First-line Afatinib in Epidermal Growth Factor Receptor–mutant Metastatic Non-small Cell Lung Cancer: a Clinical Retrospective Study
ORIGINAL ARTICLE
First-line Afatinib in Epidermal Growth Factor Receptor–mutant Metastatic Non-small Cell Lung Cancer: a Clinical Retrospective Study
DYL Chow1, TH So2, DKC Leung1, RPY Tse1, KS Lau1
1 Department of Clinical Oncology, Queen Mary Hospital, Hong Kong
2 Department of Clinical Oncology, The University of Hong Kong, Hong Kong
Correspondence: Dr DYL Chow. Department of Clinical Oncology, Queen Mary Hospital, Hong Kong. Email: dylchow@gmail.com
Submitted: 25 Aug 2021; Accepted: 15 Dec 2021.
Contributors: DYLC and KSL designed the study. DYLC acquired and analysed the data. DYLC, THS and KSL drafted the manuscript, and
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the University of Hong Kong / Hospital Authority Hong Kong West Cluster Institutional Review
Board (Ref UW 21604) and was carried out in accordance with the Declaration of Helsinki.
Abstract
Background
We sought to analyse epidermal growth factor receptor mutated (EGFR-MT) metastatic non-small
cell lung cancer (NSCLC) patients treated with afatinib as first-line therapy in a clinical setting. The outcomes of
cases, especially those harbouring rare mutations, were reviewed.
Methods
A single-centre retrospective study of 85 patients with NSCLC treated with first-line afatinib was performed.
Demographics, clinical data, and treatment information were used to assess the effects of age, mutation types (common/uncommon), Eastern Cooperative Oncology Group performance status (ECOG PS), presence of brain metastasis,
and other factors on progression-free survival (PFS) and overall survival (OS).
Results
Median age was 63 years. ECOG PS ≥2 was present in 10.6% of cases. A total of 11.8% of all cases had
brain metastasis at first presentation and 41.2% had uncommon mutations. The median PFS was 14.9 months; the
median OS was 33.9 months. 91.8% of patients experienced treatment-related adverse effects. Dose reductions
were required for 30.6% of cases. Patients with major uncommon mutations had PFS of similar lengths to those
with common mutations. Age, presence of brain metastasis, ECOG PS of ≥2 and presence of exon 20 insertions
correlated negatively with PFS and OS.
Conclusions
Afatinib is an effective first-line treatment for patients with EGFR-MT NSCLC. The drug is well
tolerated, with good response rates across a broad spectrum of patients. Given its high efficacy in major uncommon
mutations, it should be considered as first-line treatment in this subset.
Key Words: Afatinib; Carcinoma, non-small-cell lung; ErbB receptors; Mutation; Progression-free survival
中文摘要
阿法替尼一線治療表皮生長因子受體突變轉移性非小細胞肺癌:臨床回顧性研究
周一樂、蘇子謙、梁國全、謝佩楹、劉健生
背景
分析臨床使用阿法替尼作為表皮生長因子受體突變(EGFR-MT)轉移性非小細胞肺癌(NSCLC)患者的一線治療方案並檢視治療結果,尤其是帶有罕見突變的病例。
方法
對85例接受阿法替尼一線治療NSCLC的病例進行單中心回顧性研究。應用人口統計學、臨床數據和治療信息評估年齡、突變類型(常見/不常見)、ECOG體能狀態(ECOG PS)、是否有腦轉移及其他因素對疾病無惡化存活期(PFS)和總存活期(OS)的影響。
結果
年齡中位數為63歲。10.6%病例的ECOG PS得分≥2。11.8%病例在首次診斷時發現腦轉移,41.2%病例有罕見突變。PFS中位數為14.9個月;OS中位數為33.9個月。91.8%病例有與治療相關的不良反應。30.6%病例需要減少劑量。具有主要罕見突變患者的PFS與具有常見突變的患者類似。年齡、是否存在腦轉移、ECOG PS得分≥2以及是否存在20外顯子插入突變與PFS和OS呈負相關。
結論
阿法替尼是對EGFR-MT NSCLC患者有效的一線治療方案。該藥物的耐受性和緩解率均良好。鑑於其在主要罕見突變中的高效性,阿法替尼可被視為對這類患者的一線治療方案。
INTRODUCTION
Patients with non-small cell lung cancer (NSCLC)
with an epidermal growth factor receptor mutation
(EGFR-MT) are currently treated with tyrosine kinase
inhibitors (TKIs).[1] Activating mutations in the EGFR
gene causes aberrant EGFR signalling, which sensitises
tumours to targeted TKI treatment. The Food and Drug
Administration has currently approved five TKIs, with
gefitinib and erlotinib being first-generation, afatinib and
dacomitinib in the second generation, and osimertinib in
the third generation.[2]
Afatinib is an irreversible blocker of the ErbB family
of receptors (inhibiting signalling via heterodimers
and homodimers formed by ErbB1 (EGFR), ErbB2
(human epidermal growth factor receptor 2 [HER2]),
ErbB3 (HER3), and ErbB4 (HER4).[3] Randomised
controlled trials (RCTs) have shown that afatinib
significantly improved progression-free survival (PFS)
compared with standard chemotherapy.[4] [5] Adverse
events (AEs) were tolerable with few treatment
discontinuations.
Post-hoc analysis of the LUX-Lung trials[6] found afatinib had significant activity against certain uncommon mutations, including G719X, L861Q, and S768I.
Preclinical data showed that afatinib was less effective
against tumours with exon 20 insertions, or de novo
T790M mutations alone or in combination with other
mutations.
There are not as much prospective clinical data on other
EGFR TKIs targeting uncommon mutations. As a result,
afatinib is the TKI of choice in the first-line setting for
more than 80 countries to treat patients with NSCLC
with EGFR-MT.
Clinical trials typically have strict inclusion criteria, and
certain patient subgroups are frequently excluded, such
as elderly patients, patients with brain metastases, or
with an Eastern Cooperative Oncology Group (ECOG)
performance status ≥2. Given the lack of prospective data
in these patients, the goal of this study was to analyse the
available data of EGFR-MT patients treated with afatinib
in an environment similar to daily clinical practice, and
to examine and compare outcomes of individuals with
uncommon mutations.
METHODS
Cases of patients aged ≥18 years with histologically proven metastatic NSCLC that were treated with first-line
afatinib from 1 January 2015 to 30 June 2021 at
Queen Mary Hospital Hong Kong were retrospectively
reviewed. Median follow-up time was 23 months. The
data cut-off was on 31 July 2021. Those that had received
prior anticancer treatment or had primary tumours other
than in the lung were excluded. Cases were categorised
into four key groups: tumours harbouring major
uncommon point mutations (G719X, L861Q, and S768I);
exon 20 insertions; other uncommon mutations; and
common mutations (exon 19 deletion, exon 21 L858R).
Cases with brain metastasis and poor performance status
were not excluded.
The primary objective was to evaluate the safety
and efficacy of afatinib in these groups. AEs were
graded using the National Cancer Institute Common
Terminology Criteria for Adverse Events version 3.0.
Efficacy endpoints included: PFS (time from first
afatinib administration to date of progression or to date
of death, whichever came first) and overall survival (OS)
[time from diagnosis to date of death]. ‘Progression’
was defined as a worsening radiological appearance
as per standard of care at the participating institution
via Response Evaluation Criteria in Solid Tumours
(RECIST) or by clinical symptomatic progression.
Computed tomography (CT) and magnetic resonance
imaging were used for patients that underwent baseline
brain imaging assessment. Reassessment imaging was
arranged every 3 to 6 months using CT or positron
emission tomography/CT when feasible. Objective
response rates (ORRs) were judged by the authors
based on available imaging information via RECIST
criteria. EGFR mutations were detected using real-time
polymerase chain reactions.
Patients received afatinib at starting doses of 40 mg, 30 mg, or 20 mg once daily based on perceived tolerance
to treatment by the clinician. Treatment was continued
until disease progression, poor tolerability, or other
reasons requiring withdrawal. Treatment-related adverse
events (TRAEs) were managed using tolerance-guided
dose modifications. When TRAEs reversed to Grade 1
or back to baseline, treatments could be resumed at a
lower dose (in 10 mg decrements). If patients could not
tolerate 20 mg afatinib daily or if TRAEs did not return
to Grade 1 or to baseline within 6 weeks, treatment was
discontinued. The optimum dose of afatinib was defined
as the final dose that a patient received without need for
further decrements due to TRAEs.
Statistical Analysis
Data were analysed using SPSS (Windows version 23.0;
IBM Corp., Armonk [NY], United States). Kaplan–Meier estimates were used for median OS (mOS) and
median PFS (mPFS). Univariable and multivariable
analyses for prognostic factors of PFS and OS were
performed by Cox proportional hazard models. A p
value of <0.05 was considered statistically significant.
All cases were included in safety and efficacy analyses.
Exploratory subgroup analysis was conducted post hoc
and descriptive statistics are presented.
RESULTS
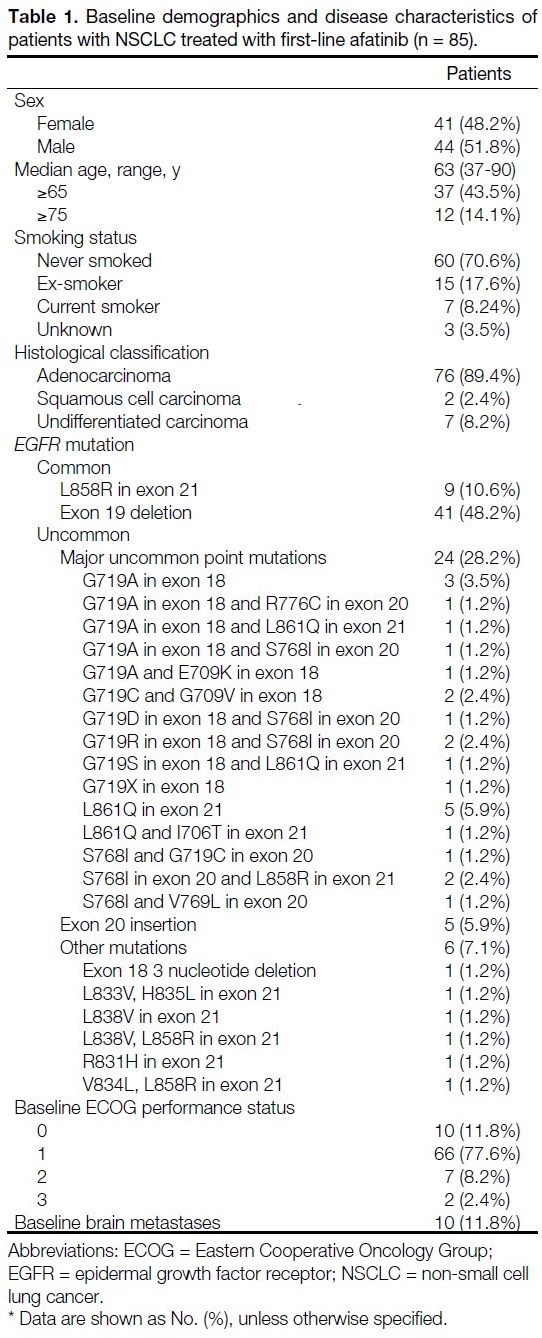
A total of 85 patients (median age 63 years, range 37-90) were treated with afatinib within the listed period
(Table 1). Within the dataset, 50 (58.8%) patients had
common mutations, whereas 35 (41.2%) patients were
harbouring uncommon mutations. Two patients with
L858R mutations had de novo T790M mutations. Major
uncommon point mutations such as G719X, L861Q and
S768I were the most frequent group, accounting for
68.6% of all uncommon mutations.
Table 1. Baseline demographics and disease characteristics of
patients with NSCLC treated with first-line afatinib (n = 85).
Efficacy
Progression-free Survival and Overall Survival
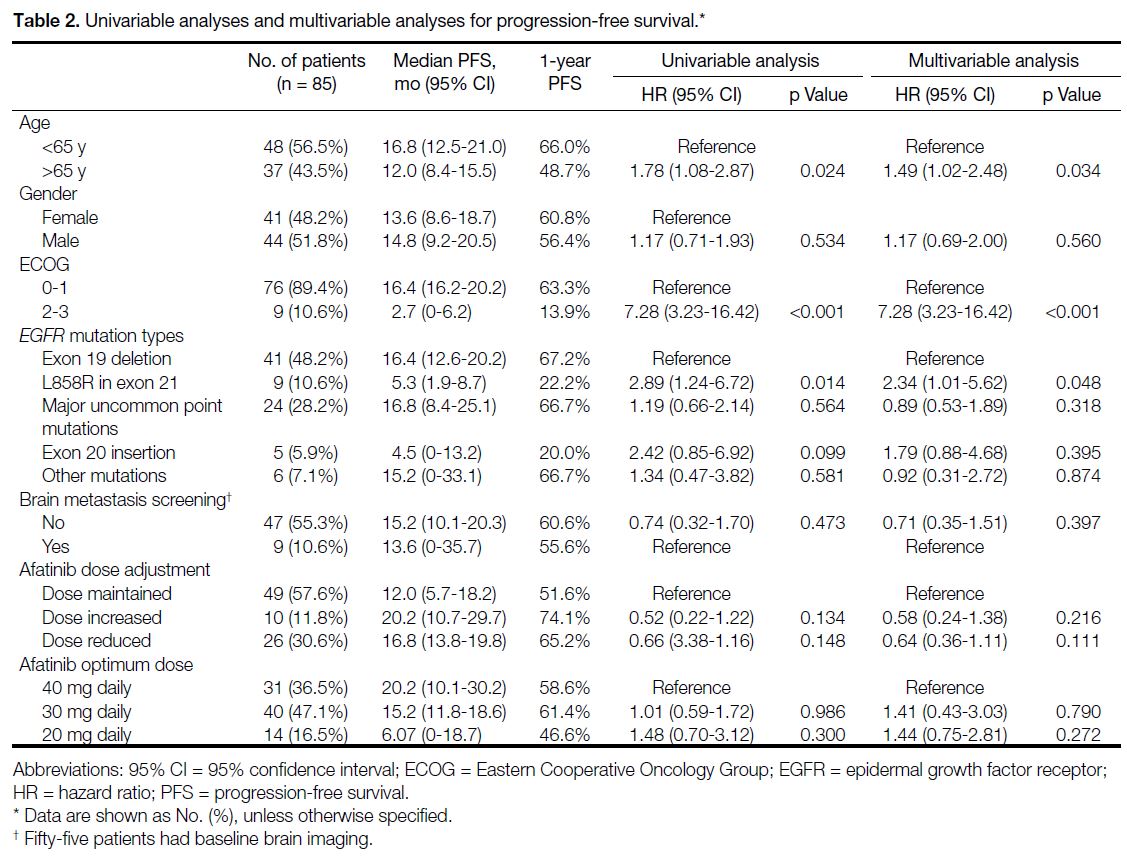
The mPFS was 14.9 months (95% confidence interval
[CI]=10.7-19.0), with 63 cases having progressed at the
time of analysis. The mPFS according to clinical and
treatment characteristics are shown in Tables 2 and 3. On
both univariable and multivariable analyses, cases with
the exon L858R point mutation had significantly shorter
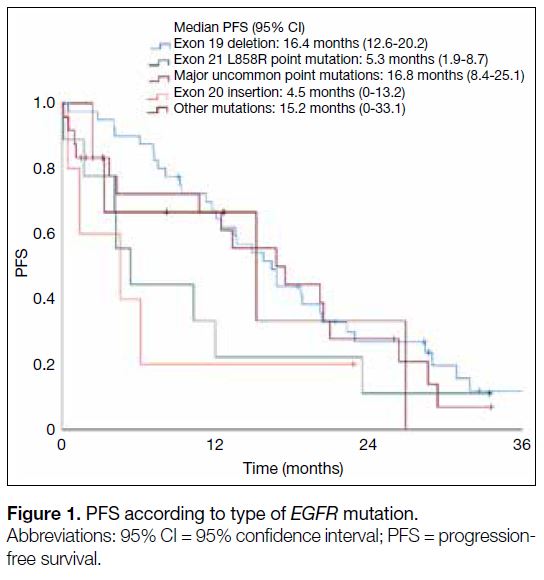
mPFS compared with those with the exon 19 deletion
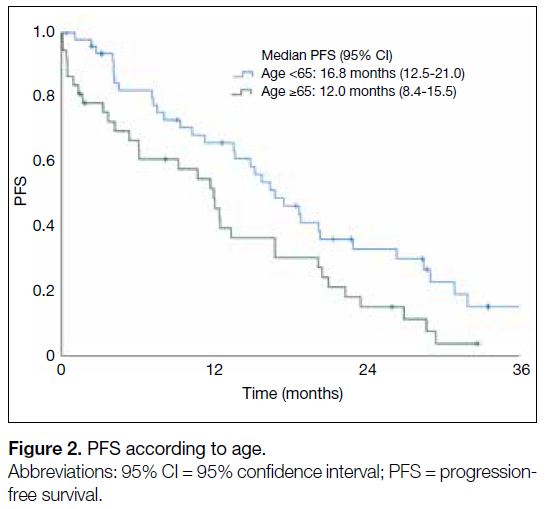
(5.3 months vs. 16.4 months; HR=2.34, 95% CI=1.01-5.62; p = 0.048) [Figure 1]. Patients aged ≥65 years (12.0
months vs. 16.8 months; HR=1.49, 95% CI=1.02-2.48;
p = 0.034) had worse mPFS than those of younger age
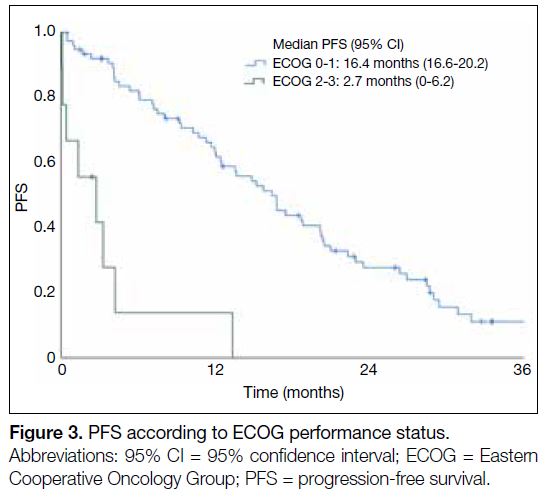
(Figure 2). Patients with ECOG performance status 2-3
(2.7 months vs. 16.4 months; HR=7.28, 95% CI=3.23-16.42; p < 0.001) had a worse mPFS than those with
better ECOG performance status (Figure 3).
Table 2. Univariable analyses and multivariable analyses for progression-free survival.
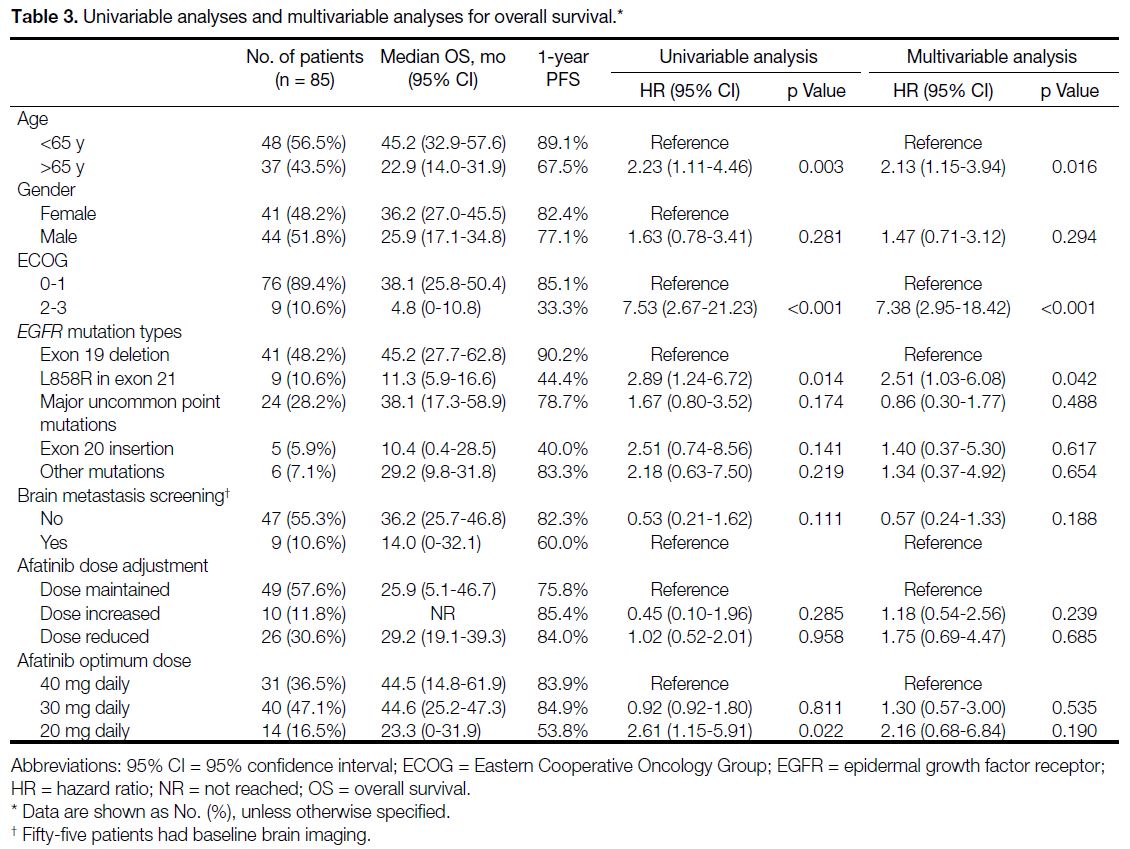
Table 3. Univariable analyses and multivariable analyses for overall survival
Figure 1. PFS according to type of EGFR mutation
Figure 2. PFS according to age
Figure 3. PFS according to ECOG performance status
Final OS data was largely congruent with PFS findings. mOS was 33.9 months (95% CI=20.6-47.2) for all cases.
Cases with exon L858R point mutation had significantly
shorter mOS compared with cases with the exon 19
deletion (11.3 months vs. 45.2 months; HR=2.51, 95%
CI=1.03-6.08; p = 0.042).
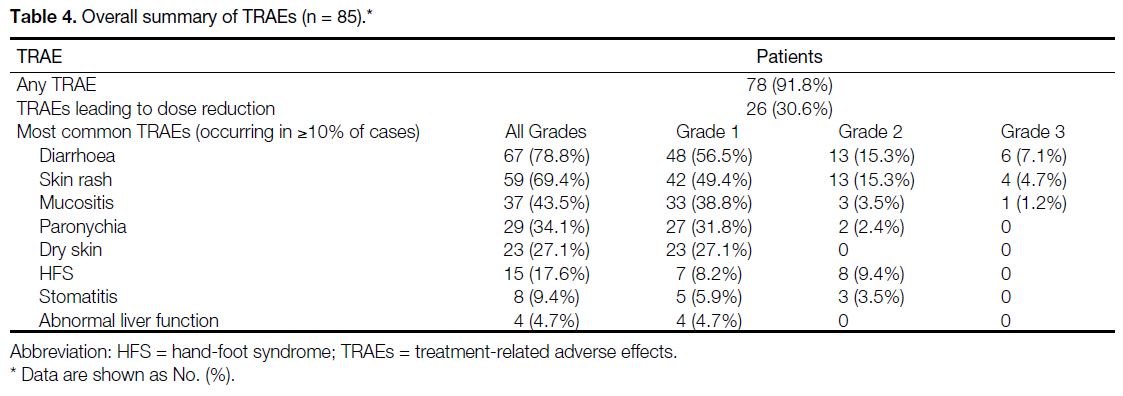
Safety and Dosage
TRAEs were common among patients, with up to 91.8% of all patients experiencing some form of TRAE (Table 4). Dose adjustments were required for patients, with up
to 54 (63.5%) patients requiring dose reductions. In total,
11.8% of all patients had dose increments. No TRAEs
resulted in death.
Table 4. Overall summary of TRAEs (n = 85)
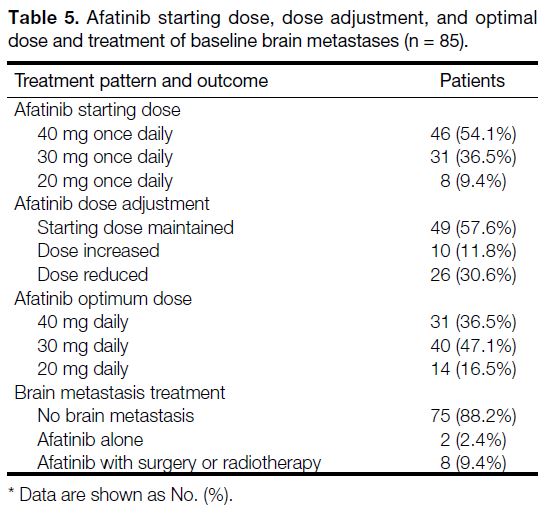
Most cases started with afatinib 40 mg daily (54.1%),
followed by 30 mg daily (36.5%) and 20 mg daily (9.4%).
Initial starting dose was maintained for 49 (57.6%)
patients (Table 5). Dose reductions were largely due
to AEs from treatment whereas dose increments were
due to initially perceived poor tolerance to treatment.
The optimum dose for most cases was 30 mg daily and
most cases with brain metastasis on diagnosis often had
surgery or radiotherapy in conjunction with afatinib
treatment.
Table 5. Afatinib starting dose, dose adjustment, and optimal
dose and treatment of baseline brain metastases (n = 85)
Objective Response Rate and Resistance
Overall, 38 of 85 cases (44.7%) had an objective
response, including one (1.2%) complete response and
37 (43.5%) partial responses. In all, 27 (31.8%) cases
had stable disease. Disease control rate was 76.5%.
Sixty-three cases eventually developed progressive
disease on afatinib. Forty-eight were retested for T790M
mutations, with 39 undergoing plasma EGFR testing
and nine with tissue sample testing. Fourteen (29.2%)
cases developed exon 20 T790M mutations. All cases
that developed T790M mutations had adenocarcinoma
exclusively. For cases that had common mutations, 19
received osimertinib as second-line treatment and nine
others received chemotherapy. Within the uncommon
mutation group, four cases received osimertinib, eight
received chemotherapy, and one continued second-line
treatment with mobocertinib.
DISCUSSION
This study was a retrospective review of a single-centre
experience of cases of metastatic NSCLC EGFR-MT
treated with first-line afatinib. Case demographics and
population subsets are comparable to other reported
studies with EGFR TKIs being used in daily clinical
practice.[7] Typically underrepresented subgroups such
as the elderly people, cases with brain metastasis, cases
with uncommon mutations, and those with ECOG
performance status ≥2 were also included in this review.
A majority of cases harboured exon 19 deletions and
up to 41.2% of all cases were harbouring uncommon
mutations. This is likely due to selection bias whereby
clinicians were influenced by the mOS results of the
LUX-Lung 3 and 6 trials, which favoured afatinib over
chemotherapy in the first-line setting,[8] and a tendency
to prescribe afatinib for patients harbouring uncommon
mutations as well.
The mPFS in this review was consistent with other clinical studies (mPFS 11.8-11.9 months) and the LUX
Lung trials (mPFS 11.0-11.1 months).[8] [9] Other studies,
such as that of Kim et al,[10] have found a substantially
longer mPFS of 19.1 months using first-line afatinib,
which could be partly due to the fact that only ECOG
performance status 0-2 cases were involved.
Our results also showed that cases with the exon 19
deletion had a significantly longer mPFS compared
with those that had exon 21 L858R mutations. Although
Kim et al[10] and Liang et al[9] have highlighted similar
outcomes where cases with exon 19 deletions had
longer mPFS and improved ORR compared to those
having exon 21 L858R mutations, two out of the nine
patients that had L858R mutations in our subgroup also
harboured de novo T790M, which may have skewed
results unfavourably.
Cases with unfavourable clinical characteristics, such as poor ECOG performance status or advanced age showed decreased mPFS compared to those without, reaching
statistical significance on univariable and multivariable
analysis. Cases with brain metastasis on screening,
however, did not show significantly worse mPFS,
contrary to the findings of Tan et al.[11]
Afatinib was demonstrated to be effective in cases with
major uncommon point mutations (G719X, L861Q and
S768I) with a response rate and mPFS comparable to
those with common EGFR mutations. This is consistent
with findings from Passaro et al[12] and Yang et al,[13]
implying that afatinib may be a suitable choice of
treatment for patients harbouring these mutations. The
Food and Drug Administration has recently expanded
the front-line indication for afatinib to cover NSCLC
with these three EGFR mutations. In contrast, exon 20
insertions had a much shorter mPFS. Recently approved
treatments, including amivantamab and mobocertinib
have shown promise in early-phase clinical studies for exon 20 insertions and could play a role in this subgroup
of patients in the future.[14] [15]
This study showed a much lower incidence of grade
>3 AEs due to afatinib compared with an incidence of
36.0% to 57.0% reported by RCTs.[4] [5] This is likely due to
the lower starting doses given within this patient group.
Early dose reductions in patients before developing
grade 3 AEs in daily practice would also explain these
findings. Of note, the incidence of the acquired T790M
mutation was lower than the rates reported in several
studies (32.1%-47.6%).[10] [16] This could possibly be due
to the fact that not all cases progressing on afatinib
were retested for T790M. Another reason could be that
certain cases that had uncommon mutations were already
resistant to afatinib, and therefore did not develop
resistance via T790M mutations.
In-vitro analysis of EGFR mutations, including uncommon and compound mutations against different
EGFR TKIs, showed that afatinib had activity against
almost all mutations tested and was more potent than
erlotinib and gefitinib. When compared with osimertinib,
afatinib also demonstrated a greater spectrum of efficacy
against uncommon EGFR mutations, while it was less
effective against T790M, as expected.[17] Clinical data,
however, are limited. Recent literature demonstrated
favourable activity with manageable toxicity in patients
with NSCLC harbouring uncommon EGFR mutations
treated with first-line osimertinib. A phase II study
by Cho et al[18] demonstrated an ORR and PFS of 53%
and 8.2 months, respectively, for patients with G719X
mutations. For afatinib, an ORR of 77.8% and a PFS
of 13.8 months were reported by Yang et al.[6] Although
cross-trial comparisons should be done with caution,
osimertinib showed a response rate in cases with G719X mutations that was comparable to that of other EGFR TKIs.[6]
Numerous factors need to be considered when choosing
a therapy, including central nervous system activity,
toxicities, and types of EGFR mutations. Clinical
efficacy of EGFR TKIs in patients with uncommon
mutations should be assessed prospectively, due to the
small number and heterogeneity of patients studied thus
far.
Clinical analyses of afatinib that include patient
characteristics such as poor ECOG performance status,
brain metastasis, old age, and uncommon mutations are
limited. This study is one of the few retrospective reviews
that examined a population similar to that encountered in
daily clinical practice. The results of our study provide
additional data on these subgroups, especially in patients
with uncommon mutations.
A few limitations should be noted. Given their
retrospective nature, data may be prone to bias during
measurement. Because radiological assessments were
performed at different radiology centres, with potentially
different methodologies, response rates and progression
were not solely measured based on RECIST criteria.
Caution is therefore needed when comparing with
published data. As there may have been inadequate
follow-up duration for patients, a fair amount of data
regarding patients’ PFS and OS were censored. The
number of patients with exon 21 L858R was also
relatively small compared to exon 19 mutations, with
exploratory subgroup analysis performed, limiting the
strength of conclusions.
In summary, afatinib is an effective first-line treatment for
patients with EGFR-MT NSCLC. The drug is generally
well-tolerated and response rates and PFS are consistent
with previous RCTs and other clinical analyses. Given
its high efficacy in major uncommon point mutations, it
should be considered as first-line treatment for patients
harbouring these mutations.
REFERENCES
1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al.
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.
N Engl J Med. 2009;361:947-57. Crossref
2. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C,
et al. Metastatic non-small cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
2018;29(Suppl 4):iv192-237. Crossref
3. Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW
2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther.
2012;343:342-50. Crossref
4. Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T,
et al. Phase III study of afatinib or cisplatin plus pemetrexed
in patients with metastatic lung adenocarcinoma with EGFR
mutations. J Clin Oncol. 2013;31:3327-34. Crossref
5. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib
versus cisplatin plus gemcitabine for first-line treatment of Asian
patients with advanced non-small-cell lung cancer harbouring
EGFR mutations (LUXLung 6): an open-label, randomised phase
3 trial. Lancet Oncol. 2014;15:213-22. Crossref
6. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M,
et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a
combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and
LUX-Lung 6. Lancet Oncol. 2015;16:830-8. Crossref
7. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II,
et al. Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl Cancer
Inst. 2005;97:339-46. Crossref
8. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N,
et al. Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung
6): analysis of overall survival data from two randomised,
phase 3 trials. Lancet Oncol. 2015;16:141-51. Crossref
9. Liang SK, Hsieh MS, Lee MR, Keng LT, Ko JC, Shih JY. Real-world
experience of afatinib as a first-line therapy for advanced
EGFR mutation-positive lung adenocarcinoma. Oncotarget.
2017;8:90430-3. Crossref
10. Kim Y, Sun J, Park K, Park SE, Lee S, Ahn M, et al. P3.01-023 first-line
afatinib for non-small cell lung cancer in real world practice.
J Thorac Oncol. 2017;12(Suppl 2):S2209. Crossref
11. Tan WL, Ng QS, Lim C, Tan EH, Toh CK, Ang MK, et al. Influence
of afatinib dose on outcomes of advanced EGFR-mutant NSCLC
patients with brain metastases. BMC Cancer. 2018;18:1198. Crossref
12. Passaro A, Mok T, Peters S, Popat S, Ahn MJ, de Marinis F. Recent
advances on the role of EGFR tyrosine kinase inhibitors in the
management of NSCLC With Uncommon, non exon 20 insertions,
EGFR mutations. J Thorac Oncol. 2021;16:764-73. Crossref
13. Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, et al.
Afatinib for the treatment of NSCLC harboring uncommon EGFR
mutations: a database of 693 cases. J Thorac Oncol. 2020;15:803-15. Crossref
14. Sabari JK, Shu CA, Park K, Leighl N, Mitchell PL, Kim S, et al.
OA04.04 amivantamab in post-platinum EGFR exon 20 insertion
mutant non-small cell lung cancer. J Thorac Oncol. 2021;16(Suppl
3):S108-9. Crossref
15. Janne PA, Neal JW, Camidge DR, Spira AI, Piotrowska Z, Horn
L, et al. Antitumor activity of TAK-788 in NSCLC with EGFR
exon 20 insertions. J Clin Oncol. 2019;37(Suppl):9007. Crossref
16. Kuiper JL, Heideman DA, Thunnissen E, Paul MA, van Wijk AW,
Postmus PE, et al. Incidence of T790M mutation in (sequential)
rebiopsies in EGFR mutated NSCLC-patients. Lung Cancer.
2014;85:19-24. Crossref
17. Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N,
et al. A method of high-throughput functional evaluation of EGFR
gene variants of unknown significance in cancer. Sci Transl Med.
2017;9:eaan6566. Crossref
18. Cho JH, Lim SH, An HJ, Kim KH, Park KU, Kang EJ, et al. Osimertinib for patients with non–small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol. 2020;38:488-95. Crossref