Underestimation of Ductal Carcinoma In Situ and Invasive Ductal Carcinoma in Specimens Obtained with Stereotactic-Guided Vacuum-Assisted Biopsy
ORIGINAL ARTICLE
Underestimation of Ductal Carcinoma In Situ and Invasive Ductal Carcinoma in Specimens Obtained with Stereotactic-Guided
Vacuum-Assisted Biopsy
ALC Chan, KH Wong, KY Tam, YY Man, PY Tang
Department of Radiology, North District Hospital and Alice Ho Miu Ling Nethersole Hospital, Hong Kong
Correspondence: Dr ALC Chan, Department of Radiology, North District Hospital and Alice Ho Miu Ling Nethersole Hospital, Hong Kong. Email: lokchi327@gmail.com
Submitted: 21 Feb 2021; Accepted: 12 May 2021.
Contributors: ALCC, KHW, KYT and PYT designed the study. ALCC and YYM acquired the data. ALCC, KHW and PYT analysed the data. ALCC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref: 2020.441). The patients were treated in accordance with the Declaration of Helsinki. Consent regarding data retrieval was waived by the Committee.
Abstract
Objective
We sought to determine the underestimation rates of ductal carcinoma in situ (DCIS) and of invasive
ductal carcinoma (IDC), diagnosed as atypical ductal hyperplasia (ADH) and DCIS, respectively, occurring with
stereotactic-guided vacuum-assisted breast biopsy (VABB) of suspicious microcalcifications.
Methods
We retrospectively reviewed cases of ADH and DCIS diagnosed by stereotactic-guided VABB between 2010
and 2019 in our institution. The biopsy results were correlated with the subsequent surgical histopathology results.
Results
A total of 44 ADH lesions and 83 DCIS lesions were sampled with stereotactic-guided VABB during the
10-year study period. All lesions were categorised as BI-RADS (Breast Imaging Reporting and Data System) 4. Most
lesions had either 6 or 12 cores taken during the biopsy. The upgrade rate of VABB-diagnosed ADH was 18.2%
(7 upgraded to DCIS and 1 to IDC out of 44 VABB diagnoses of ADH), while that of VABB-diagnosed DCIS was
9.6% (8 upgraded to IDC out of the 83 biopsy-diagnosed DCIS). Amorphous calcifications in ADH lesions were
associated with a lower rate of malignancy upgrade (p = 0.019). No other predictors of upgrade for either ADH or
DCIS were identified. When the pathology results of specimens without visible microcalcifications were reviewed
separately, we found a very low rate of upgrade in the absence of histological microcalcifications or in the presence
of a benign pathologic entity.
Conclusion
A significant proportion of stereotactic-guided VABB-diagnosed ADH and DCIS were underdiagnosed
when compared to surgical histopathology. Surgical excisional biopsy is recommended for all VABB-diagnosed
ADH and DCIS lesions for definitive pathology.
Key Words: Breast; Carcinoma, Intraductal, Noninfiltrating; Biopsy/IS; Pathology, Surgical; Neoplasms
中文摘要
用立體定向引導真空輔助活檢獲得的標本中導管原位癌和浸潤性導管癌分級的低估
陳洛之、黃健開、譚家盈、文欣欣、鄧佩儀
目的
當對於可疑微鈣化立體定向發生引導真空輔助乳房活檢(VABB)診斷非典型導管增生(ADH)和導管原位癌(DCIS)時,了解DCIS和浸潤性導管癌(IDC)分級的低估率。
方法
回顧性總結我院2010至2019年立體定向引導VABB診斷的ADH和DCIS病例。活檢結果與隨後的手術組織病理學結果相驗證。
結果
在為期十年的研究期間,使用立體定向引導的VABB對44個ADH病變和83個DCIS病變進行了採樣。所有病變都歸類為BI-RADS 4。大多數病變取了6或12個活檢核。VABB診斷ADH的升級率為18.2%(44例VABB診斷的ADH中,7例升級為DCIS,1例升級為IDC),而VABB診斷DCIS的升級率為 9.6%(83例活檢診斷的DCIS中,8例升級為IDC)。ADH病變中的無定形鈣化提示較低的惡性腫瘤升級概率(p = 0.019),並沒有發現其他影響ADH或DCIS升級的預測因素。當單獨核對沒有可見微鈣化標本的病理學結果時,在沒有組織學微鈣化或存在良性病理實體的情況下升級率非常低。
結論
與手術組織病理學相比,相當比例的立體定向引導VABB診斷的ADH和DCIS診斷不足。建議對所有VABB診斷的ADH和DCIS病變進行手術切除活檢以明確病理診斷。
INTRODUCTION
Clustered microcalcifications on mammography may
be associated with underlying breast malignancy. These
microcalcification clusters may be sonographically
visible, especially when there is an associated mass,
which enables biopsy to be performed under sonographic
guidance.[1] Sonographically occult microcalcification
clusters can be biopsied using stereotactically guided
vacuum-assisted breast biopsy (VABB). It is a minimally
invasive and cost-effective tissue sampling method,
which is safely performed in an outpatient setting as part
of the workup for suspicious breast lesions.
Atypical ductal hyperplasia (ADH) is associated with
a high risk for breast cancer, with cytopathological
appearances that resemble but fail to meet a diagnosis
of low-grade ductal carcinoma in situ (DCIS).[2] It can
coexist with ductal carcinoma in situ (DCIS) and
invasive ductal carcinoma (IDC). DCIS is the direct
precursor of IDC.[3] The histopathological distinction
between ADH and DCIS is hampered by significant
inter-observer variation, probably related to differences
in the interpretation of specific histological features and
diagnostic field selection.[4] [5]
It is known that lesions with an initial histopathologic diagnosis of ADH or DCIS using VABB may be upgraded
from ADH to DCIS or IDC, or from DCIS to IDC after
surgical excision and complete histological examination.
A previous study has shown the underestimation rate of
11-gauge VABB lies between 10% and 27% for ADH and
5% and 18% for DCIS.[6] Surgical excision is advocated
for these lesions for definitive histopathology.[7] [8] [9]
We sought to determine the underdiagnosis rate of DCIS
and IDC with stereotactic-guided VABB performed
on an Asian population in the radiology department
of our institution comprising two regional hospitals
in Hong Kong and to identify factors associated with
underdiagnosis.
METHODS
Patients
This retrospective study included 127 lesions from 126
patients from two hospitals. Institutional approval was
obtained for this retrospective study. The radiology
information database for cases of stereotactic-guided
VABB from January 2010 to December 2019 was
reviewed. Patients were referred from the breast surgical
team and underwent complete diagnostic workup with
mammography and breast ultrasound. Patients who had
suspicious microcalcifications detected on mammogram with no corresponding abnormalities identified on
ultrasonography were recommended for stereotactic-guided
VABB.
Our study included patients with ADH or DCIS
diagnosed by stereotactic-guided VABB of suspicious
microcalcifications, who had undergone subsequent
surgical excision. Cases with no corresponding surgical
histopathology correlation at sites of VABB were
excluded, instead undergoing follow-up for 2 to 9 years.
Biopsy Procedure and Postprocedural
Assessment
Stereotactic-guided VABBs were carried out in the
prone position on a biopsy table with either a 10- or
9-gauge biopsy needle (LORAD MultiCare Stereotactic
Breast Biopsy System; Hologic, Marlborough [MA],
US) equipped with a 10G EnCor biopsy needle (Bard;
Murray Hill [NJ], US) from 2010 to 2016 in one centre,
and with the Affirm Prone Biopsy Table (Hologic) and
ATEC Breast Biopsy and Excision System (Hologic)
with a 9G Eviva biopsy needle (Hologic) from 2016
to 2019 in another centre. All biopsies were performed
by one of the breast radiologists with 10 to 20 years of
experience in our institution. During the biopsy, at least
six cores were obtained by a 360-degree rotational probe,
allowing sampling from different angles without repeated
removal and re-insertion of the needle into the breast.
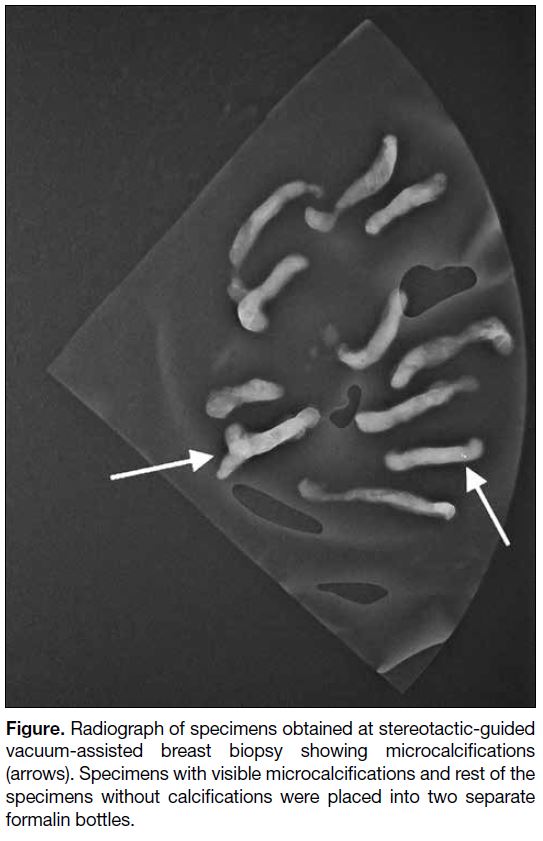
Specimen radiographs were obtained to ensure adequate
inclusion of the microcalcifications initially identified on
mammography. The specimens were separated according
to the presence or absence of microcalcifications on
the radiograph (Figure), and placed into two separate
formalin bottles, labelled as ‘with microcalcifications’
and ‘without microcalcifications’ from the same biopsy
site.
Figure. Radiograph of specimens obtained at stereotactic-guided
vacuum-assisted breast biopsy showing microcalcifications
(arrows). Specimens with visible microcalcifications and rest of the
specimens without calcifications were placed into two separate
formalin bottles.
Data Collection
Data on patients’ demographics, including age of
patients when the biopsy was performed, were collected
(Tables 1 and 2). The suspicious microcalcifications
on the preprocedural mammogram were categorised
with reference to the fifth edition of the Breast Imaging
Reporting and Data System (BI-RADS) developed by
the American College of Radiology. Location and size of
the lesions (measured as the single greatest dimension)
were documented. The dates of the preprocedural
mammogram, biopsy and surgery, and the time interval
between the preprocedural mammogram and biopsy,
and between the biopsy and surgery, were recorded. The
needle size and number of cores taken during biopsy were obtained. The post-biopsy mammogram was evaluated
to assess for the presence of residual calcifications. The
final histopathological results of the VABB samples
and subsequent surgical specimens, and the number of
ADH foci in the VABB specimens were recorded. We
reviewed the final histopathology of specimens with
and without microcalcifications obtained during the
VABB.
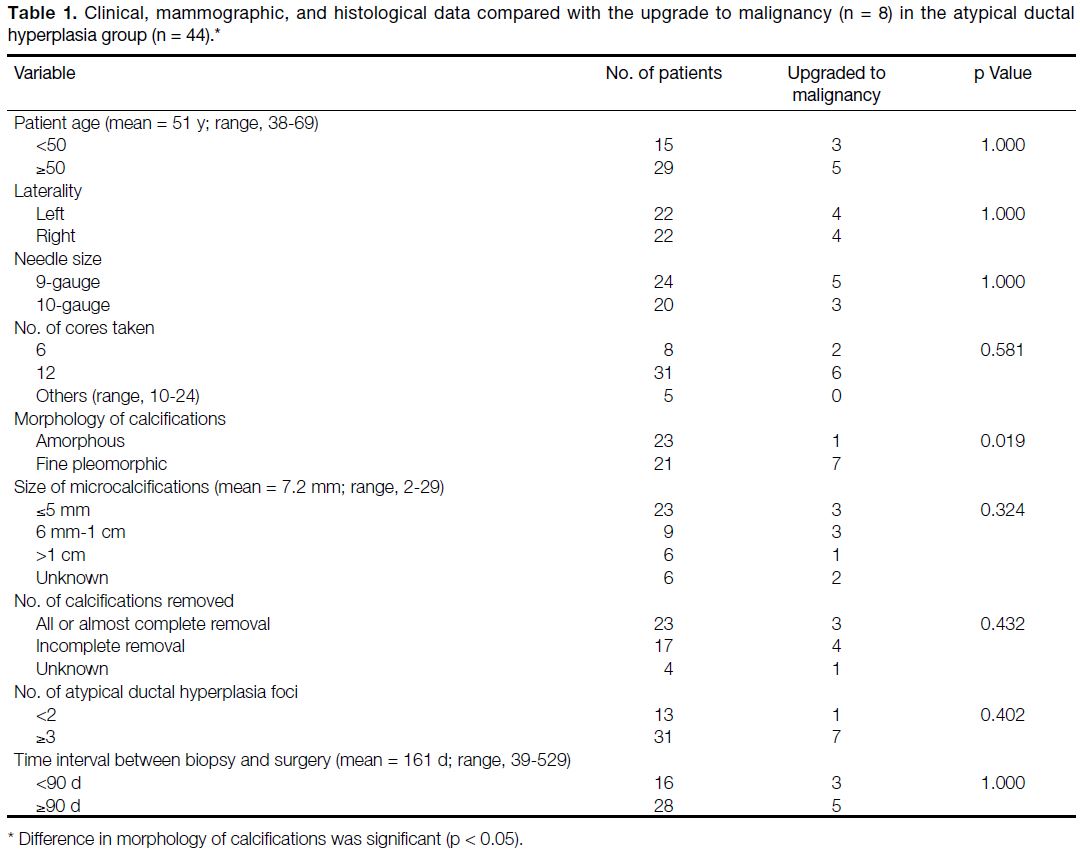
Table 1. Clinical, mammographic, and histological data compared with the upgrade to malignancy (n = 8) in the atypical ductal
hyperplasia group (n = 44).
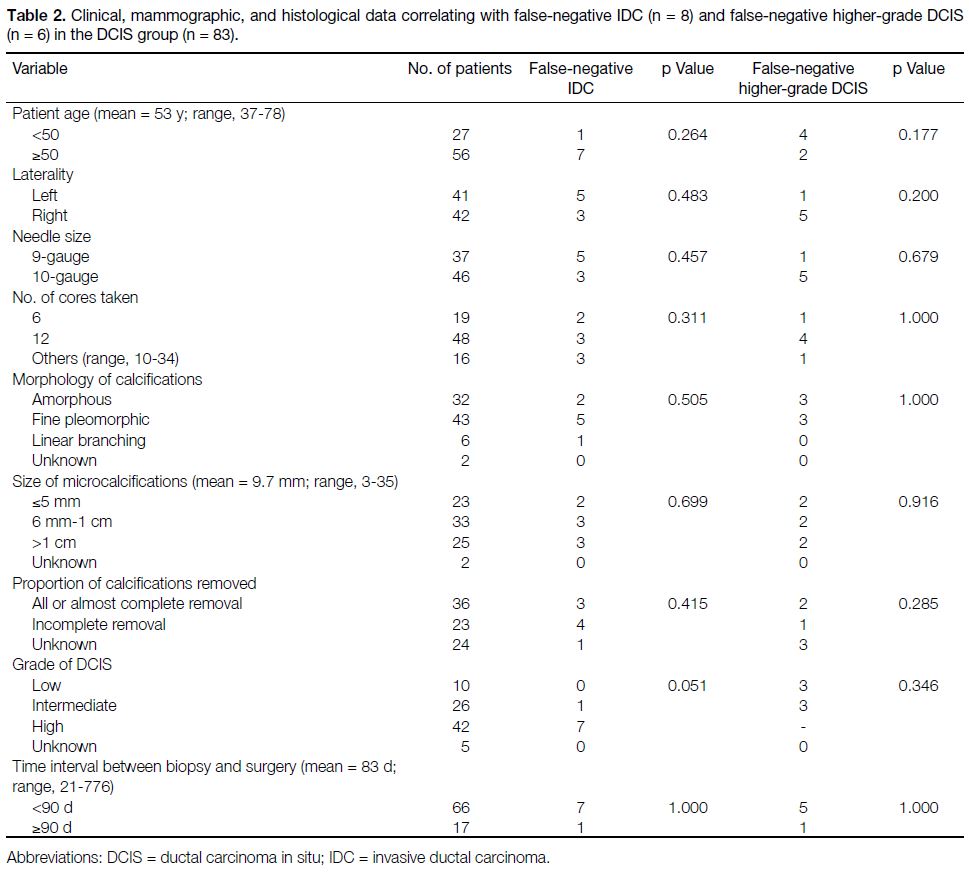
Table 2. Clinical, mammographic, and histological data correlating with false-negative IDC (n = 8) and false-negative higher-grade DCIS
(n = 6) in the DCIS group (n = 83).
Data Analysis
Underestimated DCIS or IDC diagnoses refer to lesions
with an initial VABB diagnosis of ADH that was
upgraded to DCIS or IDC, or DCIS that was upgraded
to IDC, in the surgical specimen histopathology. DCIS
cases with pathological evidence of microinvasion were
considered invasive.
Data were analysed with SPSS (Windows version 23.0;
IBM Corp, Armonk [NY], US). To identify factors that
affected underestimated DCIS and IDC diagnoses, the
association between categorical variables was evaluated using Fisher’s exact test or the Chi squared test. A p
value <0.05 was considered significant. For specimens
without microcalcifications, the positive predictive value
and negative predictive value (NPV) of the presence
of histological microcalcifications or pathology (ADH/DCIS) with respect to upgrade to DCIS/IDC in the
surgical specimen were calculated. Missing or unknown
data were excluded from statistical analysis.
RESULTS
During the 10-year study period, a total of 171 patients
were diagnosed with ADH (n = 78) and DCIS (n = 93)
by stereotactic-guided VABB, of which 35 patients in
the ADH group and 10 patients in the DCIS group with
no corresponding surgical histopathology correlation at
sites of VABB were excluded. The study finally included
43 patients (mean age = 51 years; range, 38-69) with 44
lesions in the ADH group, and 83 patients (mean age = 53 years; range, 37-78) with 83 lesions in the DCIS group.
One patient in the ADH group had bilateral lesions and
underwent two separate biopsies.
Atypical Ductal Hyperplasia Group
Clinical, mammographic, and histological data were
evaluated and correlated with the underestimation rate
(Table 1).
The mean age of the patients was 51 years, with equal
distribution of the lesions in the right and left breasts.
The size of microcalcification clusters detected on the
preprocedural mammogram ranged from 2 to 29 mm
(mean = 7.2). Out of the 44 lesions, 24 were
removed via 9-gauge needles, while 20 of them were
removed via 10-gauge needles. Most lesions had either
6 or 12 cores taken. Some lesions had more specimens
taken depending on individuals’ clinical circumstances (i.e., when microcalcifications were note detected on
the first specimen radiograph). All lesions in the ADH
group were categorised as BI-RADS 4B. Some of the
radiographs (including the postprocedural mammogram)
were not retrievable from the system and therefore some
data are missing for some patients, yet at least 52.3%
(n = 23) of lesions designated as ADH by VABB were
completely or almost completely removed during
the procedure according to available mammography.
Approximately 30% (n = 13) of these lesions contained
less than two ADH foci, while the rest (70%; n = 31) had
more than two foci of ADH in the specimen. All of these
lesions underwent subsequent surgical excision with a
mean of 161 days between VABB and surgery.
Of the 44 lesions diagnosed with ADH by VABB, the
final histopathologic diagnosis was also ADH in 36. In
eight lesions (18.2%), seven DCIS and one IDC were
diagnosed in the subsequent surgical specimen. Apart
from one case in the underestimated DCIS/IDC group
presenting with unilateral amorphous microcalcifications,
the other seven had presented with fine pleomorphic
microcalcifications (p = 0.019). All other variables
including age, laterality of the lesion, size of needle,
number of cores taken, size of microcalcifications,
complete versus incomplete removal of the calcifications,
number of ADH foci on VABB, and the time interval
between biopsy and surgery showed no significant
association with the underestimated diagnosis.
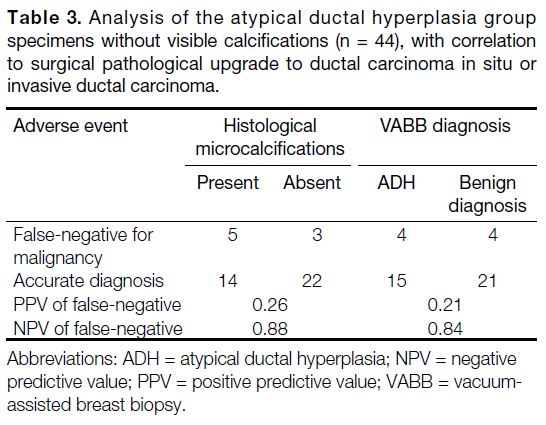
As mentioned earlier, we separated the specimens (of
the same biopsy site) according to presence or absence
of visible calcifications. In the pathology results of
those without visible calcifications, NPV was high for
malignancy in the absence of microcalcifications (0.88)
or when benign pathology (0.84) was found in the
specimens (Table 3).
Table 3. Analysis of the atypical ductal hyperplasia group
specimens without visible calcifications (n = 44), with correlation
to surgical pathological upgrade to ductal carcinoma in situ or
invasive ductal carcinoma.
Ductal Carcinoma In Situ Group
Clinical, mammographic, and histological data were
evaluated and correlated in the DCIS underdiagnosis
subgroup (Table 2).
The mean age of the patients was 53 years. The size of
microcalcification clusters detected on the preprocedural
mammogram ranged from 3 to 35 mm (mean = 9.7).
Out of the 83 lesions, 37 lesions were retrieved via
9-gauge needles and 46 lesions via 10-gauge needles. Most lesions had either 6 or 12 cores taken. Some lesions
had more specimens taken depending on individuals’
clinical circumstances. Most of the lesions (n = 75) were
BI-RADS 4B lesions while a small proportion (n = 6)
were BI-RADS 4C lesions. At least 43.3% (n = 36) of
lesions were completely or almost completely removed
during the VABB. The DCIS lesions were further
categorised into low- (n = 10), intermediate- (n = 26) or
high-grade (n = 42) lesions according to the Van Nuys
DCIS Classification. All of these lesions underwent
subsequent surgical excision with a mean of 83 days
between VABB and surgery.
Of the 83 lesions with the post-biopsy diagnosis of DCIS,
75 lesions had the same pathology and eight lesions had
IDC revealed on the subsequent surgical specimens;
hence the underdiagnosis rate of invasive carcinoma
was 9.6%. No variables, including patient age, laterality
of the lesion, size of needle, number of cores taken,
size/morphology of microcalcifications, complete or
incomplete removal of the calcifications, or the time
interval between biopsy and surgery were significantly
associated with IDC. Among the 75 DCIS lesions
without evidence of invasion on biopsy specimens, six of
them (7.2%, 6/83) were upgraded to higher DCIS grades
in the subsequent surgical specimen. This included three
lesions with an initial diagnosis of low-grade DCIS (2
of them upgraded to intermediate-grade and 1 to high-grade),
and three lesions with intermediate-grade DCIS
(upgraded to high-grade). There were again no variables
significantly associated with upgrade to a higher grade
of DCIS.
Similarly, we reviewed the pathology results of specimens
without visible microcalcifications. There were high
NPVs for pathological upgrade when no histological
microcalcifications (0.87) or benign pathology (0.94)
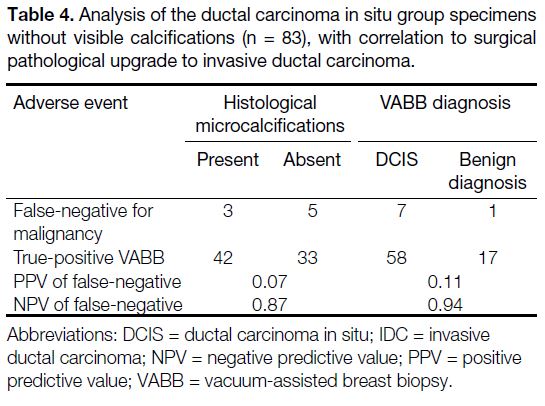
were found in the specimens (Table 4).
Table 4. Analysis of the ductal carcinoma in situ group specimens
without visible calcifications (n = 83), with correlation to surgical
pathological upgrade to invasive ductal carcinoma.
DISCUSSION
This study included a highly selected group of patients
with ADH or DCIS diagnosed by VABB, with
microcalcifications depicted by mammogram and not by
ultrasound. Stereotactic-guided VABB is a minimally
invasive and reliable technology for sampling of
mammographic microcalcifications.[10] Underestimation
of carcinoma and/or invasion associated with VABB-proven
ADH and DCIS are unavoidable. In our cohort,
18.2% of patients diagnosed with ADH by VABB had
malignancy found in the subsequent surgical specimen
and 9.6% of patients underdiagnosed with DCIS had IDC. These underdiagnosis rates were similar and
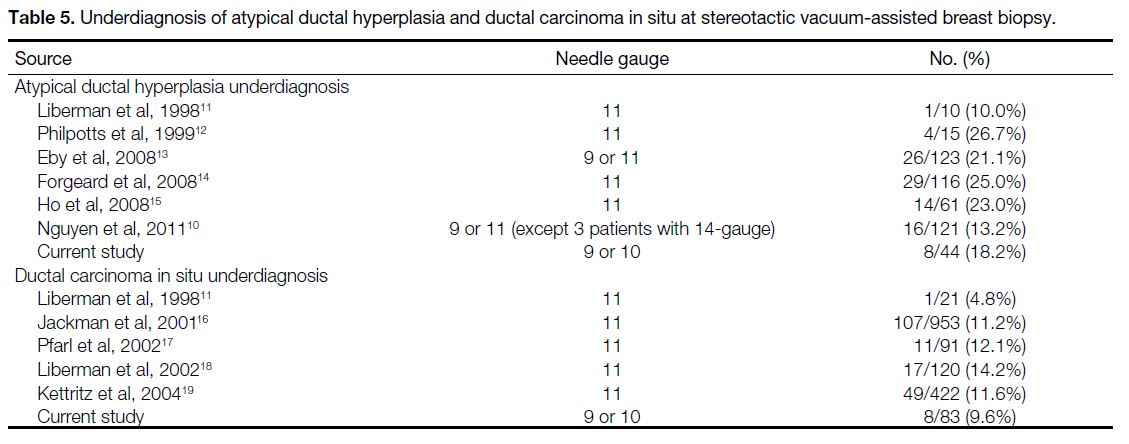
comparable to other studies (Table 5).[10] [11] [12] [13] [14] [15] [16] [17] [18] [19]
Table 5. Underdiagnosis of atypical ductal hyperplasia and ductal carcinoma in situ at stereotactic vacuum-assisted breast biopsy.
All the specimens in this study were sampled by either
9-gauge or 10-gauge needles, and inclusion of an
adequate number of microcalcifications was confirmed
on specimen radiography. Lourenco et al[6] showed no
significant difference between 11-gauge and 9-gauge
biopsy needles in the underdiagnosis of ADH and DCIS.
The use of 9- or 10-gauge biopsy needles in our study
had no significant impact on the underdiagnosis rate
(p = 1.000 and 0.679 for the ADH and DCIS groups,
respectively). Most specimens had either 6 or 12 cores
of tissues retrieved, equivalent to 180º and 360º of
probe rotation if a specimen was taken at each clock
position, respectively (the degree of probe rotation may
vary with the location of the microcalcifications and
operator preference). A few had >12 cores taken, mainly
due to difficult localisation of the lesion or lesions with
scarce microcalcifications. According to Lomoschitz
et al,[20] the highest diagnostic yield was achieved with
12 specimens per lesion, although underdiagnosis still
occurred with retrieval of 20 specimens per lesion. Our
study demonstrated no significant correlation of the
underdiagnosis rate with the number of specimens taken.
There is lack of universal consensus on predictors
associated with underdiagnosis of pathology across
various studies.[10] Our study demonstrates that the
presence of amorphous calcifications is associated with
a lower rate of malignancy underdiagnosis in ADH
lesions (p = 0.019). Oligane et al[21] showed similar
findings in stereotactic biopsy of clustered amorphous calcifications, which were rarely associated with
aggressive malignancy, yet biopsy of the amorphous
calcifications remained necessary, with a malignancy
rate of 7%. Amorphous calcifications, however, were
not a significant predictor in the DCIS underdiagnosis
subgroup (p = 0.505) or in other similar studies.[15] [22] [23]
A few studies[11] [24] [25] have demonstrated no
underdiagnoses among cases of ADH in which the entire
lesion seen on mammography was removed at VABB.
In our series, 23 ADH lesions with microcalcifications
were completely removed during the biopsy, with three
(13%) of them upgraded to DCIS on the subsequent
surgical specimen. Similarly for DCIS, three out of
36 (8.3%) lesions with microcalcifications completely
removed during biopsy were underdiagnosed. Our study
also demonstrated that lesion size was not a significant
predictor of underdiagnosis for either the ADH
(p = 0.324) or DCIS subgroups (p = 0.699) [Tables 1
and 2].
The mean time intervals between VABB and surgery
were 161 and 83 days for the ADH and DCIS groups,
respectively. While it is logical to deduce underdiagnosis
of pathology could be the result of disease progression
during the lag time between biopsy and surgery, our
results demonstrated no significant differences in the
underdiagnosis rates related to this factor. Indeed, out of
nine lesions with a final diagnosis of IDC in the DCIS
group, seven of them had had surgery done within
3 months of the biopsy.
Histologically, there was no significant difference in the underdiagnosis rate in lesions with fewer than three ADH
foci (p = 0.402) compared to lesions with greater than
three ADH foci. Among 13 lesions with fewer than three
foci in our study, one lesion containing a focus of ADH
had malignancy (intermediate-grade DCIS) detected in
the surgical specimen. This finding is in contrast to that
reported by Sneige et al[26]: within a cohort of 42 cases,
none of the 16 patients with one or two foci of ADH was
found to have DCIS or invasive cancer at surgery.
For the DCIS/IDC underdiagnosis subgroup, none of the
patients with low-grade DCIS was found to have invasive
cancer at surgery (n = 10), yet this was not statistically
significant as a predictor (p = 0.051), and was probably
related to the relatively small number of patients with
low-grade DCIS compared to the number of patients
with intermediate- (n = 26) and high-grade DCIS (n =
42). According to Meurs et al,[27] in a study of 2892 DCIS
biopsies, the underdiagnosis rate was the lowest (15%)
for low-grade DCIS compared to intermediate- (20%)
and high-grade subgroups (23%) in their model, which
was comparable to our findings.
We specifically analysed the pathological results for
specimens without visible microcalcifications. We found
high NPVs for underdiagnosis (0.84-0.94) in both ADH
and DCIS groups without visible microcalcifications on
mammography when no histological microcalcifications
or benign pathology were found in the specimens.
These results suggest that underdiagnosis is less likely
under these circumstances. Nonetheless, the presence
of histological microcalcifications or positive pathology
(ADH/DCIS according to the group) in the specimens
without visible calcifications were not useful predictors
of underdiagnosis (positive predictive value = 0.07-0.26). Recent studies[22] [23] have also demonstrated that
analysis of specimens without microcalcifications
may be beneficial in determining the likelihood of
underdiagnosis. Further studies with larger cohorts to
verify this hypothesis are necessary.
We identified a few weaknesses in this retrospective
study. Our relatively small sample size and the
retrospective nature of the study based on data,
mammography, and specimen radiograph review might
have influenced the statistical analysis. Some of the
data and radiographs could not be retrieved, resulting
in a smaller sample size for several parameters. Inter-observer
variability of histopathological analysis cannot
be excluded.
CONCLUSION
Our study showed that the DCIS/IDC underdiagnosis
rates of ADH and DCIS diagnosed with vacuum-assisted
biopsies with 9- or 10-gauge needles were 18.2% and
9.6%, respectively. Our study demonstrated that the
presence of amorphous calcifications was associated
with a lower rate of malignancy upgrade in ADH lesions.
No other predictors of underdiagnosis for both ADH
and DCIS were identified. Surgical excisional biopsy
is recommended for all biopsy-proven ADH and DCIS
lesions for definitive pathology.
REFERENCES
1. Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of
mammographically detected clustered microcalcifications.
Radiology. 2000;217:849-54. Crossref
2. East EG, Carter CS, Kleer CG. Atypical ductal lesions of the breast:
criteria, significance, and laboratory updates. Arch Pathol Lab Med.
2018;142:1182-5. Crossref
3. Bombonati A, Sgroi DC. The molecular pathology of breast cancer
progression. J Pathol. 2011;223:307-17. Crossref
4. Gomes DS, Porto SS, Balabram D, Gobbi H. Inter-observer
variability between general pathologists and a specialist in breast
pathology in the diagnosis of lobular neoplasia, columnar cell
lesions, atypical ductal hyperplasia and ductal carcinoma in situ
of the breast. Diagn Pathol. 2014;9:121. Crossref
5. Elston CW, Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP,
Bianchi S, et al. Causes of inconsistency in diagnosing and
classifying intraductal proliferations of the breast. European
Commission Working Group on Breast Screening Pathology. Eur
J Cancer. 2000;36:1769-72. Crossref
6. Lourenco AP, Mainiero MB, Lazarus E, Giri D, Schepps B.
Stereotactic breast biopsy: comparison of histologic underestimation
rates with 11- and 9-gauge vacuum-assisted breast biopsy. AJR Am
J Roentgenol. 2007;189:W275-9. Crossref
7. Schiaffino S, Calabrese M, Melani EF, Trimboli RM, Cozzi A,
Carbonaro LA, et al. Upgrade rate of percutaneously diagnosed pure
atypical ductal hyperplasia: systematic review and meta-analysis
of 6458 lesions. Radiology. 2020;294:76-86. Crossref
8. Brennan ME, Turner RM, Ciatto S, Marinovich ML, French JR,
Macaskill P, et al. Ductal carcinoma in situ at core-needle biopsy:
meta-analysis of underestimation and predictors of invasive breast
cancer. Radiology. 2011;260:119-28. Crossref
9. Esen G, Tutar B, Uras C, Calay Z, İnce Ü, Tutar O. Vacuum-assisted
stereotactic breast biopsy in the diagnosis and management of
suspicious microcalcifications. Diagn Interv Radiol. 2016;22:326-33. Crossref
10. Nguyen CV, Albarracin CT, Whitman GJ, Lopez A, Sneige N.
Atypical ductal hyperplasia in directional vacuum-assisted biopsy
of breast microcalcifications: considerations for surgical excision.
Ann Surg Oncol. 2011;18:752-61. Crossref
11. Liberman L, Smolkin JH, Dershaw DD, Morris EA, Abramson AF,
Rosen PP. Calcification retrieval at stereotactic, 11-gauge,
directional, vacuum-assisted breast biopsy. Radiology.
1998;208:251-60. Crossref
12. Philpotts LE, Shaheen NA, Carter D, Lange RC, Lee CH.
Comparison of rebiopsy rates after stereotactic core needle biopsy
of the breast with 11-gauge vacuum suction probe versus 14-gauge
needle and automatic gun. AJR Am J Roentgenol. 1999;172:683-7. Crossref
13. Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S,
Lehman CD. Is surgical excision necessary for focal atypical ductal
hyperplasia found at stereotactic vacuum-assisted breast biopsy?
Ann Surg Oncol. 2008;15:3232-8. Crossref
14. Forgeard C, Benchaib M, Guerin N, Thiesse P, Mignotte H,
Faure C, et al. Is surgical biopsy mandatory in case of atypical ductal
hyperplasia on 11-gauge core needle biopsy? A retrospective study
of 300 patients. Am J Surg. 2008;196:339-45. Crossref
15. Ho JT, Tan PH, Hee SW, Wong JS. Underestimation of
malignancy of atypical ductal hyperplasia diagnosed on 11-gauge
stereotactically guided Mammotome breast biopsy: an Asian breast
screen experience. Breast. 2008;17:401-6. Crossref
16. Jackman RJ, Burbank F, Parker SH, Evans WP 3rd, Lechner MC,
Richardson TR, et al. Stereotactic breast biopsy of nonpalpable
lesions: determinants of ductal carcinoma in situ underestimation
rates. Radiology. 2001;218:497-502. Crossref
17. Pfarl G, Helbich TH, Riedl CC, Wagner T, Gnant M, Rudas M, et al.
Stereotactic 11-gauge vacuum assisted breast biopsy: a validation
study. AJR Am J Roentgenol. 2002;179:1503-7. Crossref
18. Liberman L, Kaplan JB, Morris EA, Abramson AF, Menell JH,
Dershaw DD. To excise or to sample the mammographic target:
what is the goal of stereotactic 11-gauge vacuum-assisted breast
biopsy? AJR Am J Roentgenol. 2002;179:679-83. Crossref
19. Kettritz U, Rotter K, Schreer I, Murauer M, Schulz-Wendtland R,
Peter D, et al. Stereotactic vacuum-assisted breast biopsy in 2874
patients: a multicenter study. Cancer. 2004;100:245-51. Crossref
20. Lomoschitz FM, Helbich TH, Rudas M, Pfarl G, Linnau KF,
Stadler A, et al. Stereotactic 11-gauge vacuum-assisted breast
biopsy: influence of number of specimens on diagnostic accuracy.
Radiology. 2004;232:897-903. Crossref
21. Oligane HC, Berg WA, Bandos AI, Chen SS, Sohrabi S, Anello M,
et al. Grouped amorphous calcifications at mammography:
frequently atypical but rarely associated with aggressive
malignancy. Radiology. 2018;288:671-9. Crossref
22. Cheung YC, Chen SC, Ueng SH, Yu CC. Ductal carcinoma in
situ underestimation of microcalcifications only by stereotactic
vacuum-assisted breast biopsy: a new predictor of specimens
without microcalcifications. J Clin Med. 2020;9:2999. Crossref
23. Yu CC, Cheung YC, Ueng SH, Chen SC. Impact of non-calcified
specimen pathology on the underestimation of malignancy for
the incomplete retrieval of suspicious calcifications diagnosed as
flat epithelial atypia or atypical ductal hyperplasia by stereotactic
vacuum-assisted breast biopsy. Korean J Radiol. 2020;21:1220-9. Crossref
24. Adrales G, Turk P, Wallace T, Bird R, Norton HJ, Greene F. Is
surgical excision necessary for atypical ductal hyperplasia of the
breast diagnosed by Mammotome? Am J Surg. 2000;180:313-5. Crossref
25. Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I.
Underestimation of breast cancer with II-gauge vacuum suction
biopsy. AJR Am J Roentgenol. 2000;175:1047-50. Crossref
26. Sneige N, Lim SC, Whitman GJ, Krishnamurthy S, Sahin AA,
Smith TL, et al. Atypical ductal hyperplasia diagnosis by directional
vacuum-assisted stereotactic biopsy of breast microcalcifications.
Considerations for surgical excision. Am J Clin Pathol.
2003;119:248-53. Crossref
27. Meurs CJ, van Rosmalen J, Menke-Pluijmers MB, Ter Braak BP,
de Munck L, Siesling S, et al. A prediction model for underestimation
of invasive breast cancer after a biopsy diagnosis of ductal
carcinoma in situ: based on 2892 biopsies and 589 invasive cancers.
Br J Cancer. 2018;119:1155-62. Crossref