Incidental Computed Tomography Angiography Finding of a Delayed Asymptomatic Ascending Aortic Dissection after Transcatheter Aortic Value Implantation: a Case Report
CASE REPORT
Incidental Computed Tomography Angiography Finding of a Delayed Asymptomatic Ascending Aortic Dissection after Transcatheter Aortic Value Implantation: a Case Report
L Procaccini1,2, A Bernardini2, A Costanzi2, E Algeri2, A Gennarelli2, E Mincuzzi1,2, N Caputo2
1 Department of Neuroscience and Imaging and Clinical Sciences, Institute of Radiology, Section of Diagnostic Imaging and Therapy-Radiology Division, G. d’Annunzio University, Chieti-Pescara, Italy
2 Department of Radiology, G. Mazzini Hospital, Teramo, Italy
Correspondence: Dr L Procaccini, Department of Neuroscience and Imaging and Clinical Sciences, Institute of Radiology, Section of Diagnostic Imaging and Therapy-Radiology Division, G. d’Annunzio University, Chieti-Pescara, Italy. Email: luca.procaccini93@gmail.com
Submitted: 31 Jul 2021; Accepted: 24 Nov 2021.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki and has provided written informed consent for all treatments, procedures, and publication.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI), also
referred to as transcatheter aortic valve replacement,
is suitable for patients with severe symptomatic aortic
stenosis who are not eligible for surgical aortic valve
replacement due to high surgical risk. More recent
clinical trials have determined that TAVI is also
indicated in intermediate-risk patients and could be a
potential therapeutic choice in low-risk patients.[1] [2] The
transfemoral approach remains the preferred access
route. Vascular complications account for 2% to 17%
of all adverse events and ascending aortic dissection
(AAD) is reported in only 0.2% to 0.3% of patients.[3] We present a case of asymptomatic AAD in a patient who
underwent TAVI in 2020.
CASE REPORT
Patient Information and Clinical Findings
An asymptomatic 84-year-old woman presented to our hospital for a routine cardiological check-up to
evaluate the possibility of transcatheter tricuspid valve
repair with TriClip system (Abbott Structural Heart,
Santa Clara [CA], United States). On presentation she
was afebrile with blood pressure 150/80 mmHg, pulse
75 beats per minute and oxygen saturation 90% with
oxygen support (2 L/min). She was alert and oriented
to time, place, and person. Her medical history was
significant for hypertension, severe aortic stenosis
with a planimetric aortic valve area of 0.8 cm2, chronic
atrial fibrillation, chronic obstructive pulmonary
disease, and osteoporosis. Her surgical history revealed
previous hysteroannessiectomy for uterine fibroids and
conservative surgery and irradiation (quadrantectomy,
axillary dissection, and radiotherapy) for left-sided
breast cancer.
She had undergone TAVI in 2020 (aortogram
not available in our local picture archiving and communication system) with a CoreValve prosthetic
aortic valve (Medtronic, Minneapolis [MN], United
States), as well as invasive assessment of the coronary
tree that revealed non-significant stenosis. No immediate
complications following TAVI were reported. A
voluminous iatrogenic active bleeding haematoma at the
right access site developed 10 days after the procedure;
unfortunately, computed tomography (CT) performed
during the management of this complication did not
include review of the prosthetic valve. As a result, it
became necessary to admit her to our Interventional
Radiology Unit. A superselective angiogram of two
distal branches of the right deep femoral artery directed
towards the haematoma was performed via a 2.8-French
Progreat microcatheter (Terumo Medical Corporation,
Japan) and embolisation was successful with 500-μm
Embozene microspheres (CeloNova Biosciences,
Newnan [GA], United States). She was discharged from
hospital with no further complications until the current
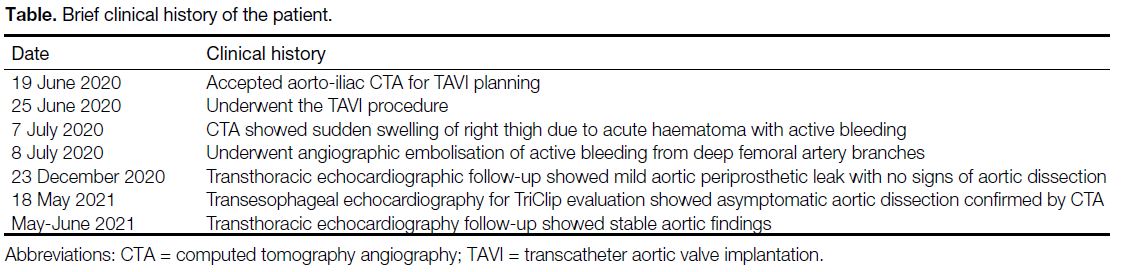
admission (Table).
Table. Brief clinical history of the patient.
Diagnostic Assessment and Therapeutic Intervention
Transoesophageal echocardiography surprisingly
showed an ascending aortic aneurysm with an intimal
flap. The explorable descending aorta was intact and
no significant pericardial effusion was depicted. The
prosthetic aortic valve was in situ and a mild anterior
paravalvular leak was demonstrated. Collateral
findings included left ventricular hypertrophy with
normal global systolic function (ejection fraction:
58%), moderate mitral regurgitation, severe tricuspid
regurgitation (end-diastolic tricuspid valve annulus
46 mm in 4-chamber view) and consequent severe left
and, mostly, right atrial dilatation. At the end of the
examination a rapid electrocardiogram was performed
and highlighted the known atrial fibrillation, left
anterior fascicular block and a pulse rate of 80 beats
per minute. No significant difference in blood pressure was recorded (155/85 mmHg). The patient was instantly
escorted to the emergency department. A retrospective
electrocardiogram-gated thoracoabdominal CT
angiography performed on a Brilliance 64-slice scanner
(Philips, The Netherlands) confirmed the presence of
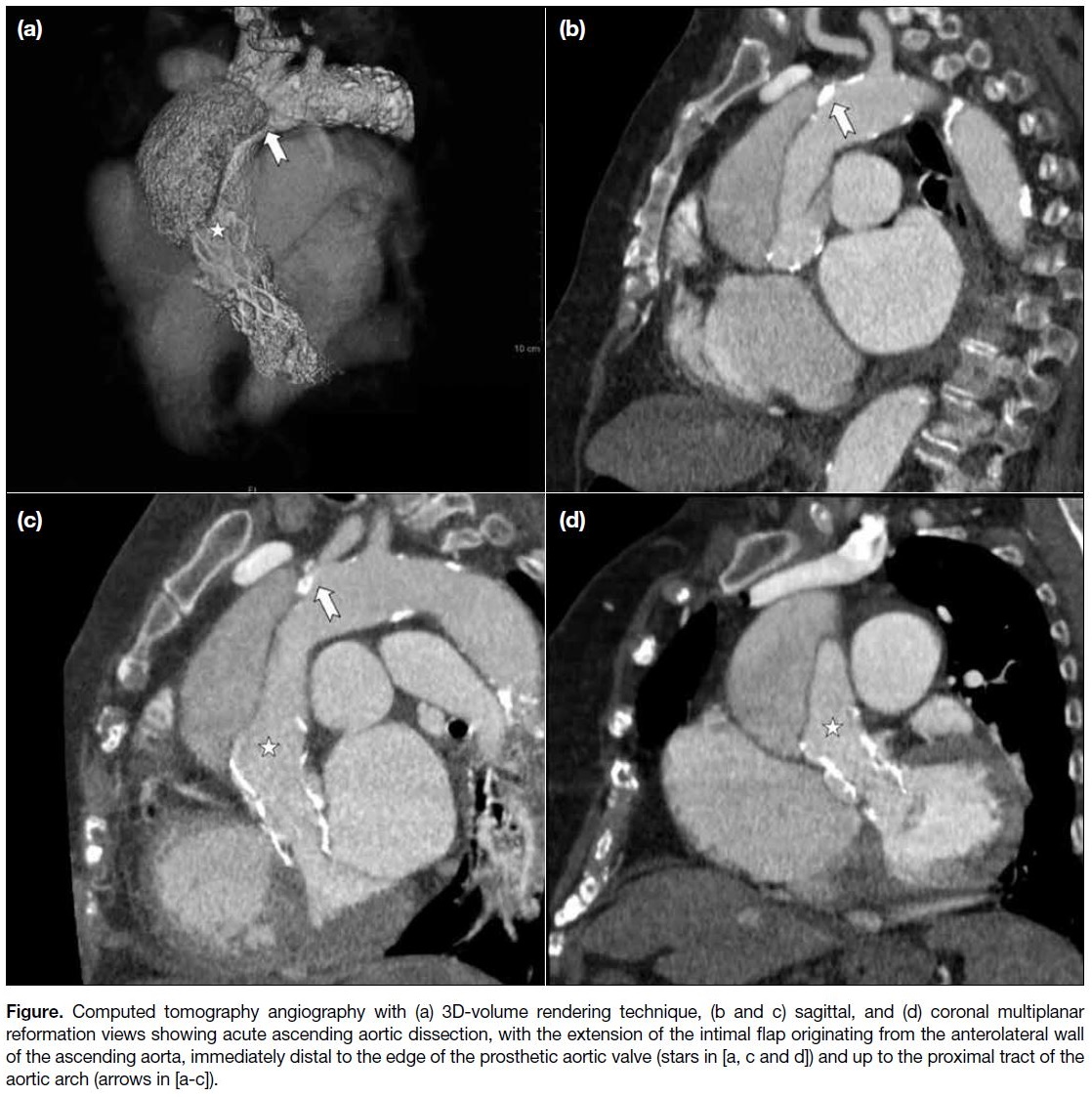
intimal flap originating from the anterolateral wall of
the ascending aorta, immediately distal to the rim of the
prosthetic valve and extending up to the proximal tract of
the aortic arch (Figure). Coronary arteries and epiaortic
vessels were not involved. No direct or indirect sign
of aortic rupture was evident and low attenuation near
water density effusion was assessed in the pericardial
recesses. Mean ascending aortic and true lumen
diameters were 58 mm and 20 mm, respectively. The
patient was hospitalised but only conservative treatment
was indicated since she was at high surgical risk and had
no symptoms.
Figure. Computed tomography angiography with (a) 3D-volume rendering technique, (b and c) sagittal, and (d) coronal multiplanar reformation views showing acute ascending aortic dissection, with the extension of the intimal flap originating from the anterolateral wall of the ascending aorta, immediately distal to the edge of the prosthetic aortic valve (stars in [a, c and d]) and up to the proximal tract of the aortic arch (arrows in [a-c]).
Follow-up and Outcomes
The patient remained haemodynamically stable on
medical therapy and was discharged from hospital
after 1 week with no apparent deficits. The follow-up
plan for this patient included close monitoring through
echocardiography that consistently demonstrated
a haemodynamically stable condition. One-month
follow-up of true and false lumen dimensions and of the
extension of the intimal flap demonstrated no change.
CT angiography was not advised due to the probability
of kidney injury. The patient continues to be kept
under surveillance through follow-up examinations and
echocardiography. No alarming signs or symptoms have
emerged to date.
DISCUSSION
AAD following TAVI is a rare but life-threatening
complication that usually occurs during the procedure.[4]
Damage to the ascending aortic wall can result from
mispositioning of the prosthetic valve, guidewire and/or delivery system manipulation, and the excessive amount of eccentric calcifications in the left ventricular
outlet tract. Delayed AAD has been infrequently
described as a complication of TAVI. In our case,
transthoracic echocardiography performed about 6
months after TAVI revealed the prosthetic valve in situ
and a mild paravalvular leak. This finding suggests that
the asymptomatic AAD was more likely a delayed and
unexpected complication rather than a previously missed
intraoperative complication. Losmanova et al[5] reported a
case of acute aortic dissection in an 81-year-old patient
3 years after TAVI, while Gerber et al[6] outlined two post-mortem examinations that determined AAD as
a cause of death at 6 and 22 days following TAVI. In
none of these cases was the asymptomatic presentation
highlighted. In this respect, the International Registry
of Acute Aortic Dissection found that 6.3% of AADs
were painless.[7] Although asymptomatic presentation has
been described in isolated case reports,[8] [9] Imamura et al[10]
showed that the true percentage of asymptomatic AAD
cases was higher (17%). In these studies, patients with
painless AAD were more likely to present with impaired
consciousness or stroke and have a higher mortality risk than AAD patients who experienced pain. Aoyama et
al[11] compared the results of conservative (medical) and
surgical treatment in an elderly population with AAD
and demonstrated that although all-cause in-hospital
death was significantly lower in surgical patients, there
was no significant difference in event-free survival. In
view of the rarity of AAD following TAVI and its mode
of presentation, we believe that follow-up protocols for
patients post-TAVI will not change, at least at our hospital.
There is no consensus to perform routine CT angiography
after TAVI.[12] [13] [14] CT can be useful to depict and evaluate
hypo-attenuated leaflet thrombosis if echocardiography
is suspicious. Transthoracic echocardiography is the
technique of choice for evaluating the prosthetic valve,
whereas CT may be appropriate for some cases.
In conclusion, AAD represents a potentially fatal
condition and may be encountered more frequently with
the current increase in number of TAVI procedures.
A high index of suspicion should be maintained in
symptomatic and asymptomatic patients.
REFERENCES
1. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med.
2017;376:1321-31. Crossref
2. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-15. Crossref
3. Naik M, McNamara C, Jabbour RJ, Gopalan D, Mikhail GW, Mirsadraee S, et al. Imaging of transcatheter aortic valve
replacement complications. Clin Radiol. 2021;76:27-37. Crossref
4. Holmes DR Jr, Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019-28. Crossref
5. Losmanova T, Tosoni I, Fahrni S, Ballmer PE. Autopsy case of aortic dissection after transcatheter aortic valve implantation (TAVI). BMJ Case Rep. 2018;2018:bcr2017220105. Crossref
6. Gerber RT, Osborn M, Mikhail GW. Delayed mortality from aortic dissection post transcatheter aortic valve implantation (TAVI): the tip of the iceberg. Catheter Cardiovasc Interv. 2010;76:202-4. Crossref
7. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Di
Eusanio M, Sechtem U, et al. Insights from the International
Registry of Acute Aortic Dissection: a 20-year experience of
collaborative clinical research. Circulation. 2018;137:1846-60. Crossref
8. Cohen R, Mena D, Carbajal-Mendoza R, Arole O, Mejia JO. A case report on asymptomatic ascending aortic dissection. Int J Angiol. 2008;17:155-61. Crossref
9. Chan JC, Fung SY, Ching OH, Lee KC, Cheung CW, Ng MY. Painless asymptomatic ascending aortic dissection with four-dimensional flow magnetic resonance imaging: a case report. Hong
Kong J Radiol. 2021;24:125-8. Crossref
10. Imamura H, Sekiguchi Y, Iwashita T, Dohgomori H, Mochizuki K, Aizawa K, et al. Painless acute aortic dissection. Diagnostic, prognostic and clinical implications. Circ J. 2011;75:59-66. Crossref
11. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg.
2018;13:129. Crossref
12. Soschynski M, Capilli F, Ruile P, Neumann FJ, Langer M, Krauss T. Post-TAVI follow-up with MDCT of the valve prosthesis: technical application, regular findings and typical local post-interventional complications. Rofo. 2018;190:521-30. Crossref
13. Salgado RA, Budde RP, Leiner T, Shivalkar B, Van Herck PL,
Op de Beeck BJ, et al. Transcatheter aortic valve replacement:
postoperative CT findings of Sapien and CoreValve transcatheter
heart valves. Radiographics. 2014;34:1517-36. Crossref
14. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter
J, Jilaihawi H, et al. Computed tomography imaging in the context
of transcatheter aortic valve implantation (TAVI)/transcatheter
aortic valve replacement (TAVR): an expert consensus document
of the Society of Cardiovascular Computed Tomography. JACC
Cardiovasc Imaging. 2019;12:1-24. Crossref