An Occult Androgen-Secreting Ovarian Tumour Revealed by NP-59 Scintigraphy: a Case Report
CASE REPORT
Hong Kong J Radiol 2023 Jun;26(2):133-7 | Epub 10 May 2023
An Occult Androgen-Secreting Ovarian Tumour Revealed by NP-59 Scintigraphy: a Case Report
SH Kwok, WT Ngai
Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr SH Kwok, Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR,
China. Email: ksh727@ha.org.hk
Submitted: 4 Sep 2022; Accepted: 15 Nov 2022.
Contributors: Both authors designed the study. SHK acquired the data. Both authors analysed the data. SHK drafted the manuscript. WTN
critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: Both authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: Ethics approval has been obtained from the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong
Kong (Ref No.: HKECREC-2022-038). Patient consent was waived by the Committee.
INTRODUCTION
Androgen-secreting tumours constitute a rare but
important cause of hyperandrogenism, the possibility of
which needs to be considered and excluded in patients
with postmenopausal, severe, or rapidly progressive
hyperandrogenism. Conventional anatomical
imaging may help localise the source of androgen
hypersecretion but is occasionally inconclusive. We
describe a postmenopausal Chinese female with
severe hyperandrogenism whose initial investigations
were unrevealing. Iodine-131 6-beta-iodomethyl-19-norcholesterol (NP-59) scintigraphy successfully
localised an occult, small androgen-secreting ovarian
steroid cell tumour that was resected with subsequent
resolution of hyperandrogenism.
CASE REPORT
A 49-year-old Chinese female presented with a 2-year
history of hirsutism. She had early menopause at the
age of 41 years but medical history was otherwise
unremarkable. Clinical examination revealed hirsutism,
male-pattern alopecia and facial acnes, while breasts and
external genitalia were normal. She was also found to be hypertensive with blood pressure measuring around
170/110 mmHg. Hormonal profile revealed markedly
elevated testosterone level of up to 33.9 nmol/L,
more than 13 times the upper limit of normal level
(<2.6 nmol/L). The rest of the hormonal profile and
tumour marker panel were unremarkable. Imaging
investigations to localise any androgen-secreting tumour
were performed.
Transvaginal ultrasonography visualised a uterus of
6-week size, but the ovaries were not clearly seen.
Contrast-enhanced computed tomography of the
abdomen and pelvis did not reveal any adrenal or
adnexal lesions, but several enhancing uterine nodules
up to 1.6 cm, thought to be fibroids, were seen. Further
18F-fluorodeoxyglucose positron emission tomography
was also negative.
A dexamethasone-suppressed NP-59 scintigraphy was
subsequently performed with intravenous administration
of 37 MBq of NP-59. To suppress physiological adrenal
uptake, oral dexamethasone 1 mg was prescribed 4 times
daily for 13 days, starting 7 days before NP-59 injection. Planar scintigraphic images of the abdomen and pelvis
were acquired from day 3 to 7 post-injection. Additional
single-photon emission computed tomography–computed tomography (SPECT-CT) images were
acquired on days 4 and 7. A positive finding was indicated
by early visualisation of focal NP-59 uptake before
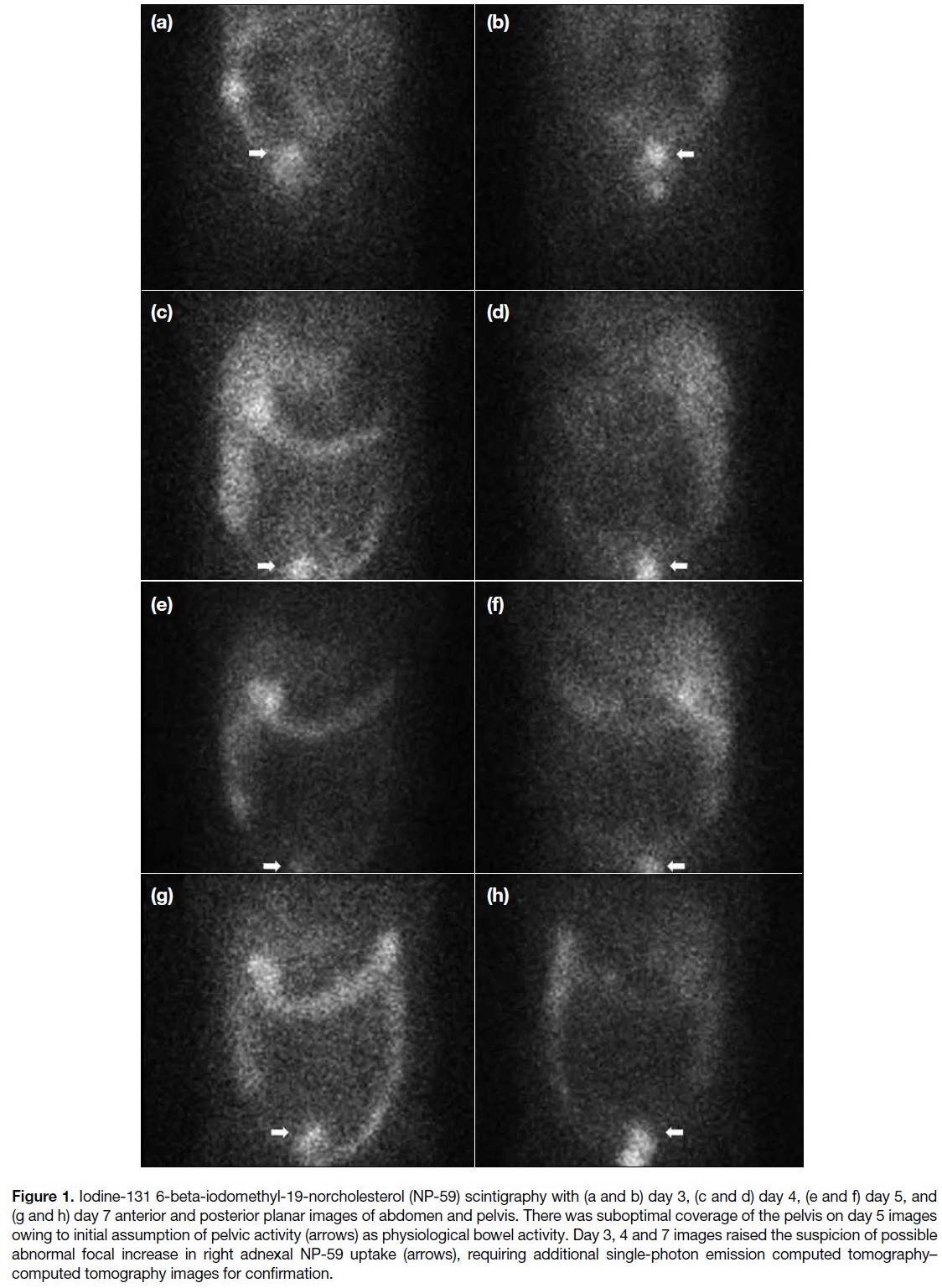
day 5. Planar scintigraphic images from day 3 showed
suspicious focal pelvic NP-59 uptake which persisted
until day 7 (Figure 1), and a right adnexal lesion with
NP-59 uptake was confirmed on SPECT-CT (Figure 2).
Figure 1. Iodine-131 6-beta-iodomethyl-19-norcholesterol (NP-59) scintigraphy with (a and b) day 3, (c and d) day 4, (e and f) day 5, and
(g and h) day 7 anterior and posterior planar images of abdomen and pelvis. There was suboptimal coverage of the pelvis on day 5 images
owing to initial assumption of pelvic activity (arrows) as physiological bowel activity. Day 3, 4 and 7 images raised the suspicion of possible
abnormal focal increase in right adnexal NP-59 uptake (arrows), requiring additional single-photon emission computed tomography–computed tomography images for confirmation.
Figure 2. Iodine-131 6-beta-iodomethyl-19-norcholesterol (NP-59) scintigraphy with day 4 (a-c) coronal computed tomography (CT), single-photon
emission computed tomography (SPECT) and fused SPECT-CT images, (d-f) sagittal CT, SPECT and fused SPECT-CT images, and
(g-i) axial CT, SPECT and fused SPECT-CT images of pelvis. SPECT and fused images show an abnormal focal increase in NP-59 uptake
at the right adnexal region (arrows) that could be distinguished from adjacent physiological large bowel activity.
The patient underwent bilateral salpingo-oophorectomy.
During the operation, the right ovary was found to be
enlarged with a 2-cm unilocular cyst containing chocolate
material. Histological findings of the right ovary were
consistent with the presence of a small steroid cell
tumour with no malignant features. Following removal
of the tumour, her serum testosterone level normalised
with resolution of virilising features. She also became
normotensive.
DISCUSSION
Hyperandrogenism may manifest clinically as hirsutism
and virilisation. Hirsutism is defined as excessive
terminal hair that appears in a male pattern in women
such as on the chin, upper lip or abdomen. Virilisation
includes clinical features of more significant androgen
excess such as clitoromegaly, deepening of the voice or
increasing muscularity.[1] Causes of hyperandrogenism
can be non-tumourous, such as polycystic ovarian
syndrome, congenital adrenal hyperplasia, ovarian
hyperthecosis, obesity, endocrinopathies, or iatrogenic;
such causes can also be tumourous, such as adrenal or
ovarian tumours.[2] Androgen-secreting tumours constitute
a rare (5.8%) cause of hyperandrogenism although they
are relatively more prevalent in postmenopausal (21.4%)
than premenopausal women (2.0%).[3]
A clinical diagnostic algorithm for investigation of
hyperandrogenism commonly includes adrenal and/or ovarian imaging to exclude an androgen-secreting
tumour, especially in case of onset after menopause,
severe clinical and/or biochemical hyperandrogenism,
rapid progression, or presence of virilisation. In particular,
very high serum testosterone (>150-200 ng/dL)
and dehydroepiandrosterone sulphate (>6000 ng/mL)
levels favour an androgen-secreting tumour of ovarian
or adrenal origin, respectively.[2]
Ultrasonography and/or magnetic resonance imaging
(MRI) are recommended imaging modalities to identify ovarian tumours.[2] Nonetheless androgen-secreting
ovarian tumours may be difficult to detect if they are small
in size. A recent study reported that ultrasonography
and MRI failed to detect four of 31 androgen-secreting
ovarian tumours (12.9%), ranging from 0.7 to 1.5 cm.[4]
Although CT and MRI are recommended for detection
of adrenal tumours,[2] incidental adrenal masses are
common and may occur in 3% to 7% of adults, most of
which are benign non-functioning adenoma.[5] Combined
ovarian and adrenal vein sampling may be considered
if ultrasonography, CT and MRI have failed to localise
the androgen-secreting tumours, although its application
has not been proven to reliably alter management. The
success rate for catheterisation of all four veins, i.e.,
bilateral adrenal and ovarian veins, has been reported to
be only 27%; hence, this technically difficult procedure
may be considered only in centres with expertise.[6]
Successful identification of androgen-secreting
tumours with 18F-fluorodeoxyglucose positron emission
tomography has been reported in only a few isolated cases.[7]
The application of NP-59 in functional imaging
commenced in the mid-1970s.[8] Steroid hormone synthesis initiates with arrival of cholesterol in
adrenocortical cells by low-density lipoprotein. Twenty
percent of NP-59 is incorporated in low-density
lipoprotein and deposited in adrenocortical cells by a
specific receptor, which does not follow the metabolic
process and thus concentrates in the adrenocortical
cells. This allows scintigraphic localisation of the
hypersecreting adrenal and ovarian tumours in primary
hyperaldosteronism, Cushing’s syndrome, and
hyperandrogenism. Previous case studies demonstrated
the usefulness of NP-59 scintigraphy in localising both
tumourous and non-tumourous ovarian and adrenal
sources of androgen excess.[9] [10] [11] Among the reported
cases, unilateral uptake was seen in androgen-secreting
ovarian and adrenal tumours, and bilateral ovarian or
adrenal uptake was seen in ovarian hyperthecosis and
congenital adrenal hyperplasia. Normal scintigraphy
was seen in peripheral conversion and increased end-organ
sensitivity, while absent uptake (i.e., loss of
normal physiological uptake) was seen in adrenocortical
carcinoma. The unique role of NP-59 scintigraphy
in localising the site of androgen hypersecretion was
highlighted in two of the reported cases of adrenal
hyperandrogenism, where the incidental abnormalities
show absence of uptake. One patient had an adrenal
lipoid cell tumour detected on CT. The other had
congenital adrenal hyperplasia with adrenal glands appearing normal on CT. Both had incidental findings
of ovarian masses, subsequently confirmed to be
polycystic ovaries that were not contributory to the
degree of hyperandrogenism. No false-positive NP-59
scintigraphic findings for hyperandrogenism have been
reported in the English literature to date. Due to the rarity
of this clinical condition, data on the diagnostic accuracy
of NP-59 scintigraphy in hyperandrogenism are scarce.
As presence of intense physiological activity along
the large bowel and its close proximity to androgen-secreting
ovarian tumours hampers evaluation by NP-59 scintigraphy, preprocedural oral laxatives for bowel
preparation have been recommended.[12] The availability
of hybrid SPECT-CT technology, in addition to planar
imaging, allows accurate delineation of any focal
abnormal adnexal uptake from adjacent large bowel
activity. The usefulness of SPECT-CT is well illustrated in this case where the focal abnormal adnexal uptake was
difficult to appreciate on serial planar images but could
be confirmed on SPECT-CT images.
There are several drawbacks to the widespread use of
NP-59 scintigraphy in evaluation of hyperandrogenism.
These include suboptimal image quality with
iodine-131, relatively high radiation, prolonged
imaging time, relatively high radiopharmaceutical cost,
and potential adverse effects associated with use of
high-dose dexamethasone as a pre-medication. Thus,
NP-59 scintigraphy is often reserved for patients with
clinical and biochemical evidence of ovarian or adrenal
hypersecretion where conventional anatomical imaging
has been unrevealing. A 18F version of NP-59 is being
developed for positron emission tomography imaging
with promising initial data in imaging cholesterol
trafficking and, specifically, uptake in adrenocortical tissue.[13] It is expected that this 18F version of NP-59 will
become available for clinical use in the near future and
provide higher image quality with lower radiation dose
to help localise the site of hormone hypersecretion.
CONCLUSION
Accurate localisation of the source of androgen
hypersecretion is critical to appropriate management
in patients with suspected androgen-secreting tumours.
This case report highlights the unique role of NP-59
scintigraphy in providing functional information and
localising the site of androgen hypersecretion, which
may not have been achievable by other non-invasive
investigations. It is an indispensable and time-honoured
nuclear medicine procedure that produces the most
significant and conclusive results in such situations.
Nevertheless the limitations of NP-59 scintigraphy limit
its use to problem-solving rather than screening purposes.
It is especially helpful in selected patients where there is
a high suspicion of ovarian or adrenal hypersecretion but
inconclusive conventional anatomical imaging.
REFERENCES
1. Martin KA, Anderson RR, Chang RJ, Ehrmann DA, Lobo RA, Murad MH, et al. Evaluation and treatment of hirsutism in
premenopausal women: an Endocrine Society clinical practice
guideline. J Clin Endocrinol Metab. 2018;103:1233-57. Crossref
2. Markopoulos MC, Kassi E, Alexandraki KI, Mastorakos G, Kaltsas G. Hyperandrogenism after menopause. Eur J Endocrinol.
2015;172:R79-91. Crossref
3. Elhassan YS, Idkowiak J, Smith K, Asia M, Gleeson H, Webster R, et al. Causes, patterns, and severity of androgen excess in 1205 consecutively recruited women. J Clin Endocrinol Metab.
2018;103:1214-23. Crossref
4. Zou M, Chen R, Wang Y, He Y, Wang Y, Dong Y, et al. Clinical and ultrasound characteristics of virilizing ovarian tumors in pre- and postmenopausal patients: a single tertiary center experience.
Orphanet J Rare Dis. 2021;16:426. Crossref
5. Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, et al. Management of incidental adrenal masses: a
white paper of the ACR Incidental Findings Committee. J Am Coll
Radiol. 2017;14:1038-44. Crossref
6. Zaman A, Rothman MS. Postmenopausal hyperandrogenism: evaluation and treatment strategies. Endocrinol Metab Clin North Am. 2021;50:97-111. Crossref
7. Wong FC, Chan AZ, Wong WS, Kwan AH, Law TS, Chung JP, et al. Hyperandrogenism, elevated 17-hydroxyprogesterone and
its urinary metabolites in a young woman with ovarian steroid
cell tumor, not otherwise specified: case report and review of the
literature. Case Rep Endocrinol. 2019;2019:9237459. Crossref
8. Prado-Wohlwend S; Grupo de Trabajo de Endocrinología de la SEMNIM. Functional imaging studies of the adrenal cortex. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2020;39:393-404. Crossref
9. Taylor L, Ayers JW, Gross MD, Peterson EP, Menon KM. Diagnostic considerations in virilization: iodomethyl-norcholesterol
scanning in the localization of androgen secreting tumors. Fertil
Steril. 1986;46:1005-10. Crossref
10. Mountz JM, Gross MD, Shapiro B, Barkan AL, Woodbury MC, Schteingart DE, et al. Scintigraphic localization of ovarian
dysfunction. J Nucl Med. 1988;29:1644-50.
11. Kazerooni EA, Sisson JC, Shapiro B, Gross MD, Driedger A, Hurwitz GA, et al. Diagnostic accuracy and pitfalls of [iodine-131]6-beta-iodomethyl-19-norcholesterol (NP-59) imaging. J Nucl
Med. 1990;31:526-34.
12. Shapiro B, Nakajo M, Gross MD, Freitas JE, Copp J, Beierwaltes WH. Value of bowel preparation in adrenocortical
scintigraphy with NP-59. J Nucl Med. 1983;24:732-4.
13. Brooks AF, Winton WP, Stauff J, Arteaga J, Henderson B, Niedbala J, et al. Development of flourinated NP-59: a revival of
cholesterol use imaging with PET. J Nucl Med. 2022;63:1949-55. Crossref