Contrast-Enhanced Ultrasonography and Its Application in Liver Interventions
PERSPECTIVE
Contrast-Enhanced Ultrasonography and Its Application in Liver Interventions
CH Ho, SM Wong, HL Wong, JCW Siu, KCH Yu, JCX Chan, HY Lau, CB Tan, YC Wong
Department of Radiology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr CH Ho, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: hch1931@ha.org.hk
Submitted: 22 Sep 2021; Accepted: 4 Feb 2022.
Contributors: All authors designed the study. CHH, SMW, HLW and JCWS acquired and analysed the data. CHH drafted the manuscript. SMW, HLW, JCWS, KCHY, JCXC, HYL, CBT and YCW critically revised the manuscript for important intellectual content. All authors had full
access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by New Territories West Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: NTWC/REC/21086). Informed consent was waived by the Committee.
Abstract
Ultrasound (US) guidance has been a fundamental tool for interventionalists to perform percutaneous procedures. A
limitation to US guidance is poor lesion visibility on conventional B-mode (brightness mode) US. Contrast-enhanced
US (CEUS) is an adjunct technique that facilitates the visualisation and localisation of lesions. We review the use
of CEUS and its application in liver interventions and describe the experience in our institution in using CEUS in
these procedures.
Key Words: Contrast media; Radiology, interventional; Ultrasonography, interventional
中文摘要
超聲造影檢查及其在肝臟介入中的應用綜述
何卓謙、王先民、黃皓廉、蕭志偉、余俊鴻、陳積聖、劉顯宇、陳崇文、王耀忠
超聲引導一直是介入醫生開展經皮手術的基本工具,它的其中一個局限性是傳統B模式(亮度模式)超聲對於病變的可見性欠佳。對比增強超聲這種輔助技術可幫助病變的檢出和定位。本文檢視對比增強超聲的使用及其在肝臟介入中的應用,並描述本院在有關操作中使用對比增強超聲的實踐。
INTRODUCTION
Ultrasound (US) guidance has been a fundamental tool
for interventionalists for various percutaneous
procedures. It has the advantages of real-time imaging,
lack of ionising radiation, and wide availability.
However, the role of US guidance is greatly limited if
the lesion has a poor visibility on conventional B-mode
(brightness mode) US.
To overcome this limitation, contrast-enhanced
ultrasound (CEUS) is an adjunct technique to facilitate
the localisation of lesions.[1] In this article, we review
the background information of US contrast agents and
techniques for performing CEUS. We also describe
the application of CEUS in liver interventions and our
experience with this technique in our institution.
ULTRASOUND CONTRAST AGENTS
US contrast agents consist of gaseous microbubbles
enclosed within shells.[2] They are injected intravenously.
The size of microbubbles ranges from 1 to 10 μm.[3]
Microbubbles respond differently under different
acoustic energies. When microbubbles are subjected
to low acoustic energy (mechanical index [MI] = 0.1-0.3), they oscillate and produce non-linear harmonic
resonances.[2] [3] Separation of the non-linear resonances
from microbubbles and linear resonances from
background soft tissue forms the basis of CEUS. These
two signals can be separated using one of several soft
tissue cancellation techniques, such as pulse inversion,
frequency, and amplitude modulation.[2] [4] Microbubbles
are vulnerable to higher acoustic energies (MI > 0.3-0.6),
which can cause cavitation and fragmentation.[2]
US contrast agents are classified into first and second
generations, depending on the solubility of the gaseous
content.[3] [5] The first-generation US contrast agents, which
consisted mostly of air, are largely obsolete due to their
instability (as they will burst easily) and high solubility
in blood. Most of the currently used second-generation
contrast agents are composed of encapsulated inert gases
with high stability and low solubility (e.g., perfluorobutane,
perfluoropropane, and sulphur hexafluoride).[5] Currently,
there are four agents that are available internationally
for use in liver imaging, including sulphur hexafluoride
within a phospholipid shell (SonoVue; Bracco Suisse
SA, Switzerland), octafluoropropane within a bilayer
phospholipid shell (Luminity; Lantheus Medical
Imaging, Inc, North Billerica [MA], United States), perfluorobutane gas coated with a chicken egg–derived
surfactant hydrogenated egg phosphatidylserine sodium
[Optison; GE HealthCare, United Kingdom], and
perflubutane enclosed in a phospholipid shell, which has
immediate blood pool and delayed Kupffer cell uptake in
the liver, which can last up to a few hours (Sonazoid[6] [7] [8];
GE HealthCare, Norway). In Asian countries, SonoVue
and Sonazoid are more commonly used.[7]
SonoVue is taken up by the blood pool. It is the only
registered US contrast agent in Hong Kong.[9] It is
currently registered in 44 countries[10] and is available in
Japan, Korea, Norway, Singapore and China, etc.[7] It is
currently an unregistered drug in Hong Kong, and the
relevant legal requirement needs to be observed before
use.[8] Details can be obtained from the Drug Office of the Department of Health.[11]
Intravenous use of US contrast agents has a very safe
profile. They are excreted via the lungs. The outer
shells are biodegradable in general owing to the fact
that they will be engulfed by macrophages in the
reticuloendothelial system.[2] They are not nephrotoxic,
and therefore can be administered in patients with renal
failure.[5] [6] It also has no effect on thyroid function as it
does not contain iodine.[5] US contrast agents have a very
low rate of anaphylactic reactions (1 in 7000 patients
or 0.014%) compared to iodinated contrast agents or
gadolinium-based contrast agents.[5] [6]
Contraindications vary among different US contrast
agents. For SonoVue, contraindications include, but are
not limited to, hypersensitivity to the active substance
or to any of the excipients (including polyethylene
glycol), known right-to-left shunts, severe pulmonary
hypertension, uncontrolled systemic hypertension, and
adult respiratory distress syndrome.[12] For Sonazoid,
contraindications include hypersensitivity to the
active substances (including perfluorobutane gas and
hydrogenated egg phosphatidylserine sodium) or to
any of the excipients. Sonazoid is derived from egg.
For patients with egg or egg products allergy, Sonazoid
should only be used if the benefit clearly outweighs
the potential hazard.[7] Care should be taken in patients
with right-to-left shunts, unstable heart conditions,
serious coronary arterial diseases or serious pulmonary
diseases.[13] Readers are advised to read the relevant product information and package insert carefully before use.
TECHNIQUE OF CONTRAST-ENHANCED ULTRASOUND
One of the unique features of CEUS is that real-time
imaging of contrast enhancement is enabled. The arterial
phase usually occurs from 10-20 seconds to 30-45
seconds after injection. The portal venous phase ensues
30-45 seconds to 2 minutes post-injection and is followed
by late phase, which ends when there is clearance of
microbubbles from the circulation which is about 4-6
minutes.[4] For Sonazoid, the Kupffer cell uptake (post-vascular)
phase usually starts 10 minutes post-injection
and can persist up to a few hours.[7] [10]
MI is the measure of acoustic power of an US beam.
To minimise the disruption of the microbubbles, CEUS
imaging is performed at low acoustic pressures with MI
ranging from 0.05 to 0.3.[4] Different contrast agents may
require different machine settings for optimal signals; for
example, SonoVue can be used with a lower MI (<0.1)
due to its softer shell, while a higher MI is needed for
Sonazoid due to its stiffer outer shell.[9] [14] Optimal MI
settings may vary from machine to machine.
The dose of US contrast agent varies with different
brands. The current recommended dose is 2.4 mL for
SonoVue (peripheral vascular use), and 0.015 mL/kg
body weight for Sonazoid.[12] [13] Both SonoVue and
Sonazoid need to be reconstituted before administration
and readers are referred to the relevant package insert
for detail information. A reminder on reconstituting
Sonazoid from our experience is although an ampoule of 10 mL sterile water is provided in the package, only
2 mL is required for reconstitution. Using a 20G or
larger catheter for contrast injection is recommended to
minimise microbubble destruction. Slow hand injection
of contrast agent over 2 to 3 seconds followed by a 5-to 10-mL saline flush is suggested.[4] Repeated contrast injection of the recommended dose can be considered if
necessary.[15]
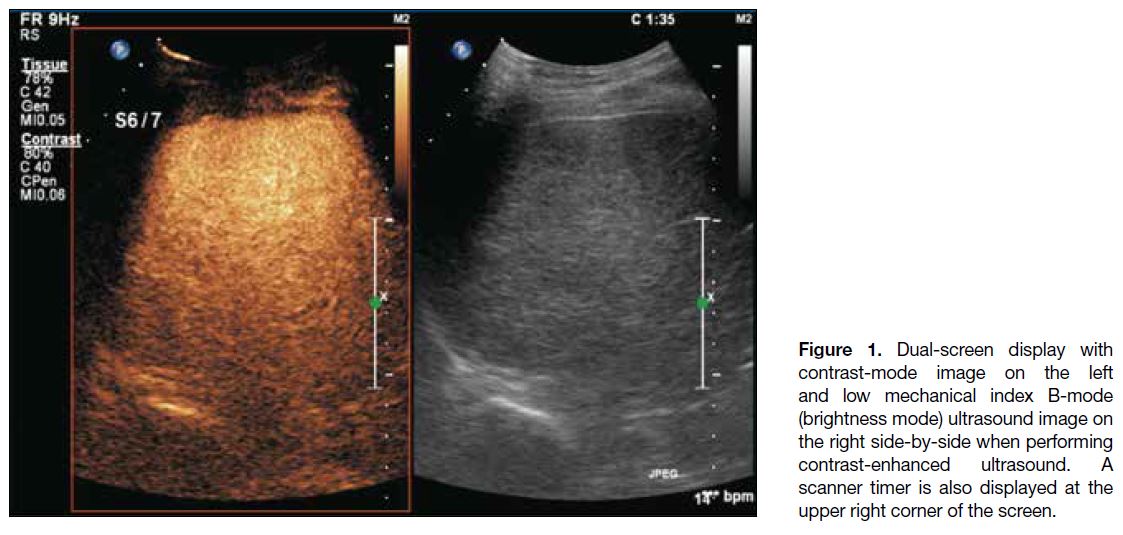
Dual-screen display with low MI B-mode and contrast-mode
images side-by-side is commonly used during
CEUS. A timer is also displayed to record the time after
contrast injection (Figure 1). Depth of penetration of
CEUS is usually less than that seen with conventional
B-mode imaging due to low MI settings. The focal zone
should be placed just deep to the target lesion.[4] [15] It is
important to avoid excessive or continuous scanning in a
single plane in order to prevent microbubble destruction,
which causes loss of contrast signal.[15] Again, repeated
contrast injection can be considered to characterise a
washed-out region for any arterial phase enhancement.[15]
Figure 1. Dual-screen display with contrast-mode image on the left and low mechanical index B-mode (brightness mode) ultrasound image on the right side-by-side when performing contrast-enhanced ultrasound. A scanner timer is also displayed at the upper right corner of the screen.
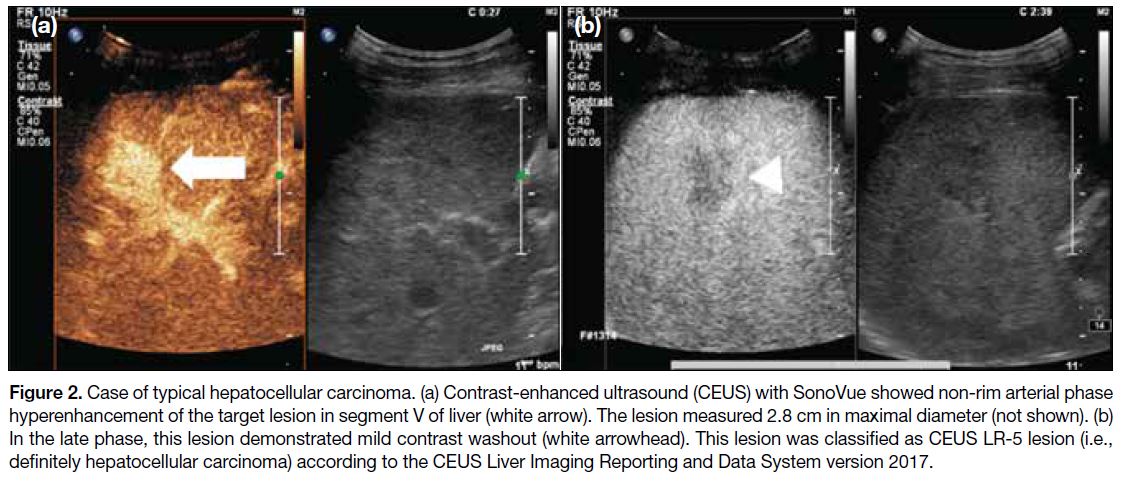
Key features of hepatocellular carcinoma with SonoVue
are arterial phase hyperenhancement followed by late
and mild washout (Figure 2). Similarly for Sonazoid,
hepatocellular carcinoma typically shows arterial phase
hyperenhancement and a defect in the Kupffer cell phase.
Figure 2. Case of typical hepatocellular carcinoma. (a) Contrast-enhanced ultrasound (CEUS) with SonoVue showed non-rim arterial phase hyperenhancement of the target lesion in segment V of liver (white arrow). The lesion measured 2.8 cm in maximal diameter (not shown). (b) In the late phase, this lesion demonstrated mild contrast washout (white arrowhead). This lesion was classified as CEUS LR-5 lesion (i.e., definitely hepatocellular carcinoma) according to the CEUS Liver Imaging Reporting and Data System version 2017.
There are many guidelines and publications describing
the use of CEUS in characterising focal liver lesions.
A complete description of lesion enhancement patterns and a lexicon are beyond the scope of this article.
Readers are referred to the Guidelines and Good Clinical
Practice Recommendations for CEUS in the Liver from
WFUMB (World Federation for Ultrasound in Medicine
and Biology), and CEUS of the liver: technical and
lexicon recommendations from the American College
of Radiology CEUS Liver Imaging Reporting and Data
System working group for further information.[4] [6] The
current American College of Radiology CEUS Liver
Imaging Reporting and Data System (LI-RADS) version
2017 only described the use of pure blood pool agents,
and the use of Sonazoid will be addressed in the next
version.[4]
APPLICATION OF CONTRAST-ENHANCED ULTRASOUND IN LIVER INTERVENTIONS
CEUS can enhance lesion conspicuity for percutaneous
interventions, especially when they are not well depicted
on conventional B-mode US.[16] Common uses of CEUS
in liver interventions include guiding percutaneous
biopsy and tumour ablation.
For indeterminate lesions on computed tomography
(CT) or magnetic resonance (MR) imaging, CEUS may
provide further diagnostic information to characterise
the lesions. For example, indeterminate lesions showing
absence of arterial hyperenhancement on CT or MR
may be due to mistiming of the arterial phase imaging.
Using CEUS can eliminate this problem since it is real-time
continuous imaging.[4] [6] [7] [17] Therefore, CEUS can be a problem-solving tool and may obviate the need for
biopsy for indeterminate lesions on CT or MR.
CEUS is often employed in guiding percutaneous biopsy
of focal liver lesions. It is helpful in both increasing
lesion conspicuity and evaluating the viable vascularised
portion of the lesion. In the recent guidelines issued by
WFUMB, CEUS guidance for focal liver lesion biopsy
should be attempted when the lesions are invisible or
inconspicuous on conventional B-mode imaging and
should be considered in lesions with potential necrotic
areas or if previous biopsy resulted in necrotic material.[6]
A two-dose procedure is recommended. The first dose of
US contrast is used for characterising the target lesion and
planning the needle path, and the second dose is used for
the real-time CEUS guidance during interventions.[6] The
safety and feasibility of using CEUS with SonoVue and
Sonazoid in focal liver lesion biopsy have been reported
in multiple studies.[16] [18] [19] With the use of CEUS, the need
to abort the procedure and convert to CT guidance is
potentially reduced. It also helps to confirm the target
lesion in cases of advanced cirrhosis where multiple
background cirrhosis-related nodules are common or
of concurrent benign liver lesions (e.g., haemangioma),
and therefore minimises mistargeting. Vascular
complications after biopsy, such as pseudoaneurysm
formation, can be detected by CEUS, avoiding the need
for contrast-enhanced CT.[20]
CEUS is also valuable in guiding liver tumour ablation.
Similar to guiding percutaneous biopsy of focal liver
lesions, CEUS can increase lesion conspicuity, allow
real-time needle guidance to the lesion during the
procedure, and minimise mistargeting to other lesions. In
a randomised controlled trial reported by Minami et al,[21]
there was a significantly higher complete ablation rate (94.7% vs. 65.0%) and a smaller number of treatment
sessions when using CEUS guidance with Levovist in
liver tumour ablation for lesions poorly depicted on
conventional B-mode US. After ablation, gas clouds
form in the treatment bed; they are markedly echogenic and obscure the ablation zone, but usually resolve after
10 to 15 minutes, and CEUS can then be performed
post-ablation to evaluate for residual disease around
the ablation zone.[22] [23] [24] [25] Re-intervention can be performed
in the same setting if indicated. Performing immediate postprocedural CEUS with Definity can significantly
reduce the incidence of residual tumour (0% vs.
16.7%) shown in a retrospective study by Lekht et al.[24]
Mauri et al[25] also reported using CEUS with Sonovue
after liver tumour ablation, detecting residual tumour in
29.0% of the ablations. They were able to repeat ablation
immediately, with later CT showing 96.6% success.
Nishigaki et al[23] reported the successful use of Sonazoid
in detecting residual tumour and securing minimal
ablative margins immediately after ablation. These show
the effectiveness of immediate post-ablation CEUS
in determining the adequacy of the ablation, which
can potentially improve patient survival and clinical
outcome.
SonoVue is the only registered US contrast agent
in Hong Kong.[9] It can be used in guiding different
liver interventions as described. However, its short
enhancement period may not be ideal for liver
interventions, especially in liver tumour ablation where
the procedural time is usually long. Sonazoid provides a
unique advantage with the prolonged Kupffer cell phase,
which can last up to a few hours, providing a longer time
window for real-time CEUS guidance.
We have recently introduced CEUS with Sonazoid
in our institution. For patients referred to us for
percutaneous liver tumour ablation, we would carry out
a consultation in our interventional radiology clinic.
During the consultation, we routinely perform a US of
the index lesion for preprocedural planning. CEUS can
be considered at the same juncture if the lesion cannot
be clearly visualised on conventional B-mode US. If
the lesion becomes more conspicuous after contrast
administration and the time window of visibility appears
technically feasible for percutaneous ablation, then
CEUS-guided percutaneous ablation is scheduled. In
our experience, the early Kupffer phase (10-30 minutes
post-injection) provides a good intervention window.
Avoiding unnecessary continuous scanning is important
to minimise microbubble destruction. A second dose of
contrast injection is also helpful if contrast signal loss
occurs. CEUS with Sonazoid during interventional
radiology clinic consultation can be safely performed
in outpatient setting. In our institution, patients would
be discharged following a 10- to 15-minute observation
after administration of Sonazoid.
In our experience, CEUS with Sonazoid improves the
detection and conspicuity of liver lesions (Figure 3).
It enables real-time US guidance for lesions that are not conspicuous on conventional B-mode US when
performing percutaneous liver procedures, obviating
the need for CT guidance. This reduces the radiation
exposure to patients, and possibly decreases the
procedural time and complexity. CEUS has also a role
in percutaneous liver tumour ablation to detect any
residual tumour immediate post-ablation (Figure 4), and re-intervention can be easily performed in the same
setting if any residual tumour is detected. There are also
no serious adverse reactions reported after intravenous
administration of Sonazoid in our institution.
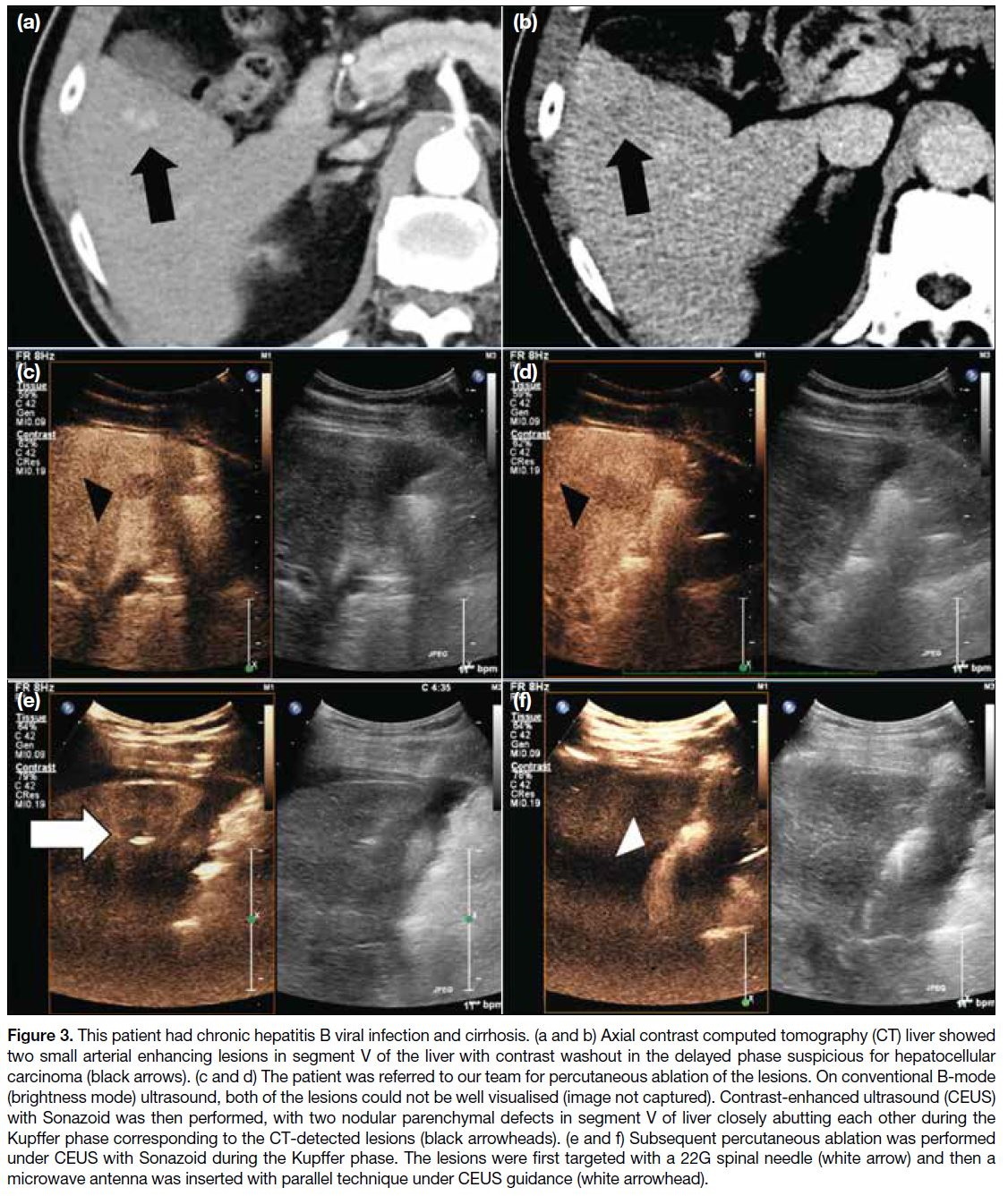
Figure 3. This patient had chronic hepatitis B viral infection and cirrhosis. (a and b) Axial contrast computed tomography (CT) liver showed
two small arterial enhancing lesions in segment V of the liver with contrast washout in the delayed phase suspicious for hepatocellular
carcinoma (black arrows). (c and d) The patient was referred to our team for percutaneous ablation of the lesions. On conventional B-mode
(brightness mode) ultrasound, both of the lesions could not be well visualised (image not captured). Contrast-enhanced ultrasound (CEUS)
with Sonazoid was then performed, with two nodular parenchymal defects in segment V of liver closely abutting each other during the
Kupffer phase corresponding to the CT-detected lesions (black arrowheads). (e and f) Subsequent percutaneous ablation was performed
under CEUS with Sonazoid during the Kupffer phase. The lesions were first targeted with a 22G spinal needle (white arrow) and then a
microwave antenna was inserted with parallel technique under CEUS guidance (white arrowhead).
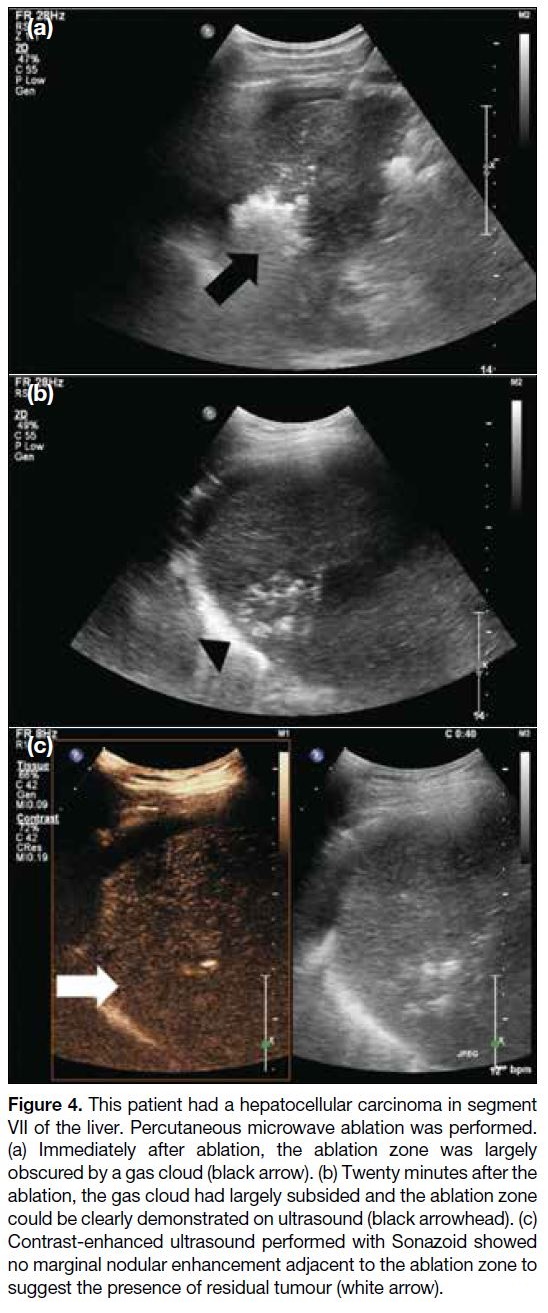
Figure 4. This patient had a hepatocellular carcinoma in segment
VII of the liver. Percutaneous microwave ablation was performed.
(a) Immediately after ablation, the ablation zone was largely
obscured by a gas cloud (black arrow). (b) Twenty minutes after the
ablation, the gas cloud had largely subsided and the ablation zone
could be clearly demonstrated on ultrasound (black arrowhead). (c)
Contrast-enhanced ultrasound performed with Sonazoid showed
no marginal nodular enhancement adjacent to the ablation zone to
suggest the presence of residual tumour (white arrow).
However, there are still some limitations of CEUS in
guiding liver interventions from our experience. CEUS
has a detection limit for deep lesions since the penetration
may not be adequate with the presence of microbubbles
and low MI settings. Similar to conventional B-mode
US, there is also limitation for CEUS to detect lesions
located at the liver dome.
CONCLUSION
CEUS is a safe and effective tool for liver interventions. It improves the visibility of lesions for needle guidance
and is particularly useful when the lesions are small or not
conspicuous on conventional B-mode US. The unique
feature of Kupffer cell uptake of Sonazoid provides a
longer time window for real-time guidance during liver
interventions.
REFERENCES
1. Kim YJ, Lee MW, Park HS. Small hepatocellular carcinomas:
ultrasonography guided percutaneous radiofrequency ablation.
Abdom Imaging. 2013;38:98-111. Crossref
2. Baun J. Contrast-enhanced ultrasound: a technology primer. J Diagn Med Sonogr. 2017;33:446-52. Crossref
3. Ignee A, Atkinson NS, Schuessler G, Dietrich CF. Ultrasound contrast agents. Endosc Ultrasound. 2016;5:355-62. Crossref
4. Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, et al. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working
group. Abdom Radiol (NY). 2018;43:861-79. Crossref
5. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015;34:3-18. Crossref
6. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN,
Cantisani V, et al. Guidelines and Good Clinical Practice
Recommendations for Contrast-Enhanced Ultrasound (CEUS)
in the Liver–update 2020 WFUMB in cooperation with
EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol.
2020;46:2579-604. Crossref
7. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using Sonazoid.
Ultrasonography. 2020;39:191-220. Crossref
8. Shunichi S, Hiroko I, Fuminori M, Waki H. Definition of contrast enhancement phases of the liver using a perfluoro-based microbubble agent, perflubutane microbubbles. Ultrasound Med
Biol. 2009;35:1819-27. Crossref
9. Drug Office, Department of Health, Hong Kong SAR Government. List of Registered Pharmaceutical Products. SONOVUE FOR INJ 8ΜCL/ML. Available from: https://www.drugoffice.gov.hk/eps/drug/productDetail/en/consumer/120026. Accessed 21 Dec 2021.
10. Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, et al. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical
evidence for SonoVue and Sonazoid. Abdom Radiol (NY). 2020;45:3779-88. Crossref
11. Drug Office, Department of Health, Hong Kong SAR Government.
Frequently asked questions. Available from: https://www.drugoffice.gov.hk/eps/do/en/pharmaceutical_trade/guidelines_forms/faq.html. Accessed 21 Dec 2021. Crossref
12. European Medicines Agency. SonoVue: EPAR — product
information. Available from: https://www.ema.europa.eu/en/documents/product-information/sonovue-epar-product-information_en.pdf. Accessed 19 Dec 2021. Crossref
13. Health Sciences Authority of Singapore. Summary report of benefit-risk
assessment: Sonazoid powder and solvent for dispersion for
injection, 16 microlitre per vial. Available from: https://www.hsa.gov.sg/docs/default-source/hprg-tpb/summary-reports/sonazoid-summary-report-06-jan-21.pdf. Accessed 19 Dec 2021. Crossref
14. Numata K, Luo W, Morimoto M, Kondo M, Kunishi Y, Sasaki T, et al. Contrast enhanced ultrasound of hepatocellular carcinoma. World J Radiol. 2010;2:68-82. Crossref
15. Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F, et al. How to perform contrast-enhanced ultrasound (CEUS). Ultrasound Int Open. 2018;4:E2-15. Crossref
16. Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH, et al.
Real-time contrast-enhanced ultrasound-guided biopsy of focal
hepatic lesions not localised on B-mode ultrasound. Eur Radiol.
2010;20:2047-56. Crossref
17. Jo PC, Jang HJ, Burns PN, Burak KW, Kim TK, Wilson SR.
Integration of contrast-enhanced US into a multimodality approach
to imaging of nodules in a cirrhotic liver: how I do it. Radiology.
2017;282:317-31. Crossref
18. Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Real-time contrast-enhanced sonographically guided biopsy or radiofrequency ablation
of focal liver lesions using perflurobutane microbubbles (Sonazoid):
value of Kupffer-phase imaging. J Ultrasound Med. 2015;34:411-21. Crossref
19. Spârchez Z, Radu P, Kacso G, Spârchez M, Zaharia T, Al Hajjar N.
Prospective comparison between real time contrast enhanced and
conventional ultrasound guidance in percutaneous biopsies of liver
tumors. Med Ultrason. 2015;17:456-63. Crossref
20. Huang DY, Yusuf GT, Daneshi M, Husainy MA, Ramnarine R, Sellars ME, et al. Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using US
contrast agents. Radiographics. 2017;37:652-64. Crossref
21. Minami Y, Kudo M, Chung H, Kawasaki T, Yagyu Y, Shimono T,
et al. Contrast harmonic sonography-guided radiofrequency
ablation therapy versus B-mode sonography in hepatocellular
carcinoma: prospective randomized controlled trial. AJR Am J
Roentgenol. 2007;188:489-94. Crossref
22. Bansal S, Gui J, Merrill C, Wong JK, Burak KW, Wilson SR.
Contrast-enhanced US in local ablative therapy and secondary
surveillance for hepatocellular carcinoma. Radiographics.
2019;39:1302-22. Crossref
23. Nishigaki Y, Hayashi H, Tomita E, Suzuki Y, Watanabe N,
Watanabe S, et al. Usefulness of contrast-enhanced ultrasonography
using Sonazoid for the assessment of therapeutic response to
percutaneous radiofrequency ablation for hepatocellular carcinoma.
Hepatol Res. 2015;45:432-40. Crossref
24. Lekht I, Gulati M, Nayyar M, Katz MD, Ter-Oganesyan R,
Marx M, et al. Role of contrast-enhanced ultrasound (CEUS)
in evaluation of thermal ablation zone. Abdom Radiol (NY).
2016;41:1511-21. Crossref
25. Mauri G, Porazzi E, Cova L, Restelli U, Tondolo T, Bonfanti M, et al.
Intraprocedural contrast-enhanced ultrasound (CEUS) in liver
percutaneous radiofrequency ablation: clinical impact and health
technology assessment. Insights Imaging. 2014;5:209-16. Crossref