Underdiagnosed Wernicke’s Encephalopathy in Children: Spectrum of Imaging Findings in Three Local Cases
PICTORIAL ESSAY
Underdiagnosed Wernicke’s Encephalopathy in Children: Spectrum of Imaging Findings in Three Local Cases
YS Lee1, KC Wong2, EYL Kan2
1 Department of Radiology, Tuen Mun Hospital, Hong Kong
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong
Correspondence: Dr YS Lee, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: lys273@ha.org.hk
Submitted: 27 Jun 2021; Accepted: 5 Oct 2021.
Contributors: YSL designed the study. KCW acquired the data. YSL analysed the data and drafted the manuscript. EYLK critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by HKCH Research Ethics Committee (Ref No.: HKCH-REC-2021-021) and conducted in
compliance with the Declaration of Helsinki.
INTRODUCTION
Although vitamin B1 deficiency is increasingly being
recognised in ill adults, Wernicke’s encephalopathy
(WE) remains underrecognised in children. We present
three local cases of paediatric WE observed over a 2-year period.
Importantly, WE was not considered a primary
differential diagnosis at initial presentation. This article
aims to raise awareness of paediatric WE since time
from recognition to thiamine replacement determines
prognosis and mortality. We also illustrate some of its
magnetic resonance imaging (MRI) findings to promote
early radiological detection of the disease.
CASE REPORTS
Three patients with WE, aged between 12 and 17 years,
are described. All three patients were prescribed total
parenteral nutrition (TPN) prior to development of WE.
All were examined with MRI. In all cases, diagnosis was
confirmed by symptom regression, serum transketolase
increase, and/or improved MRI findings following thiamine administration. Details of these three cases are
illustrated in the Table.
Table. Case summary.
Case 1
A 12-year-old boy was undergoing chemotherapy for
osteosarcoma. One week prior to presentation, he was
started on TPN due to recurrent severe vomiting. He
then developed confusion and athetoid movements.
Computed tomography revealed subtle hypodensity in
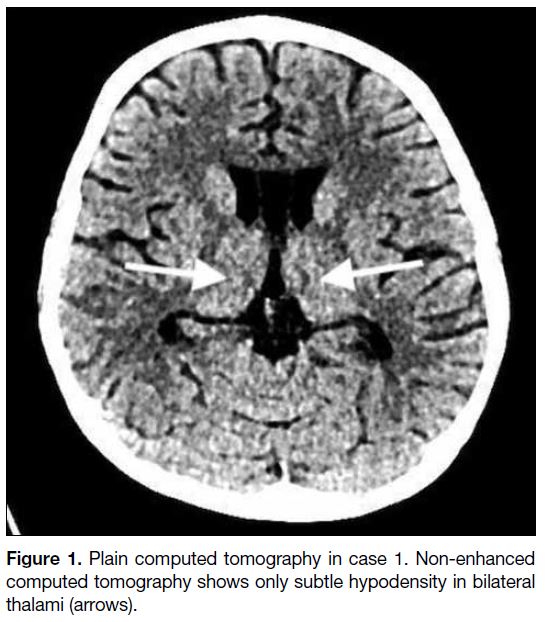
bilateral thalami (Figure 1). MRI revealed symmetrical
T2 hyperintensity and restricted diffusion at the
dorsomedial thalami (Figure 2). After the possibility of
WE was raised, he was given high doses of thiamine
(>1000 mg daily) and slowly resumed oral feeding.
Clinically, the child regained his usual functional and
neurological status with no residual neurological deficits.
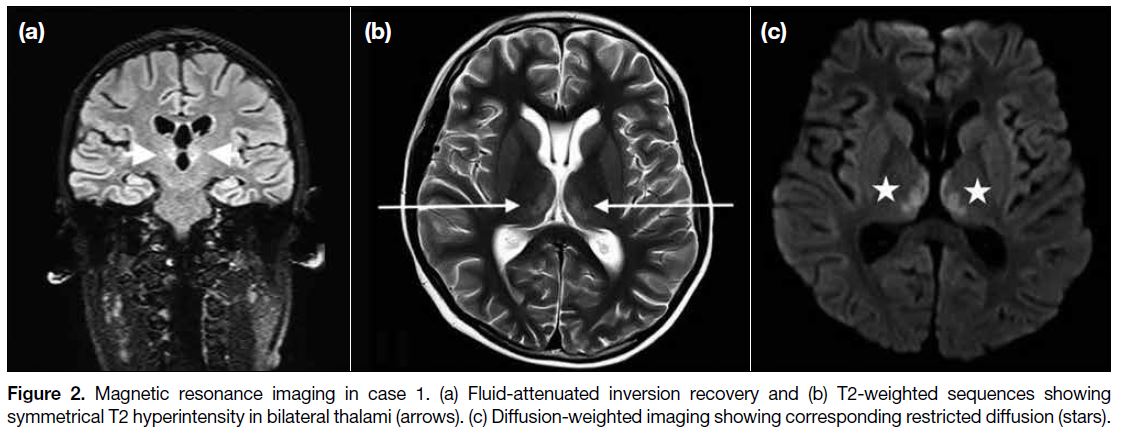
Follow-up MRI 6 months later showed resolution of the
thalamic signal abnormalities (Figure 3).
Figure 1. Plain computed tomography in case 1. Non-enhanced computed tomography shows only subtle hypodensity in bilateral thalami (arrows).
Figure 2. Magnetic resonance imaging in case 1. (a) Fluid-attenuated inversion recovery and (b) T2-weighted sequences showing symmetrical T2 hyperintensity in bilateral thalami (arrows). (c) Diffusion-weighted imaging showing corresponding restricted diffusion (stars).
Figure 3. Follow-up magnetic
resonance imaging in case 1 after 6 months of presentation. There is resolution of thalamic signal abnormalities on both (a) T2-weighted and (b) diffusion-weighted imaging sequences.
Case 2
A 17-year-old girl was undergoing chemotherapy for
osteosarcoma. She presented insidiously with dullness and generalised flaccid tone after being on TPN for
2 months due to poor intake. She was admitted in a
decorticate posture, had fixated gaze and generalised
areflexia. MRI showed T2 hyperintensity and restricted
diffusion at mammillary bodies, dorsomedial thalami,
periaqueductal grey matter, and around the third
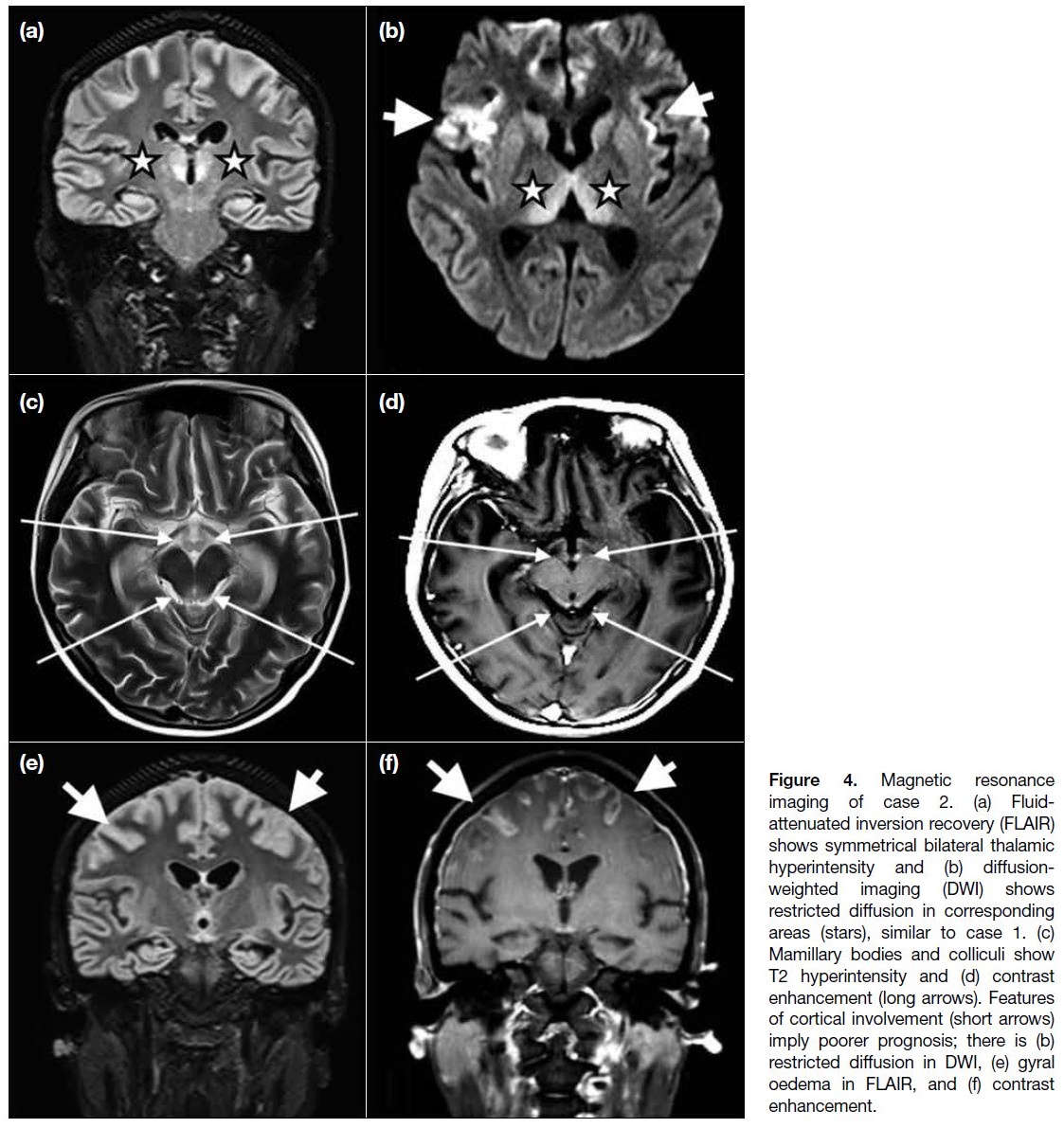
ventricle on fluid-attenuated inversion recovery. The mammillary bodies and colliculi showed subtle contrast
enhancement. Notably, signal abnormalities were also
detected in parts of bilateral cerebral cortices (Figure 4).
Thiamine (1500 mg daily) was given and enteral feeding
was resumed. Nonetheless despite improvement in
mental status, she regained very minimal voluntary
motor control and remained bedbound.
Figure 4. Magnetic resonance
imaging of case 2. (a) Fluid-attenuated inversion recovery (FLAIR) shows symmetrical bilateral thalamic hyperintensity and (b) diffusion-weighted imaging (DWI) shows restricted diffusion in corresponding areas (stars), similar to case 1. (c) Mamillary bodies and colliculi show T2 hyperintensity and (d) contrast enhancement (long arrows). Features of cortical involvement (short arrows) imply poorer prognosis; there is (b) restricted diffusion in DWI, (e) gyral oedema in FLAIR, and (f) contrast enhancement.
Case 3
A 12-year-old boy was on monoclonal antibody therapy
for recurrent atypical haemolytic uraemic syndrome. He
had experienced bouts of pancreatitis over the years and
was put on bowel rest and TPN episodically. He developed
lethargy, unsteady gait and vertical nystagmus 1 week
after the current episode of TPN. Compared with MRI
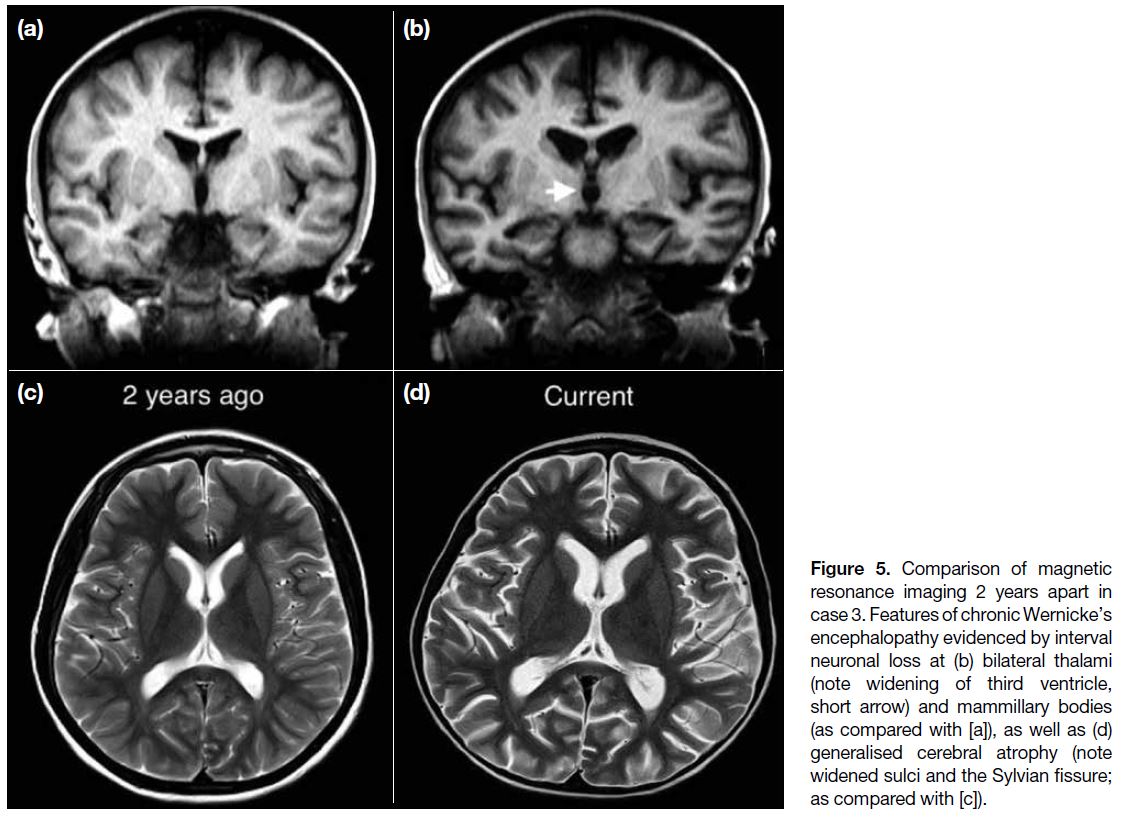
images 2 years previously, there was generalised cerebral
atrophy, with volume loss most significant at bilateral
thalami (evidenced by widening of the third ventricle),
mammillary bodies, colliculi, and hippocampi (Figure 5). Features and interval changes were highly suggestive
of chronic WE. This suspicion was substantiated by
interviews with the carer who reported past episodes of
abnormal behaviour in the form of disinhibition and limb
twitching. He was prescribed thiamine (1000 mg daily)
and resumed enteral feeding. His gait disturbance and eye
signs soon subsided but he remained underperforming in
academic and social aspects.
Figure 5. Comparison of magnetic resonance imaging 2 years apart in case 3. Features of chronic Wernicke’s encephalopathy evidenced by interval neuronal loss at (b) bilateral thalami (note widening of third ventricle, short arrow) and mammillary bodies (as compared with [a]), as well as (d) generalised cerebral atrophy (note widened sulci and the Sylvian fissure; as compared with [c]).
DISCUSSION
WE is a potentially fatal acute neuropsychiatric disease caused by thiamine (vitamin B1) deficiency. Thiamine,
in its biologically active form thiamine pyrophosphate, is
an essential coenzyme in several biochemical pathways
in the brain. The body’s reserve of thiamine can be
readily depleted over 2 to 3 weeks[1] after which brain
lesions develop, usually restricted to selective, vulnerable
regions with high thiamine content and turnover.
Epidemiology
Although WE is a relatively well-recognised disease
entity in alcoholic adults, it remains underrecognised
in children. There has been increasing academic and
clinical interest in WE in sick children over the last
two to three decades, but as many as 58% of paediatric
WE cases have been missed at clinical examination and recognised only on autopsy.[2] This lack of awareness of
WE in the paediatric patient group may be due to poor
clinical familiarity, non-classic presentation, and atypical
imaging features.
Thiamine deficiency in childhood is most often
associated with cancer.[2] Other recognised causes are
prolonged parenteral nutrition without supplementation
of thiamine, gastrointestinal surgery, and other systemic
diseases.[3] Seear et al[4] reported that as many as four of six
children undergoing chemotherapy, and 10 of 80 children
receiving intensive care were deficient in thiamine. Such
prevalence is much higher than previously believed;
therefore, clinical awareness and a low threshold of
suspicion are vital.
Clinical Presentation
Early detection of subclinical thiamine deficiency is
difficult as symptoms in children can be vague and
non-specific such as headache, fatigue, irritability, and
decline in growth rate.[1] Textbooks describe a classic triad
of confusion, ataxia and ophthalmoplegia in only 16% to
21% of adult patients at presentation, with up to 19% having none of these symptoms.[2] [5] More consistently
though, 82% of WE patients will experience some
degree of altered mental status ranging from confusion,
sluggishness and apathy to coma and death.[5] Other less
common presentations include stupor, hypotension and
tachycardia, hypothermia and seizures, all of which can
be related to insults to the hypothalami and thalami.[1]
Imaging Features
Neuroimaging is the most valuable method in diagnosing
WE. In all our patients, the radiologist was the first to
propose a diagnosis of WE.
A normal computed tomography of the brain cannot
exclude WE since changes are subtle or even undetectable.
The most useful imaging modality is MRI that has a
high specificity of 93% and an acceptable sensitivity
of 53%.[6] As demonstrated in our cases, typical MRI
findings of acute WE are symmetrical signal intensity
alterations (usually in the form of T2 hyperintensity
and restricted diffusion as shown in Figure 2) in the
dorsomedial thalami, mammillary bodies, tectal plate,
and periaqueductal area. Selective involvement of the
cerebellum (particularly the vermis), cranial nerve nuclei,
red nuclei, cerebellar dentate nuclei, fornix, splenium,
and cerebral cortex has been previously described.[7]
Interestingly, basal ganglia involvement, which has not
been reported in adults, has been observed in up to 55% of paediatric WE patients.[2] [3] This finding may be related
to the high thiamine-dependent metabolism of nuclear-basal
regions in children. Importantly, albeit uncommon,
mammillary body contrast enhancement (Figure 4) can
be the only sign of WE.[8] [9] Cortical involvement in WE
(Figure 4) usually implies poorer prognosis, as shown by
the inferior outcome in case 2.[10]
In chronic WE, the brain can show necrosis, gliosis, and
neuronal loss.[11] As illustrated in case 3 (Figure 5), these
changes can be gradual and subtle. Thus, it is salient that
a comparison has to be made with prior imaging studies
to detect temporal differences. MR spectroscopy, if
performed, will reveal the expected lactate doublet and
decreased N-acetylaspartate peak at the periaqueductal
lesion, reflecting anaerobic metabolism and necrosis.[10]
Blood Tests
Traditionally, blood tests with measurement of
serum thiamine, thiamine pyrophosphate effect and transketolase have been performed to diagnose WE.
These tests are now considered inadequate for diagnosis
due to their poor sensitivity and specificity.[12] In addition,
a normal serum thiamine level does not necessarily
exclude the presence of WE.[13] In our cases, serum
thiamine was not measured, and transketolase level and
thiamine pyrophosphate effect showed varied changes in
each case following thiamine replacement (Table).
Imaging Differential Diagnoses
There are other disease entities that can show similar
MRI features to WE. These include paramedian thalamic
infarction, ventriculoencephalitis, demyelinating disease,
Leigh disease, primary cerebral lymphoma, Behçet’s
disease, variant Creutzfeldt–Jakob disease, other
metabolic disturbances, and intoxication. When the
clinical history lacks a predisposing factor for thiamine
deficiency, or when response to thiamine replacement is
unclear, these differential diagnoses should be considered.
Management
Since the timing of thiamine replacement determines
outcome, WE is regarded as a medical emergency. In all
cases when the disorder is suspected, thiamine therapy
should be initiated immediately. There is currently no
consensus on thiamine dosage or route of administration
for individuals with WE, but high-dose intravenous
infusion is common.
A retrospective study by Wrenn et al[14] found no
significant allergic reactions in more than 300,000
patients treated with parenteral thiamine. Given
its generally safe profile, some institutes advocate
administration of prophylactic thiamine supplements
to patients with predisposing factors or suggestive
neurological symptoms.[15]
CONCLUSION
In this article, we have demonstrated the spectrum of
MRI findings of WE. In all three cases, the radiologist
was the first to propose WE as a differential diagnosis.
We suspect that these cases may just be the tip of the
iceberg in terms of the prevalence of malnutrition in
children with long-term illnesses, in particular cancers.
Further investigations are warranted to reveal the true
prevalence of paediatric malnutrition, which is currently
presumed rare, in this world city.
In the case of WE, since the time to thiamine replacement determines prognosis, we recommend radiologists
maintain a high level of suspicion when imaging
children with abnormal behaviour, especially if there is
a recent history of parenteral nutrition without thiamine
replacement. Awareness of this entity and its findings
can facilitate early diagnosis and timely management to
improve disease outcome.
REFERENCES
1. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings
and recent advances in diagnosis and management. Lancet Neurol.
2007;6:442-55. Crossref
2. Vasconcelos MM, Silva KP, Vidal G, Silva AF, Domingues RC,
Berditchevsky CR. Early diagnosis of pediatric Wernicke’s
encephalopathy. Pediatr Neurol. 1999;20:289-94. Crossref
3. Zuccoli G, Siddiqui N, Bailey A, Bartoletti SC. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology. 2010;52:523-9. Crossref
4. Seear M, Lockitch G, Jacobson B, Quigley G, MacNab A.
Thiamine, riboflavin, and pyridoxine deficiencies in a population
of critically ill children. J Pediatr. 1992;121:533-8. Crossref
5. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the
Wernicke-Korsakoff complex: a retrospective analysis of 131
cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry.
1986;49:341-5. Crossref
6. Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola
J, Urbano-Marquez A. Usefulness of CT and MR imaging in
the diagnosis of acute Wernicke’s encephalopathy. AJR Am J
Roentgenol. 1998;171:1131-7. Crossref
7. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192:501-8. Crossref
8. Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B,
Giadás TC, et al. Wernicke encephalopathy: MR findings at clinical
presentation in twenty-six alcoholic and nonalcoholic patients.
AJNR Am J Neuroradiol. 2007;28:1328-31. Crossref
9. Shogry ME, Curnes JT. Mamillary body enhancement on MR as the only sign of acute Wernicke encephalopathy. AJNR Am J Neuroradiol. 1994;15:172-4.
10. Kornreich L, Bron-Harlev E, Hoffmann C, Schwarz M, Konen O, Schoenfeld T, et al. Thiamine deficiency in infants: MR findings in the brain. AJNR Am J Neuroradiol. 2005;26:1668-74.
11. Kril JJ. Neuropathology of thiamine deficiency disorders. Metab Brain Dis. 1996;11:9-17. Crossref
12. Talwar D, Davidson H, Cooney J, St JO’Reilly D. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000;46:704-10. Crossref
13. Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408-18. Crossref
14. Wrenn KD, Slovis CM. Is intravenous thiamine safe? Am J Emerg Med. 1992;10:165. Crossref
15. Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy
in the Accident and Emergency Department. Alcohol Alcohol.
2002;37:513-21. Crossref