Eosinophilic Meningitis and Pneumonitis due to Angiostrongyliasis: a Case Report
CASE REPORT
Eosinophilic Meningitis and Pneumonitis due to Angiostrongyliasis: a Case Report
YM Leng, Allen Li
Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong
Correspondence: Dr YM Leng, Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong. Email:
Submitted: 22 May 2020; Accepted: 7 Oct 2020.
Contributors: Both authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: Both authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by New Territories West Cluster Research Ethics Committee of Hospital Authority (Ref No.: NTWC/REC/20052). The patient was treated in accordance with the tenets of the Declaration of Helsinki and has provided written informed consent for all treatments and procedures. Patient consent for this study was waived by the Committee.
INTRODUCTION
Angiostrongylus cantonensis infection is one of the most common causes of eosinophilic meningitis in
Southeast Asia and the Pacific Basin.[1] The organism was
first described in 1935 by Chinese parasitologist Hsin-tao
Chen. It was first found in the cerebrospinal fluid
(CSF) of a Japanese patient who died of eosinophilic
meningoencephalitis in Taiwan in 1944.[2] Since then,
there has been an increase in the global distribution of
reported cases. Sporadic cases in travellers who have
returned from endemic areas have been reported.
Infection with A cantonensis can arise from ingestion of
food items contaminated by intermediate or definitive
hosts. Neural tissue can be targeted after infection. Lung
involvement is less commonly encountered clinically.
To the best of our knowledge, this is the first reported
local case of A cantonensis infection with both central
nervous system and lung involvement.
CASE PRESENTATION
A 29-year-old Chinese man with a history of epileptic
seizure since childhood was admitted to Tuen Mun
Hospital with a 5-day history of severe cerebellar ataxia, preceded by a 3-day history of fever and mild dry cough
after travelling to Japan. On admission, he was afebrile
with a right-hand intentional tremor, pass pointing
sign, and tandem walking instability. No neck rigidity
was detected. His Glasgow Coma Scale score was 15.
Computed tomography (CT) of the brain on admission
was essentially normal.
Initial lumbar puncture revealed elevated opening
pressure and a CSF white blood count of 257/μL,
with 96% lymphocytes and 4% polymorphonuclear
leucocytes. Cryptococcal antigen, bacterial culture
and sensitivity test, CSF Japanese encephalitis virus
antigen, Mycobacterium tuberculosis polymerase chain
reaction (PCR), and CSF viral PCR test results were
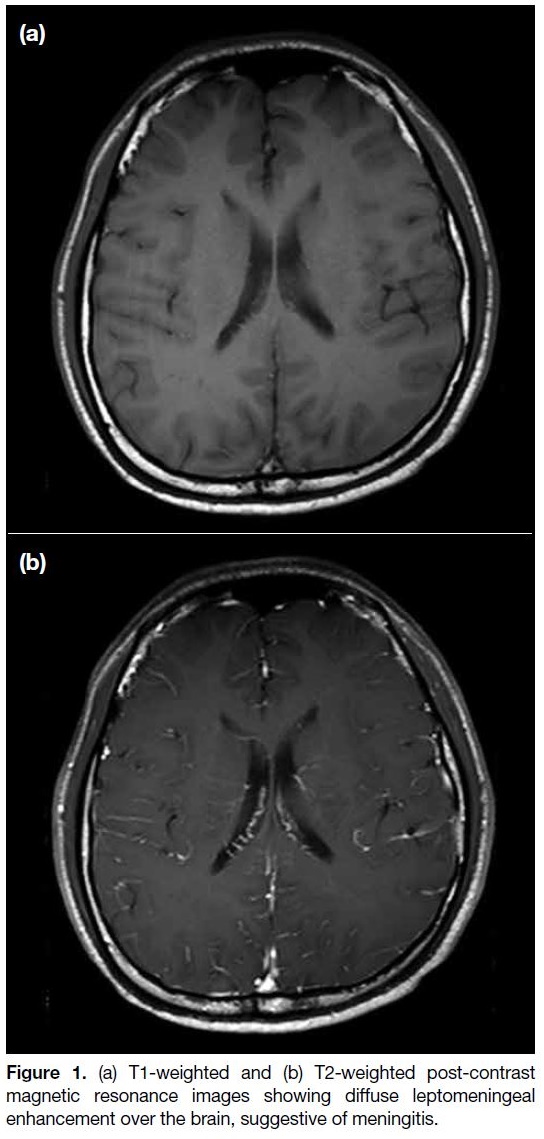
all negative. Subsequent magnetic resonance imaging
(MRI) of the brain (Figure 1) showed diffusely increased
leptomeningeal enhancement, suggestive of meningitis.
MRI of the whole spine showed no abnormal signal
along the spinal cord. A new lumbar puncture revealed
escalating white cell count from 257 to 2049/μL. Repeat
CSF viral, bacterial, and fungal test results were again
all negative.
Figure 1. (a) T1-weighted and (b) T2-weighted post-contrast
magnetic resonance images showing diffuse leptomeningeal
enhancement over the brain, suggestive of meningitis.
However, peripheral blood eosinophil count
progressively increased to 33.3% (normal <6%). A third
lumbar puncture demonstrated eosinophilia, and a PCR
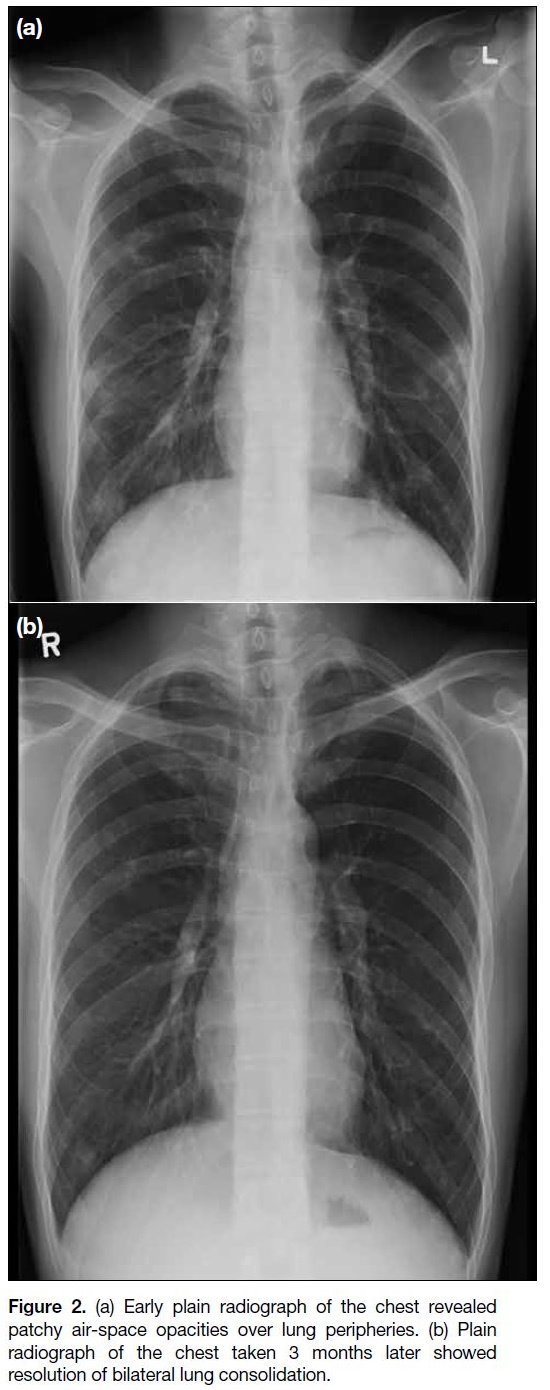
test for Angiostrongylus spp. was positive. Plain chest
radiograph on admission had revealed patchy infiltrates
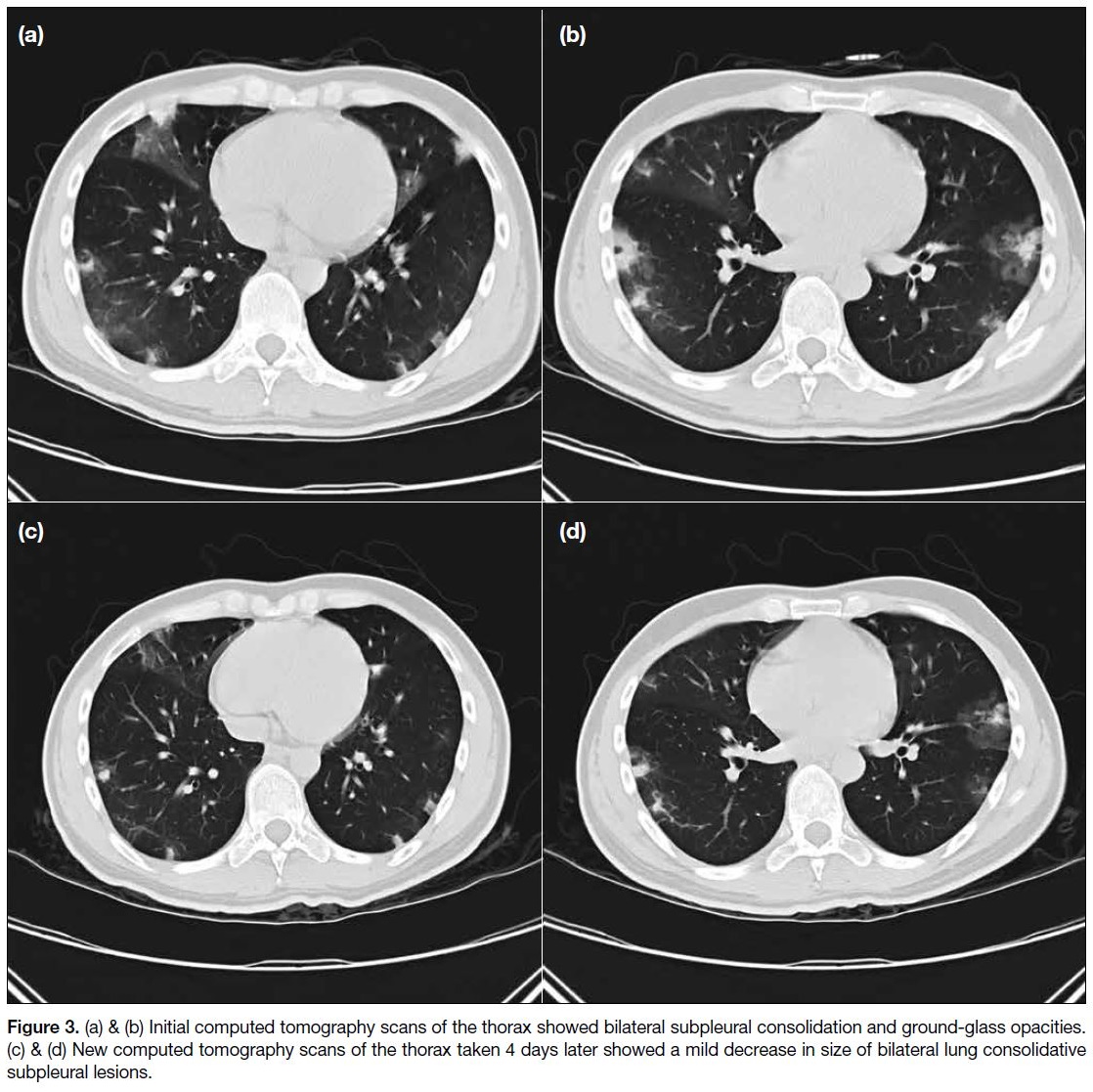
over peripheral lung fields (Figure 2). CT scan of the
thorax showed bilateral subpleural consolidation and
ground-glass opacities (Figure 3). Of note was the
presence of the halo sign in some areas, that is ground-glass
opacity surrounding some of the consolidative lesions.
Figure 2. (a) Early plain radiograph of the chest revealed
patchy air-space opacities over lung peripheries. (b) Plain
radiograph of the chest taken 3 months later showed
resolution of bilateral lung consolidation.
Figure 3. (a) & (b) Initial computed tomography scans of the thorax showed bilateral subpleural consolidation and ground-glass opacities.
(c) & (d) New computed tomography scans of the thorax taken 4 days later showed a mild decrease in size of bilateral lung consolidative
subpleural lesions.
The patient was prescribed empirical antibiotics,
antiviral treatment, and analgesics with gradual clinical
improvement. He was not immunocompromised after work-up and was discharged after 4 weeks, with follow-up
CT scan of the thorax during admission (Figure 3)
and chest radiograph after discharge showing resolution
of the lung lesions (Figure 2). His fever had resolved,
and no headache or neurological signs were evident.
Peripheral eosinophil count was decreasing.
DISCUSSION
A cantonensis primarily infects rat lungs. First-stage larvae migrate to the pharynx where they are swallowed
and excreted via the faeces. Intermediate hosts such as
snails ingest the faeces of rodents contaminated by these first-stage larvae that then moult twice within the mollusc
to develop into second- and third-stage larvae. The third-stage
larva is the infecting form of the parasite. Human
infection can occur after ingestion of undercooked
snails, or through ingestion of unwashed salads or raw
vegetable juice contaminated with infective third-stage
larvae.[3]
Once infective third-stage larvae are ingested, they can
invade the gastrointestinal system and cause enteritis.
The larvae then pass through the liver and lungs before
reaching the nervous system. Symptoms that include cough, sort throat, and fever develop as the nematode
moves through the lungs. The neurological manifestations
of A cantonensis infection include eosinophilic
meningitis, encephalitis or encephalomyelitis, radiculitis,
cranial nerve abnormalities, and ataxia.
Angiostrongyliasis should be considered in patients with
high eosinophil count in blood and/or CSF. The diagnosis
of angiostrongyliasis is confirmed by detection of A
cantonensis antigen or antibody in blood or CSF. PCR
for antigen detection in CSF has recently been developed
and may help to confirm diagnosis at an earlier stage.[4] In
our case, the patient had a high eosinophil count in blood
and CSF. The CSF PCR confirmed the diagnosis.
MRI examination of the brain and spinal cord in our
patient showed diffuse leptomeningeal enhancement of
the brain only. There was no abnormal enhancement of
the spinal cord on MRI study. In a case series with 17
MRI examinations of the brain and spinal cord in five
patients with angiostrongyliasis, multiple enhancing
nodules in the brain and linear enhancement in the
leptomeninges were the main imaging findings.[5] Spinal
cord involvement with enhancement of the nerve roots
can also occur but is rare.[5] In a study of 27 patients
who were clinically diagnosed with angiostrongyliasis,
MRI findings included leptomeningeal enhancement,
thickening of the meninges, intracranial nodules, and
perimeningeal vascular thickening.[6]
The CT thorax in our patient showed diffuse bilateral
subpleural consolidations with ground-glass halo sign.
In another study, CT thorax in 15 patients revealed
pulmonary nodular lesions and ground-glass opacity
lesions located in the subpleural area, which are
characteristic signs of the disease.[7] In a case series of
12 patients with lung involvement, CT findings included
small nodules with or without a halo of ground-glass
attenuation, patchy areas of ground-glass attenuation
and focal thickening of bronchovascular bundles
located mainly in the peripheral lungs.[8] A large series of
81 patients with A cantonensis infection showed
concordant imaging findings of focal leptomeningeal
enhancement on MRI brain (n = 36) and lung nodules
and ground-glass opacity (n = 18).[9]
The diagnosis of angiostrongyliasis can be difficult
and requires a high level of suspicion. Clinical history
is very important, particularly a history of travel to
endemic regions or ingestion of commonly infested
host. The diagnosis should be suspected in patients with eosinophilic meningoencephalitis with elevated
eosinophils in the blood and/or CSF. MRI brain finding
of leptomeningeal enhancement is non-specific. A
cantonensis can be associated with focal brain lesions on
imaging but less commonly than with other helminthic
infections of the central nervous system (gnathostomiasis
or neurocysticercosis).[10] [11] [12] Lung findings of subpleural
consolidation and ground-glass opacities, some with
halo sign, are also non-specific and can be found in
various diseases ranging from infection or neoplasia to
inflammatory conditions. Although many cases are self-limiting,
severe neurological sequelae and even death
may occur.
In summary, we report a case of angiostrongyliasis
involvement of the brain and lung. Early recognition and
accurate detection of A cantonensis can improve patient
outcomes. It is important to raise public awareness of
disease risk factors in endemic areas and international
travellers to prevent further transmission.
REFERENCES
1. Baheti NN, Sreedharan M, Krishnamoorthy T, Nair MD, Radhakrishnan K. Neurological picture. Eosinophilic meningitis
and an ocular worm in a patient from Kerala, south India. J Neurol
Neurosurg Psychiatry. 2008;79:271. Crossref
2. Nomura S, Lin H. First clinical case of Haemostrongylus ratti. Taiwan No Ikai. 1945;3:589-92.
3. Tsai HC, Lee SS, Huang CK, Yen CM, Chen ER, Liu YC. Outbreak of eosinophilic meningitis associated with drinking raw vegetable juice in southern Taiwan. Am J Trop Med Hyg. 2004;71:222-6. Crossref
4. Ansdell V, Wattanagoon Y. Angiostrongylus cantonensis in travelers: clinical manifestations, diagnosis, and treatment. Curr Opin Infect Dis. 2018;31:399-408. Crossref
5. Jin E, Ma D, Liang Y, Ji A, Gan S. MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clin Radiol. 2005;60:242-50. Crossref
6. Yang B, Yang L, Chen Y, Lu G. Magnetic resonance imaging findings and clinical manifestations in cerebral angiostrongyliasis
from Dali, China. Brain Behav. 2019;9:e01361. Crossref
7. Cui Y, Shen M, Meng S. Lung CT findings of angiostrongyliasis cantonensis caused by Angiostrongylus cantonensis. Clin Imaging. 2011;35:180-3. Crossref
8. Cheng J, Huang H, Wang X, Wu A, Yu Z, Xu F, et al. Angiostrongylus cantonensis: chest CT findings [in Chinese]. Chin J Radiol. 1999;33:371-3.
9. Wang J, Zheng X, Yin Z, Qi H, Li X, Ji A, et al. A clinical analysis of 81 cases of Angiostrongylus cantonensis in Beijing [in Chinese]. Chin J Intern Med. 2008;1:50-1.
10. Diaz JH. Recognizing and reducing the risks of helminthic eosinophilic meningitis in travelers: differential diagnosis, disease management, prevention, and control. J Travel Med. 2009;16:267-75. Crossref
11. Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621-30. Crossref
12. Ramirez-Avila L, Slome S, Schuster FL, Gavali S, Schantz PM, Sejvar J, et al. Eosinophilic meningitis due to Angiostrongylus and Gnathostoma species. Clin Infect Dis. 2009;48:322-7. Crossref