Radar Localisation of Non-palpable Breast Lesions in a Chinese Population: a Pilot Study
ORIGINAL ARTICLE CME
Radar Localisation of Non-palpable Breast Lesions in a Chinese Population: a Pilot Study
SC Woo, T Wong, CM Chau, WY Fung, RLS Chan, AWT Yung, JKF Ma
Department of Radiology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr SC Woo, Department of Radiology, Princess Margaret Hospital, Hong Kong. Email: stephaniecheriwoo@gmail.com
Submitted: 31 Mar 2021; Accepted: 24 Aug 2021.
Contributors: All authors designed the study. SCW and TW acquired and analysed the data, and drafted the manuscript. All authors critically
revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by Kowloon West Cluster Research Ethics Committee of Hospital Authority (Ref: KW/EX-21-027(156-09)). The requirement for patient consent was waived.
Abstract
Introduction
We performed a retrospective review of a radar-based breast lesion localisation system (Savi Scout;
Cianna Medical, Merit Medical Systems, Inc., South Jordan [UT], United States) in a Chinese population.
Methods
Placement success (final target-to-reflector distance <10 mm), retrieval success, margin clearance, and
re-excision rates were reviewed in the cases of 23 Chinese patients who underwent guided nonpalpable breast lesion
excision from October 2019 to December 2020 using the system in a single institution.
Results
Twenty-three reflectors were placed under sonographic (n=13; 57%) or stereotactic (n=10; 44%) guidance
to localise 23 target lesions. There was no delayed migration for the 20 reflectors placed before the day of surgery.
Placement success was achieved in 21 (91%). Mean final target-to-reflector distance was 3 mm. Of the 23 lesions,
two (9%) required alternative localisation owing to reflector distance ≥10 mm away from the target. Retrieval
success was achieved in 22 (96%). Deactivation of a reflector was noted in one case. Of these 23 lesions, three were
excised for therapeutic intent, of which one required re-excision due to close margins. There were no procedure-related
complications.
Conclusion
This radar-based localisation system is a safe and effective device for guiding the excision of non-palpable
breast lesions in a Chinese population. Its advantages, such as the fact that it causes minimal artefacts on
magnetic resonance imaging, may render it a superior alternative in selected patients.
Key Words: Breast; Breast neoplasms; Mammography; Mastectomy, segmental; Ultrasonography
中文摘要
華人人群不可觸及乳腺病變的雷達定位:初步研究
鄔潔欣、黃婷、周智敏、馮惠鈺、陳樂詩、翁維德、馬嘉輝
引言
我們對一種基於雷達的乳腺病變定位系統(Savi Scout)進行一項華人回顧性研究。
方法
對2019年10月至2020年12月期間在單中心接受該系統引導式不可觸乳腺病灶切除術的23名華人患者有關植入成功率(最終目標與反射器距離<10 mm)、回取成功率、邊緣清除率和再切除率進行回顧。
結果
13個反射器(57%)於超聲引導下放置,10個反射器(43%)於立體定向引導下放置,共定位23個目標病灶。手術當天之前放置的20個反射器沒有延遲移位。21例(91%)放置成功。平均最終目標與反射器的距離為3 mm。由於反射器與距離目標間≥10 mm,2個病變(9%)需要其他方式定位。22例(96%)回取成功。1例反射器失活。23個病灶中,3例因治療目的而切除,其中1例因切緣較近須重新切除。沒有手術相關的併發症。
結論
這雷達系統安全有效,能引導華人乳房不可觸及病灶的切除。其優勢克服其他定位方法的一些限制,例如減少磁共振成像偽影,對特定患者可成為良好替代方案。
INTRODUCTION
Excision of non-palpable breast lesions is traditionally
performed by preoperative image-guided wire
localisation. It is the most widely used method for
localisation and has been the standard technique for
decades.[1] However, this technique has several drawbacks
including patient discomfort and possible wire transection
and displacement. Furthermore, it poses limitations in
scheduling flexibility, as the procedure has to be done
on the same day as the surgery. It also limits the surgical
approach and necessitates larger amounts of healthy
breast tissue to be excised due to the presence of the
wire.[2] [3] [4] [5] [6] Hence, newer techniques have been developed to
overcome the limitations of wire localisation, including
radioguided occult lesion localisation, radar reflector
localisation (Savi Scout; Cianna Medical, Merit Medical
Systems, Inc., Aliso Viejo [CA], US), magnetic seed
localisation (Magseed; Endomagnetics, Cambridge,
United Kingdom) and radiofrequency identification tag
localisation (LOCalizer; Hologic, Marlborough [MA],
US).[1]
At our institution, we reviewed the magnetic seed
localisation technique, which gave promising preliminary
results.[7] In this paper, we report on our evaluation of the
radar-based localisation device. The methodology used
and the outcomes analysed were similar to those of the
prior study.
The radar-based surgical guidance system received
510(k) US Food and Drug Administration approval in
December 2014. It was introduced in Hong Kong in late
2019. Our goal was to evaluate the safety and efficacy of
radar-based localisation of non-palpable breast lesions.
To the best of our knowledge, this is the first publication
on radar-based localisation in a Chinese population.
METHODS
A single-institution retrospective review of 23 women
who underwent radar-based localisation (Savi Scout)[8]
for non-palpable breast lesions from October 2019 to
December 2020 was conducted. Patients were selected
in consensus by breast radiologists and breast surgeons
through reviewing images on target visibility and target
depth, and whether the patients had any nickel allergy
or cardiac implants. Patients who had a reflector placed
but underwent no surgery, and patients who underwent
mastectomy instead of lumpectomy were excluded.
Localisation Procedure
The localisation procedure is largely similar to that
described in the study of magnetic seed localisation by
Fung et al,[7] as it is performed in the same institution and
by the same group of radiologists.
Percutaneous image-guided reflector placement was
performed by one of the four breast radiologists at our institution with 3 to 19 years of experience in performing
image-guided breast localisation, or by a breast radiology
trainee who was directly supervised by one of the
breast radiologists, using a sterile single-use preloaded
16-gauge needle (7.5 or 10 cm long).
During ultrasound-guided placement, the patient was
positioned supine and rolled slightly towards the
contralateral side of the involved breast, with a wedge
placed under the ipsilateral shoulder, with the ipsilateral
arm abducted over the patient’s head, to spread the
breast thickness evenly. Target-to-reflector distance
was evaluated in real time. During stereotactic-guided
placement, patients either lay ipsilateral or contralateral
decubitus or sat erect to facilitate breast compression by
the stereotactic apparatus, and target-to-reflector distance
was measured on post-placement mammograms in the
mediolateral and craniocaudal projections.
For patients with reflectors inserted prior to the day of
surgery, additional ultrasound and/or mammography
was performed on the day of surgery for assessment of
any delayed target migration. Significant migration was
defined as a final target-to-reflector distance ≥10 mm
more than on the initial images generated during
reflector placement. In the case of significant migration,
an alternative localisation method was employed.
Radar-based guided excision was performed with
the depth of the reflector from skin first assessed by
ultrasound. For the excision, the surgeon positioned the
patient supine, with the ipsilateral arm abducted 90°.
The reflector was localised intraoperatively by breast surgeons with the use of the handpiece and console as
described above. Specimen radiographs were acquired in
all cases to confirm target lesion removal. Radial margins
of the target lesion were also evaluated on specimen
radiograph.
Outcome Analysis
We followed the outcome analysis described in a study
of magnetic seed localisation by Fung et al.[7]
Placement success rate and retrieval success rate with
95% confidence intervals (CIs) were calculated.
Placement success was defined as a final target-to-reflector
distance ≤10 mm in any plane on images on
the day of surgery.[9] For cases that achieved placement
success, the final target-to-reflector distances were
further subdivided into <2 mm, 2 to 5 mm, and 6 to 9 mm. Retrieval success was defined by localisation
by the handpiece of the presence of the reflector in
the first specimen radiograph. Patient demographics,
preoperative pathology (if any), and surgical indications
were reviewed through the electronic patient records.
The target lesions were categorised into two groups:
those resected with therapeutic intent and those resected
with diagnostic intent.
Among the cases with therapeutic intent, margin
clearance was assessed. Margin clearance was defined
as ≥2 mm disease-free margins. The re-excision rate
due to inadequate margin clearance was analysed.
Complications related to reflector deployment and
surgeries were recorded.
RESULTS
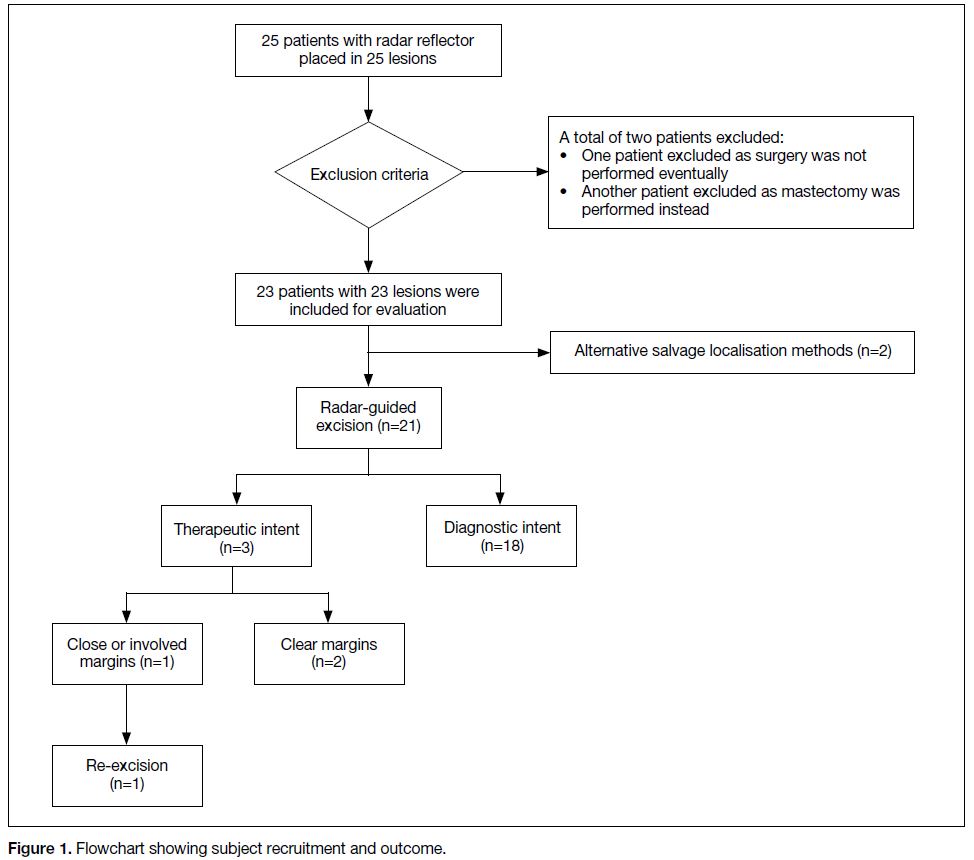
A total of 25 patients were selected for reflector
placement during the study period. Two patients were
excluded (Figure 1); one patient because the surgery was
not performed as she was diagnosed with concomitant
Stage IV lung cancer after the reflector placement.
Another patient was excluded as the breast tumour
was subsequently found to have rapidly enlarged and
the patient opted for mastectomy. A total of 23 female
patients remained, with 23 reflectors placed (Table 1).
The mean age of the patients was 55 years (range, 27-74).
Figure 1. Flowchart showing subject recruitment and outcome.
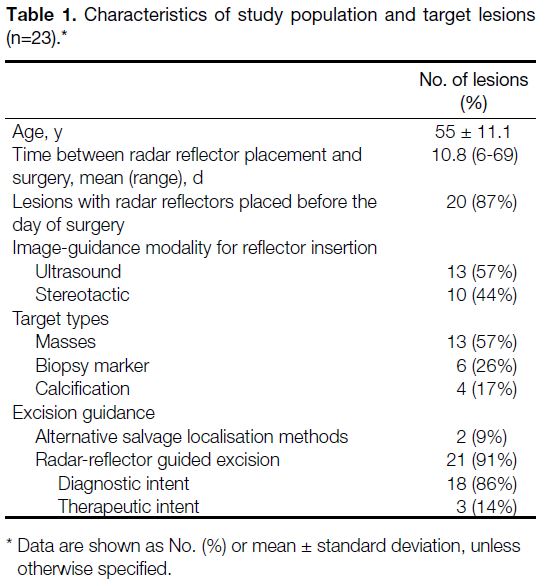
Table 1. Characteristics of study population and target lesions (n=23).
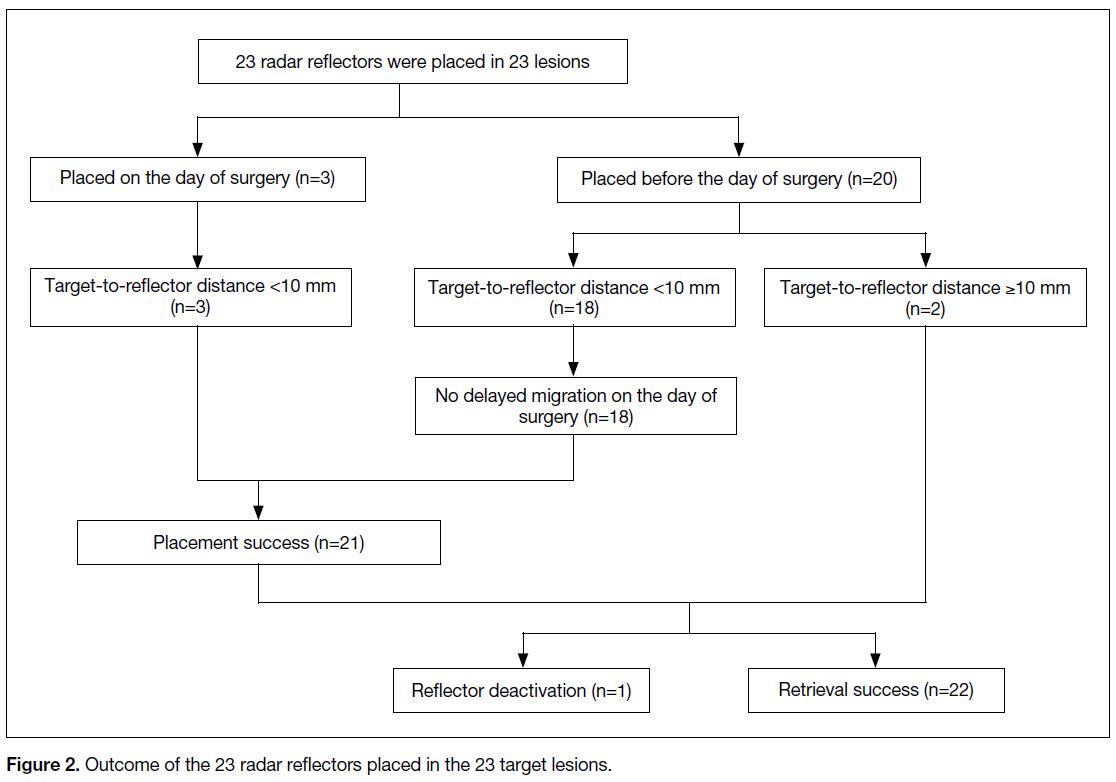
In total, 23 reflectors were placed to localise 23 target lesions (Figure 2). Three of them (13%) were placed
on the day of surgery, while 20 (87%) reflectors were
inserted 6 to 69 days (mean 10.8 ± 13.6) before the day
of surgery in an out-patient setting.
Figure 2. Outcome of the 23 radar reflectors placed in the 23 target lesions.
Of 23 reflectors, 13 (57%) were placed under
sonographic guidance (Figure 3), and 10 (44%) were
placed under stereotactic guidance (Figure 4). The most
common type of target lesion was a mass (n=13; 57%).
The second commonest type was biopsy markers (n=6; 26%), and the remainder were microcalcifications (n=4; 17%).
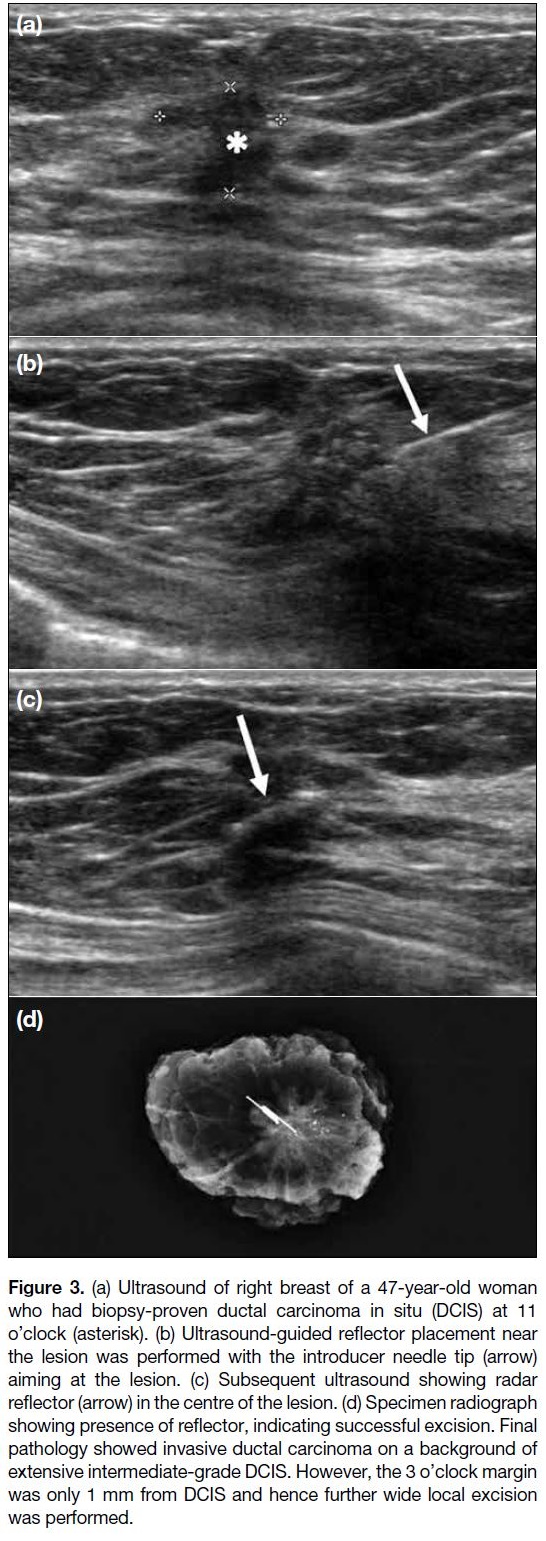
Figure 3. (a) Ultrasound of right breast of a 47-year-old woman
who had biopsy-proven ductal carcinoma in situ (DCIS) at 11
o’clock (asterisk). (b) Ultrasound-guided reflector placement near
the lesion was performed with the introducer needle tip (arrow)
aiming at the lesion. (c) Subsequent ultrasound showing radar
reflector (arrow) in the centre of the lesion. (d) Specimen radiograph
showing presence of reflector, indicating successful excision. Final
pathology showed invasive ductal carcinoma on a background of
extensive intermediate-grade DCIS. However, the 3 o’clock margin
was only 1 mm from DCIS and hence further wide local excision
was performed.
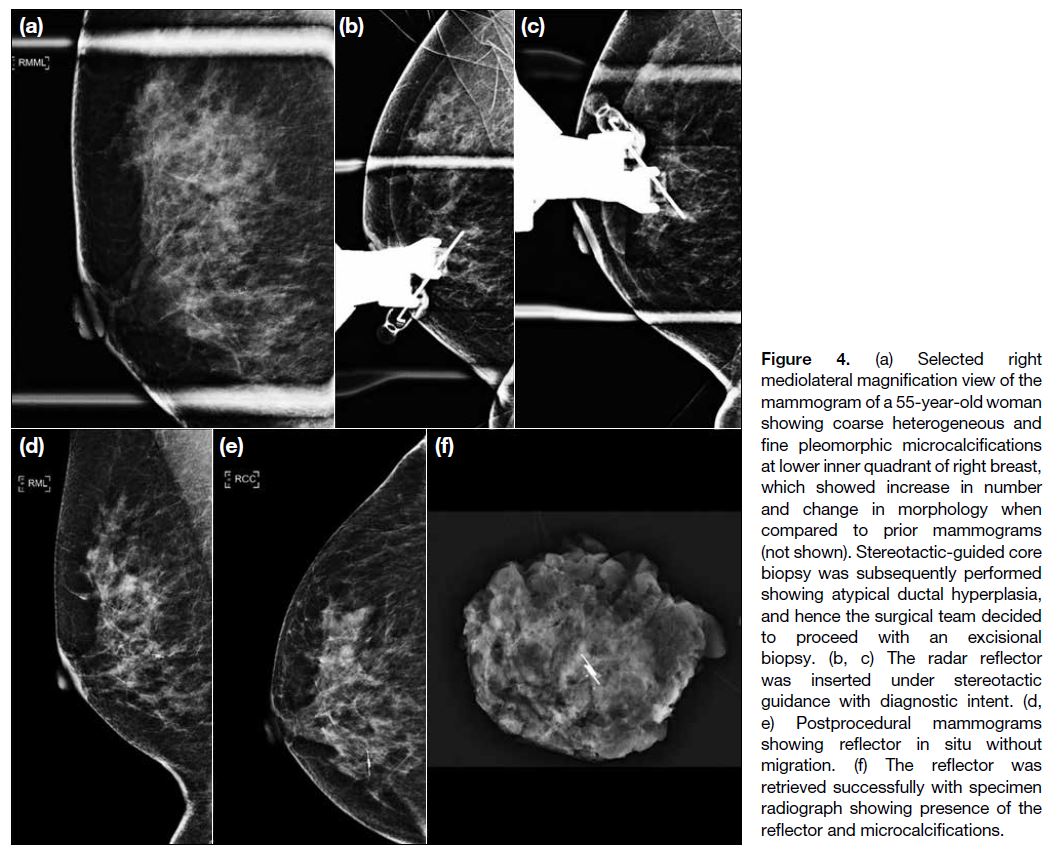
Figure 4. (a) Selected right mediolateral magnification view of the mammogram of a 55-year-old woman showing coarse heterogeneous and fine pleomorphic microcalcifications at lower inner quadrant of right breast, which showed increase in number and change in morphology when compared to prior mammograms (not shown). Stereotactic-guided core biopsy was subsequently performed showing atypical ductal hyperplasia, and hence the surgical team decided to proceed with an excisional biopsy. (b, c) The radar reflector was inserted under stereotactic guidance with diagnostic intent. (d, e) Postprocedural mammograms showing reflector in situ without migration. (f) The reflector was retrieved successfully with specimen radiograph showing presence of the reflector and microcalcifications.
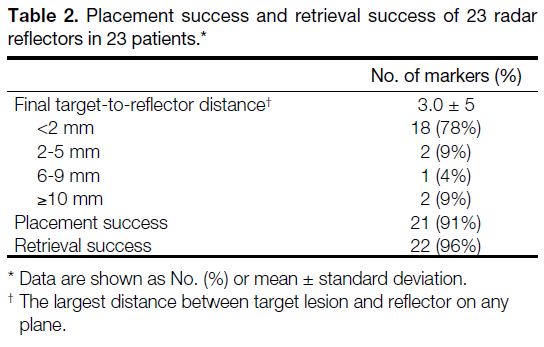
Among the 23 reflectors, 18 (78%) were within 2 mm,
two (9%) were 2 to 5 mm, and one (4%) was 6 to
9 mm from the target. Two reflectors (9%) had an initial
target-to-reflector distance ≥10 mm, unassociated with
delayed migration, and underwent localisation by other
methods. There was no delayed migration (0%) in any of
the 20 reflectors placed before the day of surgery. Hence, placement success was achieved in 21 (91%) reflectors
(95% CI=73.2%-97.6%). The mean final target-to-reflector
distance was 3.0 ± 5 mm (Table 2). These
21 lesions had guided excision of the lesions as planned,
with sonographic depth of the reflectors of 6 mm to
19 mm (mean 11 ± 3.4) from the skin surface.
Table 2. Placement success and retrieval success of 23 radar reflectors in 23 patients
Deactivation of the reflector was noted in one case.
The remaining 22 (96%) reflectors were localised by
the handpiece and appeared on the initial specimen
radiographs (95% CI = 79.0%-99.2%).
Among the 21 lesions successfully excised, 18 (86%)
were excised with diagnostic intent and three (14%) with
therapeutic intent.
For the three lesions excised with therapeutic intent,
one of them showed invasive ductal carcinoma on both
preoperative biopsy and final surgical pathology. The
second case showed atypical lobular hyperplasia with
a microscopic focus of low-grade ductal carcinoma
in situ (DCIS) on preoperative biopsy, and final
surgical pathology revealed lobular carcinoma in situ
without definite DCIS. The last case showed DCIS on
preoperative biopsy, while final surgical pathology came
back to be invasive ductal carcinoma with a background
of extensive DCIS. This lesion had narrow margins of
<1 mm and required re-excision (Figure 3). Hence the
margin clearance rate was 66.7% and re-excision rate
was 33.3%. No procedure-related complications were
recorded.
DISCUSSION
Among 23 patients, placement success was achieved
in 21 (91%) and retrieval success was achieved in
22 (96%). Such results are in line with prior literature,
which revealed high placement success (99% to
100%)[10] [11] [12] [13] [14] [15] and high retrieval success (95%-100%).[10] [11] [12] [13] [14] [15]
Our re-excision rate, however, was 33%, which was
higher than in prior literature which ranges from 7% to
20%.[10] [12]
Reflector migration has been uncommonly reported in
prior literature. A prior study reports a 0% migration
rate.[16] Three patients had reflectors placed on the day
of surgery during our initial experience so as to ensure
familiarisation of the radiologists and surgeons with
the workflow of the new device. Among all of the
20 reflectors which were placed before the day of surgery,
none of them showed delayed migration. This supports
the fact that delayed migration is a rare occurrence, and
that decoupling of surgery and radiology scheduling is
feasible. The reflectors can be placed at least 30 days
before the surgery. This eliminates the need to reserve radiology appointments for same-day hookwire
placements, and allows placement of reflectors at the
convenience of the patient as well as the of the radiology
department. Patients can be put as the first case of the
operation list, thus minimising presurgical fasting and
risk of vasovagal syncope as compared to that if same-day
localisation were needed.[6]
One of the advantages of a radar-based system over
hookwire and even other novel localisation techniques
such as Magseed and radiofrequency identification tag
is that it is suitable for long-term implantation with
minimal susceptibility artefacts on magnetic resonance
imaging (MRI).[1] [16] [17] MRI is often needed after cases
which require neoadjuvant chemotherapy to reassess the
tumour for treatment response and feasibility of breast
conservation surgery. The reflector does not impede the
subsequent imaging assessment of the tumour due to its
minimal artefacts.[17] This advantage was illustrated in
one of our cases of preoperative biopsy-proven invasive
ductal carcinoma. The patient underwent neoadjuvant
chemotherapy for tumour shrinkage after reflector
placement (Figure 5). The reflector was placed 69 days
before surgery as neoadjuvant chemotherapy was needed
for tumour shrinkage before surgical excision. Repeat
MRI after neoadjuvant chemotherapy was performed
and showed the reflector in situ, with no significant
artefacts that interfered with image interpretation. No
migration of the reflector was observed, and the tumour
was successfully excised with adequate margins. This is
one of the main advantages of the radar-based system,
as other devices may cause a relatively more significant
artefact. The reflector can also aid surgical detection of
the tumour after satisfactory shrinkage without the need
of additional localisation procedures, which can save the
resources needed for another intervention, and reduce
patient’s anxiety and pain.
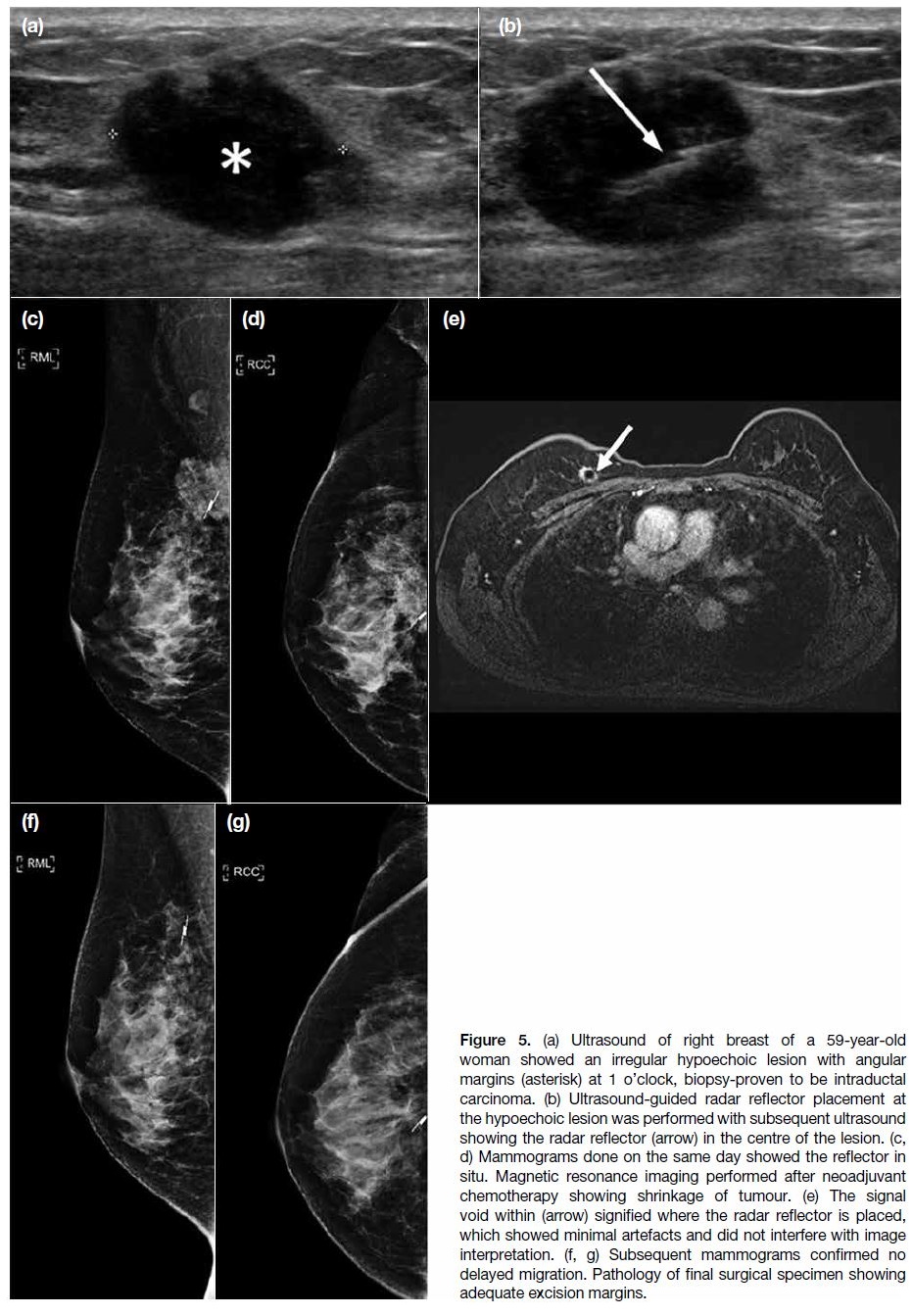
Figure 5. (a) Ultrasound of right breast of a 59-year-old
woman showed an irregular hypoechoic lesion with angular
margins (asterisk) at 1 o’clock, biopsy-proven to be intraductal
carcinoma. (b) Ultrasound-guided radar reflector placement at
the hypoechoic lesion was performed with subsequent ultrasound
showing the radar reflector (arrow) in the centre of the lesion. (c,
d) Mammograms done on the same day showed the reflector in
situ. Magnetic resonance imaging performed after neoadjuvant
chemotherapy showing shrinkage of tumour. (e) The signal
void within (arrow) signified where the radar reflector is placed,
which showed minimal artefacts and did not interfere with image
interpretation. (f, g) Subsequent mammograms confirmed no
delayed migration. Pathology of final surgical specimen showing
adequate excision margins.
Another advantage of the radar-based system is that it
does not require any radioactive materials as compared
to other localisation techniques such as radioguided
occult lesion localisation. This technique has to be
performed on the same day or a day before the surgery as
the radiotracer material decays with time. Moreover, use
of radiotracer material requires Nuclear Medicine unit
support, and is subject to radiation safety regulations.[17]
Hence comparatively, a radar-based system is more
readily available, especially in smaller centres with no
nuclear medicine support and does not pose any radiation
exposure to the patient and personnel.[1]
There are several caveats for radar-based localisation.
Although the reflector is safe to use in a strong magnetic
field, the console, handpiece/cable assembly, and the
delivery system are not.[8] [13] [18] This poses a potential limitation for use of the Scout system in lesions that
are only visualised on MRI and require MRI-guided placement.
In addition, the radar-based system has a depth limit of
6 cm for detectability,[1] [8] which is a potential limitation
for its use. However, we do not expect signal detection
to be a problem even in slightly thicker breasts as
long as we push the handpiece firmly against the
chest wall, as proven in a previous study that detected
reflectors up to 8 cm deep.[15] When we selected patients
for sonographically guided reflector placement, the
sonographic depths of the lesions were <6 cm from
the skin surface. For stereotactic-guided placement, we
selected patients with the shortest distance of the target
lesion from the skin surface <6 cm on mammogram.
However, we did not encounter any patients who had to be excluded due to such a depth limit. In our study,
the average sonographic depth of the reflector from the
skin surface was 1.1 cm ± 0.3 cm. The deepest reflector
that we have placed was 1.9 cm from the skin surface.
Chinese patients tend to have thinner breasts[19]; however,
further studies are required to confirm whether this depth
limitation poses less of a challenge in this population.
Halogen and older model operating room lights have
been shown to interfere with detection of the reflector.[15]
According to the experience of the surgeons in our
centre, halogen operating room lights did interfere with
signal detection of the reflector, and by simply directing
them in another direction, we enabled detection of the
transcutaneous signal. This is in line with the experience
of researchers, who reported that by simply shielding,
dimming, or redirecting the halogen lights slightly away
from the breast while using the handpiece, accurate
detection of the reflector was achieved.[15] Halogen lights
are also going out of favour, with more operating rooms
using LED lighting, which do not interfere with radar-based
localisation.[15]
A prior study also described a case of detection signal
loss after an electrocautery device came into contact
with the reflector.[14] Although the reflector has since
been modified with addition of an electrostatic discharge
diode to minimise the risk of reflector inactivation, it
does not entirely eliminate the possibility.[20] In our study,
there was a case with loss of reflector signal during the
operation, which was likely due to electrocautery use
during skin incision, which deactivated the device at a
superficial location in the subareolar region of the breast
(Figure 6). After identifying the location of the reflector,
the usual practice in our centre is that surgeons raise a
skin flap with an electrocautery device and dissect at
the edges of the reflector. In this case, loss of reflector
signal was caused by inadvertent direct contact of the
electrocautery with the reflector, because the reflector
was close to the skin. An initial specimen radiograph
did not show the presence of the reflector. However,
the reflector was noted to be palpable and was removed
together with the target biopsy marker as confirmed
on subsequent specimen radiograph. Extra caution
is suggested for the use of electrocautery device to
raise the skin flap, particularly when the reflector in a
superficial location, to avoid inadvertent loss of signal.
However, this limitation is probably of less significance,
as reflector damage by electrocautery would suggest that
the surgeon has reached the lesion[21] and can visualise or
at least palpate the reflector, as in our case. Apart from deactivation through direct contact with electrocautery,
there is a risk of transection of the radar-based antenna
during dissection,[20] albeit uncommon. We did not
encounter any cases of such, but caution should be taken
during operation.
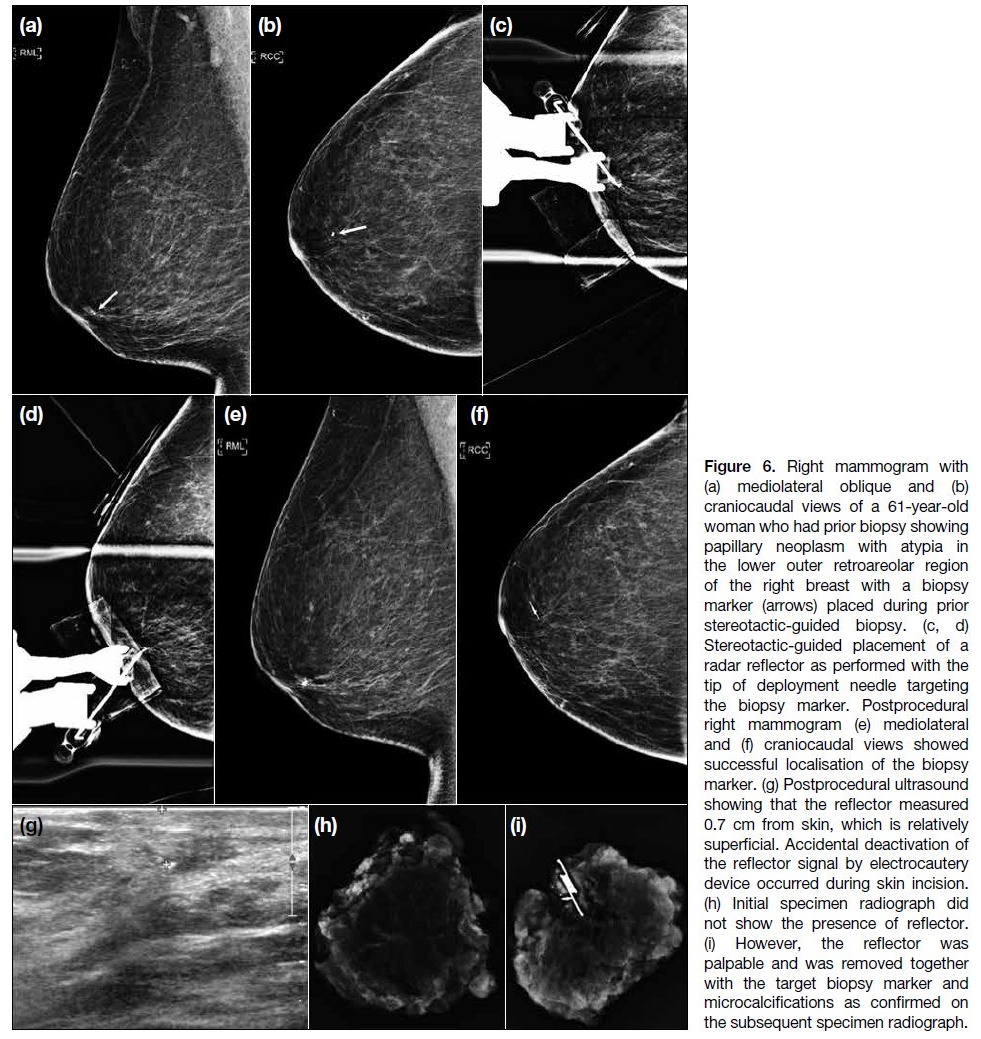
Figure 6. Right mammogram with (a) mediolateral oblique and (b) craniocaudal views of a 61-year-old woman who had prior biopsy showing papillary neoplasm with atypia in the lower outer retroareolar region of the right breast with a biopsy marker (arrows) placed during prior stereotactic-guided biopsy. (c, d) Stereotactic-guided placement of a radar reflector as performed with the tip of deployment needle targeting the biopsy marker. Postprocedural right mammogram (e) mediolateral and (f) craniocaudal views showed successful localisation of the biopsy marker. (g) Postprocedural ultrasound showing that the reflector measured 0.7 cm from skin, which is relatively superficial. Accidental deactivation of the reflector signal by electrocautery device occurred during skin incision. (h) Initial specimen radiograph did not show the presence of reflector. (i) However, the reflector was palpable and was removed together with the target biopsy marker and microcalcifications as confirmed on the subsequent specimen radiograph.
Cost is another factor when considering different
localisation techniques. The radar-based localisation
system requires an initial capital purchase and uses
several disposable items per procedure. Although it is
significantly more expensive than hookwire localisation,[1]
there are potential savings from more efficient operating
room and radiology appointment bookings, because they
do not have to be arranged on the same day. For cases
which necessitate neoadjuvant chemotherapy, radar-based
localisation also eliminates the need of additional
localisation procedures, which is also a source of
potential savings. Further cost analysis in the future will
be helpful to investigate the financial aspect of different
localisation techniques.
Patient selection for usage of the radar-based system
must be done carefully. Patients with nickel allergy are
contra-indicated to reflector insertion while extra caution
is needed for patients with cardiac implants.[8] The radar
reflector antennae are made of nitinol, which is an alloy
of nickel and titanium, hence should not be inserted into
patients with nickel allergies. For patients with cardiac
implants, there is a theoretical risk that the micro-impulse
radar signal may interfere with the intended
function of any internal or external cardiac implants,
hence the manufacturers advise to contact the cardiac
implant manufacturer for instructions before using the
radar-based system.[8]
There are also practical aspects which should be taken into consideration during usage of the radar-based
system. It is a procedure that requires more dexterity
compared to other localisation techniques. For one of
the cases with target-to-reflector distance of ≥10 mm,
it was performed under sonographic guidance from an
inferior approach and resulted in the reflector being
1 cm superior to the centre of target lesion immediately
after the placement. Sonographic guided skin marking
was performed on the day of surgery. The reflector was
detected and removed together with the target lesion
successfully. This was performed during our initial
experience, with no significant haematoma detected.
This is likely related to the relative lack of experience of
the operator at the initial phase, and the more technically
demanding nature of the device.
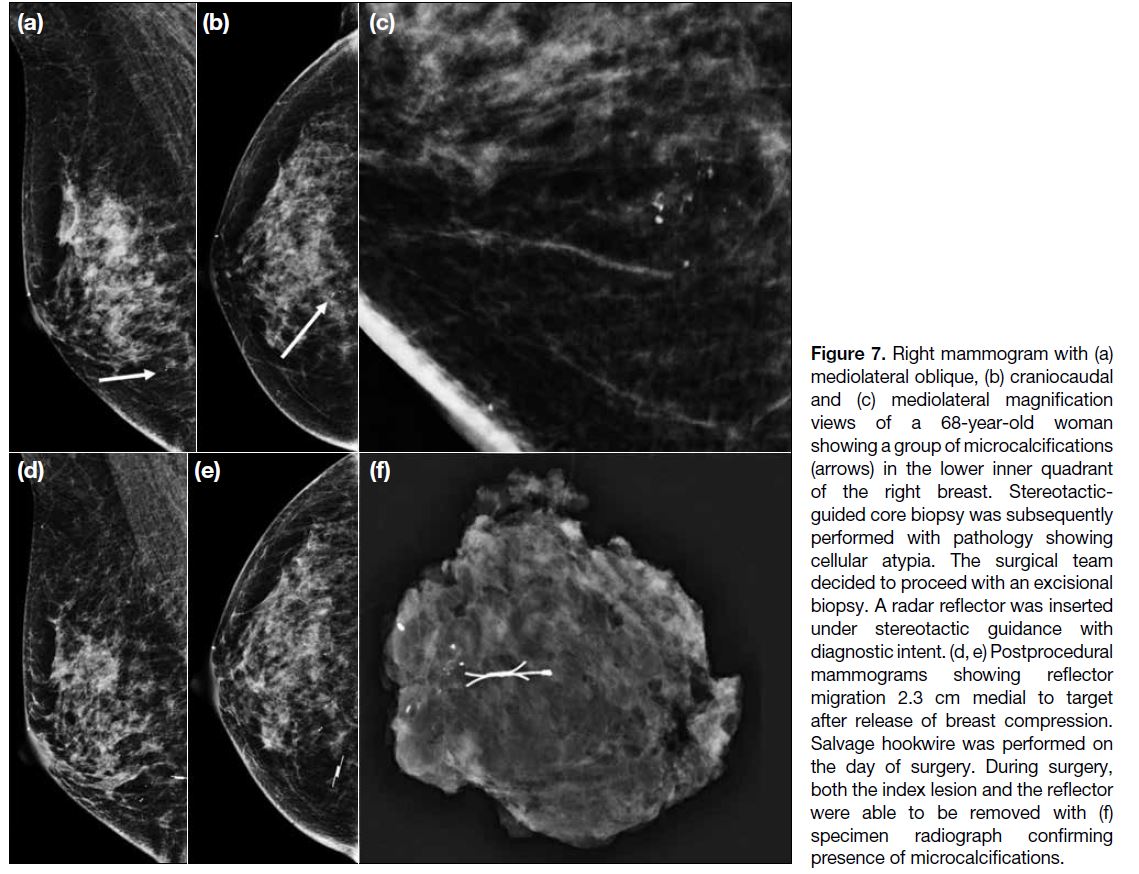
The other case with target-to-reflector distance ≥10 mm
was performed under stereotactic guidance by a
mediolateral approach (Figure 7). For detection of
any immediate marker migration, postprocedural
mediolateral and craniocaudal mammograms are
routinely done in our centre to document the initial
accuracy of reflector placement. The reflector was found
to have migrated 2.3 cm medially from the target lesion (microcalcifications) on postprocedural orthogonal
mammograms after release of breast compression. The
direction of migration occurred along the direction of
breast compression, with no clinically or radiologically
significant haematoma detected, and hence we propose
that this could be related to accordion effect (the
migration of a device when compression is released,
and the breast expands to its original shape and size).[20] A salvage hookwire procedure was performed for this
patient on the day of surgery. During surgery, both the
lesion and the reflector were able to be removed with
a specimen radiograph confirming the presence of
microcalcifications with no further calcifications at the
margins, and histology confirming DCIS. Some studies
advise partial release immediately prior to reflector or
marker deployment to minimise the accordion effect.[19]
The reflector antennae with their offset configuration
serve the additional function of securing the reflector in
tissue.[13] However, reflector migration still occurred in
one of our cases.
Figure 7. Right mammogram with (a) mediolateral oblique, (b) craniocaudal and (c) mediolateral magnification views of a 68-year-old woman showing a group of microcalcifications (arrows) in the lower inner quadrant of the right breast. Stereotactic-guided core biopsy was subsequently performed with pathology showing cellular atypia. The surgical team decided to proceed with an excisional biopsy. A radar reflector was inserted under stereotactic guidance with diagnostic intent. (d, e) Postprocedural mammograms showing reflector migration 2.3 cm medial to target after release of breast compression. Salvage hookwire was performed on the day of surgery. During surgery, both the index lesion and the reflector were able to be removed with (f) specimen radiograph confirming presence of microcalcifications.
Prior studies have reported dense objects between
reflector and handpiece, such as calcified masses or
haematomas, causing weakened signal detection.[22] None
of our cases had any significant haematomas. However,
from prior literature, this limitation can be overcome
by placing the reflector immediately adjacent to the haematoma rather than within it and marking the skin
for the surgeon if transcutaneous signal is weak, as the
audible signal is usually augmented following skin
incision.[13]
Our study is limited as it is a single-institution
retrospective study without direct comparison to
hookwire localisations or other localisation techniques
in our centre. There is potential selection bias as
patients were selected for radar-based localisation in a
multidisciplinary meeting. Our small sample size limits
our analysis for migration and margin clearance rates. We
did not include any patients with more than one reflector
inserted for bracketing or for multiple lesions in the same
breast; hence, no information can be provided on that
aspect. The manufacturer recommends at least 2.5 cm
distance between reflectors, while prior literature reports
placing reflectors as close as 1.7 cm apart with successful
detection of distinct reflector signals.[16] Apart from the efficacy of radar-based localisation for multiple lesions,
our study also did not evaluate patient satisfaction,
specimen weight, cosmetic outcome, or mean duration
of deployment, which are both potential advantages for
radar-based localisation when compared to traditional
hookwire localisation. A prospective randomised
controlled trial with larger sample size and evaluation
of more parameters will be needed to compare radar-based
localisation with hookwire and other localisation
techniques.
CONCLUSION
Results of our study suggest that the radar-based
localisation system is safe and effective for guiding the
excision of non-palpable breast lesions in a Chinese
population. Its unique advantages, which overcome
certain limitations of other localisation methods may
render it a superior alternative in selected patients.
Further studies should be performed to validate these
findings.
REFERENCES
1. Kapoor MM, Patel MM, Scoggins ME. The wire and beyond:
recent advances in breast imaging preoperative needle localization.
Radiographics. 2019;39:1886-906. Crossref
2. Montrey JS, Levy JA, Brenner RJ. Wire fragments after needle localization. AJR Am J Roentgenol. 1996;167:1267-9. Crossref
3. Kopans DB, DeLuca S. A modified needle hookwire technique
to simplify preoperative localization of occult breast lesions.
Radiology 1980;134:781. Crossref
4. Hall FM, Kopans DB, Sadowsky NL, Homer MJ. Development of
wire localization for occult breast lesions: Boston remembrances.
Radiology. 2013;268:622-7. Crossref
5. Besic N, Zgajnar J, Hocevar M, Rener M, Frkovic-Grazio S,
Snoj N, et al. Breast biopsy with wire localization: factors
influencing complete excision of nonpalpable carcinoma. Eur
Radiol. 2002;12:2684-9. Crossref
6. Homer MJ. Transection of the localization hooked wire during
breast biopsy. AJR Am J Roentgenol. 1983;141:929-30. Crossref
7. Fung WY, Wong T, Chau CM, Yu EL, Chan TS, Chan RL, et al.
Safety and efficacy of magnetic seed localisation of non-palpable
breast lesions: pilot study in a Chinese population. Hong Kong
Med J. 2020;26:500-9. Crossref
8. Merit Medical SCOUT® Surgical Guidance System: instructions
for use. Available from: https://www.merit.com/merit-oncology/localization/breast-soft-tissue-loc.... Accessed 15 Jan 2021.
9. Sibbering M, Watkins R, Winstanley J, Patnick J, editors. Quality
Assurance Guideline for Surgeons in Breast Cancer Screening
(NHSBSP publication No. 20). 4th ed. Sheffield: NHS Cancer
Screening Programmes; 2009.
10. Srour MK, Kim S, Amersi F, Giuliano AE, Chung A. Comparison
of wire localization, radioactive seed, and Savi scout® radar for
management of surgical breast disease. Breast J. 2020;26:406-13. Crossref
11. Jadeja PH, Mango V, Patel S, Friedlander L, Desperito E, Ayala-Bustamante E, et al. Utilization of multiple SAVI SCOUT surgical
guidance system reflectors in the same breast: A single-institution
feasibility study. Breast J. 2018;24:531-4. Crossref
12. Patel SN, Mango VL, Jadeja P, Friedlander L, Desperito E,
Wynn R, et al. Reflector-guided breast tumor localization versus
wire localization for lumpectomies: A comparison of surgical
outcomes. Clin Imaging. 2018;47:14-7. Crossref
13. Mango VL, Wynn RT, Feldman S, Friedlander L, Desperito E,
Patel SN, et al. Beyond wires and seeds: reflector-guided breast
lesion localization and excision. Radiology. 2017;284:365-71. Crossref
14. Cox CE, Garcia-Henriquez N, Glancy MJ, Whitworth P, Cox JM,
Themar-Geck M, et al. Pilot study of a new nonradioactive surgical
guidance technology for locating nonpalpable breast lesions. Ann
Surg Oncol. 2016;23:1824-30. Crossref
15. Cox CE, Russell S, Prowler V, Carter E, Beard A, Mehindru A,
et al. A prospective, single arm, multi-site, clinical evaluation of
a nonradioactive surgical guidance technology for the location
of nonpalpable breast lesions during excision. Ann Surg Oncol.
2016;23:3168-74. Crossref
16. Tayeh S, Muktar S, Heeney J, Michell MJ, Perry N, Suaris T, et al.
Reflector-guided localization of non-palpable breast lesions: the
first reported European Evaluation of the SAVI SCOUT® System.
Anticancer Res. 2020;40:3915-24. Crossref
17. Hayes MK. Update on preoperative breast localization. Radiol Clin
North Am. 2017;55:591-603. Crossref
18. Mango V, Ha R, Gomberawalla A, Wynn R, Feldman S. Evaluation
of the SAVI SCOUT Surgical Guidance System for localization
and excision of nonpalpable breast lesions: a feasibility study. AJR
Am J Roentgenol. 2016;207:W69-72. Crossref
19. Esserman LE, Cura MA, DaCosta D. Recognizing pitfalls in early
and late migration of clip markers after imaging-guided directional
vacuum-assisted biopsy. Radiographics. 2004;24:147-56. Crossref
20. Jeffries DO, Dossett LA, Jorns JM. Localization for breast surgery:
the next generation. Arch Pathol Lab Med. 2017;141:1324-9. Crossref
21. Kasem I, Mokbel K. Savi Scout® radar localisation of non-palpable
breast lesions: systematic review and pooled analysis of 842 cases.
Anticancer Res. 2020;40:3633-43. Crossref
22. Falcon S, Weinfurtner RJ, Mooney B, Niell BL. SAVI SCOUT® localization of breast lesions as a practical alternative to wires:
outcomes and suggestions for trouble-shooting. Clin Imaging.
2018;52:280-6. Crossref