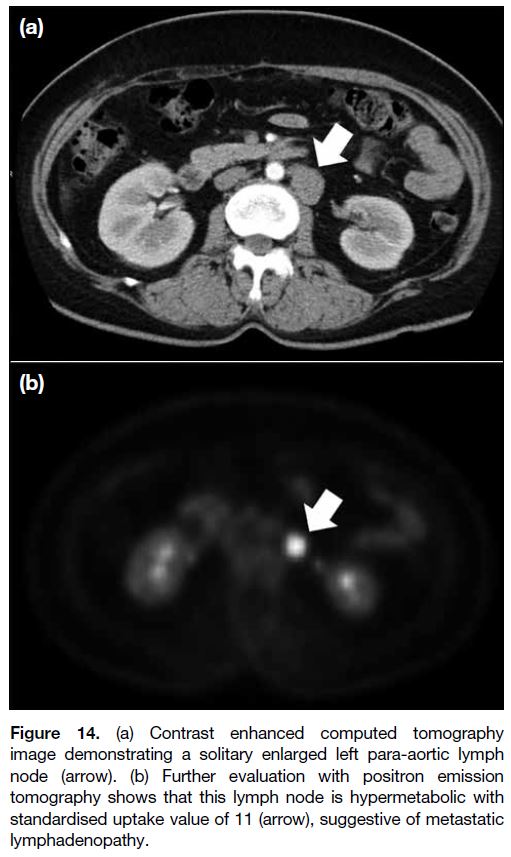
Lymphatic spread commonly involves the para-aortic
lymph nodes. An enlarged node with >1 cm short
axis suggests malignant lymphadenopathy.[13] Apart
from increased nodal size, necrosis or clustering of
lymph nodes are also suspicious features of metastatic
involvement. Any enlarged lymph node at the suprarenal para-aortic, portacaval, porta hepatis or celiac axis
should be specified because these sites may preclude
surgery (Figures 13 and 14).
Figure 13. Malignant para-aortic lymphadenopathy above the level
of the renal vein (circle in [a]) and enlarged paracaval lymph node
(arrow in [b]). These are considered surgically difficult sites.
Figure 14. (a) Contrast enhanced computed tomography
image demonstrating a solitary enlarged left para-aortic lymph
node (arrow). (b) Further evaluation with positron emission
tomography shows that this lymph node is hypermetabolic with
standardised uptake value of 11 (arrow), suggestive of metastatic
lymphadenopathy.
Lung Base
Assessment of the lung bases on preoperative CT is
crucial since these extraperitoneal sites are not accessible
by laparoscopy. The cardiophrenic lymph node is
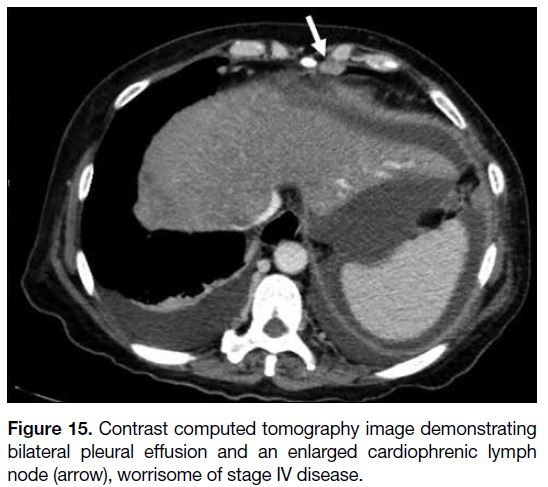
considered enlarged when the short axis is >5 mm[7] [15] and
may suggest stage IV disease. This precludes surgery.
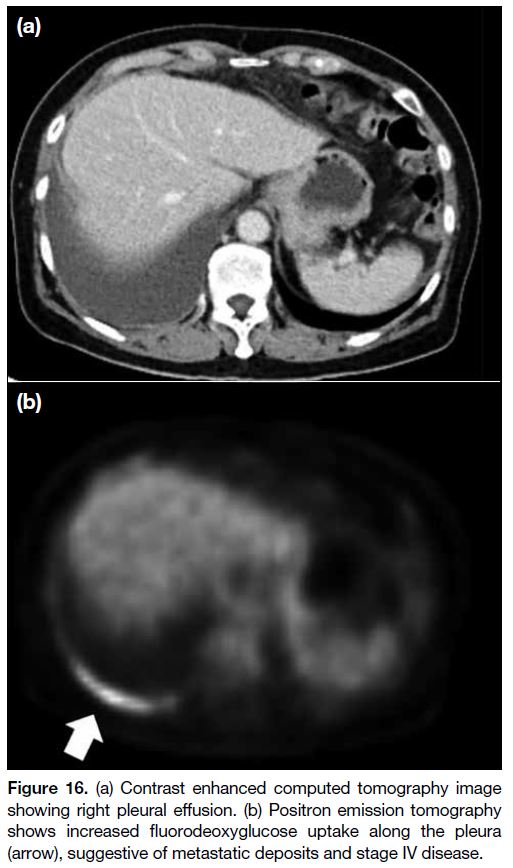
Any pleural effusion should be further investigated
to look for stage IV disease that is deemed inoperable
(Figures 15 and 16).
Figure 15. Contrast computed tomography image demonstrating
bilateral pleural effusion and an enlarged cardiophrenic lymph
node (arrow), worrisome of stage IV disease.
Figure 16. (a) Contrast enhanced computed tomography image
showing right pleural effusion. (b) Positron emission tomography
shows increased fluorodeoxyglucose uptake along the pleura
(arrow), suggestive of metastatic deposits and stage IV disease.
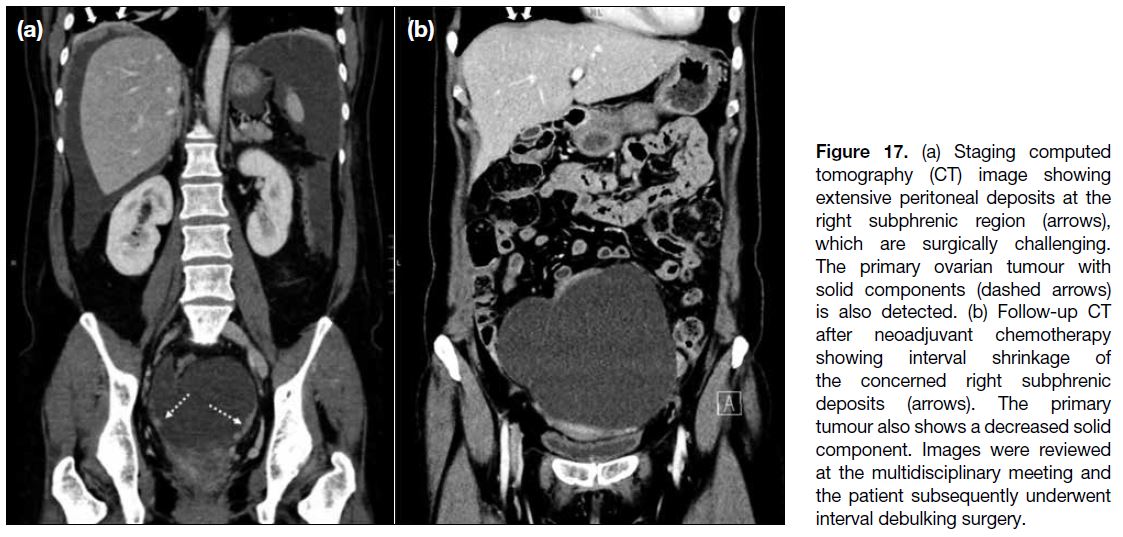
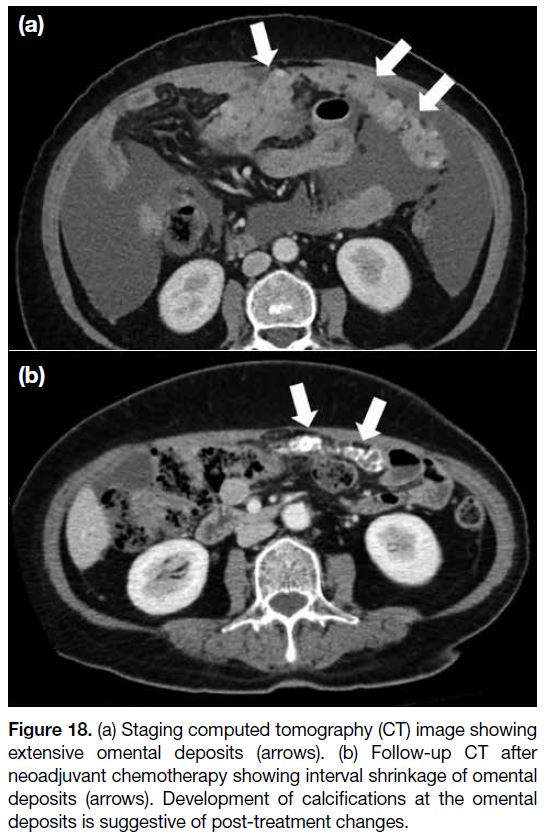
Assessment for Interval Debulking Surgery
At our institution, follow-up imaging is performed after
three cycles of neoadjuvant chemotherapy in patients with inoperable disease. Images are reviewed at the
multidisciplinary meeting for consideration of interval
debulking surgery (Figures 17 and 18).
Figure 17. (a) Staging computed tomography (CT) image showing extensive peritoneal deposits at the right subphrenic region (arrows), which are surgically challenging. The primary ovarian tumour with solid components (dashed arrows) is also detected. (b) Follow-up CT after neoadjuvant chemotherapy showing interval shrinkage of the concerned right subphrenic deposits (arrows). The primary tumour also shows a decreased solid component. Images were reviewed at the multidisciplinary meeting and the patient subsequently underwent interval debulking surgery.
Figure 18. (a) Staging computed tomography (CT) image showing
extensive omental deposits (arrows). (b) Follow-up CT after
neoadjuvant chemotherapy showing interval shrinkage of omental
deposits (arrows). Development of calcifications at the omental
deposits is suggestive of post-treatment changes.
Cancer of the ovaries, fallopian tubes and peritoneum
share similar morphological and clinical features. In
patients with advanced disease, a tubal or ovarian origin
can be difficult to delineate because tumour growth may
obscure the primary site.[6] Preoperative assessment for
these patients should be similar.
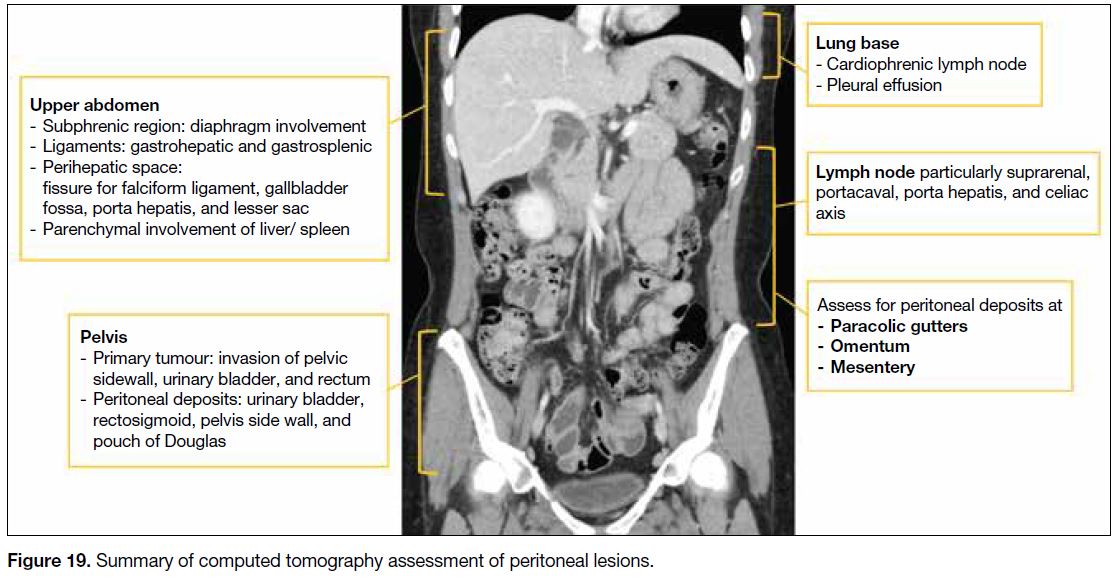
Figure 19 summarises CT assessment of peritoneal
lesions and the Table lists potentially non-resectable disease.
Figure 19. Summary of computed tomography assessment of peritoneal lesions.
Table. Summary of potentially non-resectable diseases.
CONCLUSION
Pretreatment imaging is helpful to evaluate abdominopelvic disease burden in patients with ovarian,
fallopian tube and peritoneal cancers, and to identify
patients at risk of having suboptimal debulking surgery
in whom laparotomy would be futile. It has been shown
that CT has high accuracy in detecting peritoneal lesions.
Assessment of peritoneal involvement can be improved
by understanding the peritoneal anatomy, route of tumour
dissemination, as well as common sites of peritoneal
deposits. The role of radiologists in the multidisciplinary
team is to alert clinicians to the presence of lesions that
may complicate surgery or preclude optimal debulking.
Patient-centred management should be discussed
at the multidisciplinary meeting to decide which of
cytoreductive surgery or neoadjuvant chemotherapy is
most appropriate.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I,
Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 2021;71:209-49. Crossref
2. Hong Kong Cancer Registry. Overview of Hong Kong Cancer
Statistics of 2020. Hong Kong Hospital Authority; Oct 2021.
Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202020.pdf.
Assessed 26 Apr 2023.
3. Berek J, Kehoe ST, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet. 2018;143 Suppl 2:59-78. Crossref
4. Forstner R, Sala E, Kinkel K, Spencer J; European Society of Urogenital Radiology. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20:2773-80. Crossref
5. Saida T, Tanaka YO, Matsumoto K, Satoh T, Yoshikawa H, Minami M. Revised FIGO staging system for cancer of the
ovary, fallopian tube, and peritoneum: important implications for radiologists. Jpn J Radiol. 2015;34:117-24. Crossref
6. Javadi S, Ganeshan DM, Qayyum A, Iyer RB, Bhosale P. Ovarian
cancer, the revised FIGO staging system, and the role of imaging.
AJR Am J Roentgenol. 2016;206:1351-60. Crossref
7. Nougaret S, Addley HC, Colombo PE, Fujii S, Al Sharif SS, Tirumani SH, et al. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics. 2012;32:1775-800. Crossref
8. Falzone L, Scandurra G, Lombardo V, Gattuso G, Lavoro A, Distefano AB, et al. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int J Oncol. 2021;59:53. Crossref
9. Spencer JA. A multidisciplinary approach to ovarian cancer at diagnosis. Br J Radiol. 2005;78 Spec No 2:S94-102. Crossref
10. Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40:371-7. Crossref
11. Ahmed SA, Abou-Taleb H, Yehia A, El Malek NA, Siefeldein GS, Badary DM, et al. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal
carcinomatosis index score in primary ovarian cancer. Acad Radiol.
2019;26:1650-8. Crossref
12. Pannu HK, Bristow RE, Montz FJ, Fishman EK. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics. 2003;23:687-701. Crossref
13. Sahdev A. CT in ovarian cancer staging: how to review and report with emphasis on abdominal and pelvic disease for surgical planning. Cancer Imaging. 2016;16:19. Crossref
14. Sugarbaker PH. Surgical responsibilities in the management of
peritoneal carcinomatosis. J Surg Oncol. 2010;101:713-24. Crossref
15. Kolev V, Mironov S, Mironov O, Ishill N, Moskowitz CS, Gardner GJ, et al. Prognostic significance of supradiaphragmatic lymphadenopathy identified on preoperative computed tomography
scan in patients undergoing primary cytoreduction for advanced
epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20:979-84. Crossref