Utility of Liver Imaging Reporting and Data System v2018 Ancillary Features for the Diagnosis of Hepatocellular Carcinoma in LR-4 Lesions Using Contrast-enhanced Magnetic Resonance Imaging
ORIGINAL ARTICLE CME
Utility of Liver Imaging Reporting and Data System v2018 Ancillary Features for the Diagnosis of Hepatocellular Carcinoma in LR-4 Lesions Using Contrast-enhanced Magnetic Resonance
Imaging
K Lim, H Kwon, J Cho, D Kim, S Kim, E Kang
Department of Radiology, Dong-A University Hospital, Busan, Republic of Korea
Correspondence: Prof. H Kwon, Department of Radiology, Dong-A University Hospital, Busan, Republic of Korea. Email: risual@dau.ac.kr
Submitted: 14 Feb 2021; Accepted: 13 May 2021.
Contributors: HK designed the study. KL acquired the data. JC and SK analysed the data. DK drafted the manuscript. EK critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: The study was supported by Dong-A University Research Fund.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by Dong-A Research Ethics Committee (Ref: DAUHIRB-20-087). The requirement for informed consent was waived because of the retrospective nature of the study. The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed consent for all treatments and procedures.
Abstract
Objective
To evaluate the diagnostic performance of Liver Imaging Reporting and Data System (LI-RADS) version
2018 ancillary features for the diagnosis of hepatocellular carcinoma (HCC) from LR-4 (‘probably HCC’) lesions
using gadoxetic acid–enhanced magnetic resonance imaging.
Methods
This retrospective study evaluated 166 LR-4 lesions including ancillary features in 114 high-risk cases
imaged with gadoxetic acid–enhanced magnetic resonance imaging between March 2015 and December 2017. Two
radiologists evaluated the imaging features using LI-RADS v2018. All lesions were confirmed as HCC or benign
lesions by pathological assessment or >2 years of follow-up imaging. The diagnostic contribution of ancillary features
was assessed using simple and multivariable logistic regression and generalised estimating equations.
Results
In all, 114 HCCs (68.7%) and 52 benign lesions (31.3%) were confirmed. Simple logistic regression analysis
revealed that mild to moderate T2 hyperintensity (p = 0.014), restricted diffusion (p < 0.001), and intralesional
fat (p = 0.018) were statistically significant for differentiating HCCs from benign lesions; however, multivariable
logistic analysis revealed that only restricted diffusion was statistically significant (adjusted odds ratio = 9.703,
p < 0.001). Restricted diffusion had lower sensitivity (48.2%) and higher specificity (90.4%) for the diagnosis of HCC;
however, the diagnostic values improved when combined with mild to moderate T2 hyperintensity and hepatobiliary
phase hypointensity (sensitivity: 73.8%, specificity: 80.8%).
Conclusion
Among ancillary LI-RADS v2018 imaging features, restricted diffusion is the diagnostic feature most
accurately distinguishing HCCs from benign abnormalities in LR-4 lesions.
Key Words: Carcinoma, hepatocellular; Diagnostic imaging; Gadolinium ethoxybenzyl DTPA; Liver; Magnetic resonance imaging
中文摘要
肝臟成像報告和數據系統2018版輔助徵象對MRI從LR-4病變轉診為肝細胞癌的意義
K Lim、H Kwon、J Cho、D Kim、S Kim、E Kang
目的
評估肝臟成像報告和數據系統(LI-RADS)2018版輔助徵象對釓塞酸增強MRI從LR-4病變(“可能是 HCC”)轉診為肝細胞癌(HCC)的意義。
方法
本回顧研究分析2015年3月至2017年12月期間釓塞酸增強MRI 114例高危病例中的166個包含輔助徵象的LR-4病變。兩名放射科醫生使用LI-RADS 2018版評估成像特徵。所有病灶均通過病理或超過2年的隨訪影像學證實為HCC或良性病變。使用單變量和多變量邏輯迴歸和廣義估計方程評估輔助徵象對於診斷的貢獻。
結果
共確診114個HCC(68.7%)和52個良性病變(31.3%)。單變量邏輯迴歸分析顯示輕度至中度T2高信號(p = 0.014)、彌散受限(p < 0.001)和病灶內脂肪(p = 0.018)在區分HCC和良性病變具統計學意義;而多變量邏輯分析顯示,只有彌散受限具統計學意義(經調整比值比 = 9.703,p < 0.001)。彌散受限對HCC的診斷敏感性較低(48.2%),特異性較高(90.4%),然而當結合輕度至中度T2高信號和肝膽期低信號時,敏感性為73.8%,特異性為80.8%。
結論
在LI-RADS 2018版輔助影像學徵象中,彌散受限最能區分HCC與LR-4良性病變。
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer in adults, and the most
common cause of mortality in cirrhotic patients.[1] In
2011, the American College of Radiology introduced
the Liver Imaging Reporting and Data System (LI-RADS)
to standardise the acquisition, interpretation,
and reporting of enhanced magnetic resonance imaging
(MRI) or computed tomography imaging of liver lesions
in patients at high risk of HCC. The most updated LI-RADS
v2018 includes five major categories (LR-1 to
LR-5) based on imaging features that reflect their relative
probability of being benign or HCC.[2] The accuracy of
the system for diagnosing HCC in the category ‘LR-4
(probably HCC)’ has been reported to be approximately
73% to 74%.[3] [4] When LR-4 is reported, it is generally
recognised as HCC requiring a pathological diagnosis
or treatment, but the diagnostic accuracy of the LR-4
group is often a clinical dilemma to initiate immediate
treatment without a pathological diagnosis.
When determining a LI-RADS v2018 category using
MRI, five major features are considered: nonrim arterial phase hyperenhancement (APHE), nonperipheral
washout, enhancing capsule, size, and threshold growth;
ancillary features are additional imaging findings
designed to improve detection accuracy and increase
reliability. Ancillary features are not intended to be used
without major features regardless of their abundance.
They can be used optionally at the discretion of the
radiologist when category adjustment is necessary, and
only one category can be upgraded or downgraded. But
any ancillary features cannot be used to upgrade to LR-5;
upgrading an LR-4 to an LR-5 cannot currently be
performed because there is not enough specificity for the
diagnosis of HCC.[2] Thus, in the current LI-RADS, it can
be said that LR-4 is made up of a rather heterogeneous
group (ranging from upgrades from LR-3 to remained
lesions that have not been upgraded to LR-5).
Since early diagnosis of HCC is important to increase
the likelihood of treatment, it is important to increase
the diagnostic accuracy of the LR-4 category. Several
recent studies have reported the diagnostic performance
of LI-RADS ancillary features.[5] [6] [7] However, the
diagnostic performance of category adjustment and the importance of each contributing ancillary feature have
not been sufficiently studied, particularly in observations
upgraded from LR-3 or downgraded from LR-4.
The goal of this study was to evaluate the diagnostic
performance of LI-RADS v2018 ancillary features for
improving the diagnostic accuracy for HCC in LR-4
lesions using gadoxetic acid–enhanced MRI. We also
investigated whether using a certain combination of
ancillary features could improve the diagnostic accuracy
of the LR-4 category.
METHODS
Study Population
We retrospectively searched consecutive cases at
high risk for HCC who underwent gadoxetic acid–enhanced MRI between March 2015 and December
2017. Inclusion criteria were as follows: (1) LR-3 and
LR-4 lesions categorised on the basis of major imaging
features, with one or more ancillary imaging features; (2) lesions confirmed as HCC or benign lesions through
pathological diagnosis or subsequent imaging over
2 years. Exclusion criteria were as follows: (1) lesions
difficult to characterise because of small size (<5 mm)
or suboptimal image quality; (2) multifocal lesions (>5);
(3) LR-M (probably or definitely malignant but not HCC
specific) and LR-TIV (tumour in vein) lesions; and (4)
administration of locoregional therapy before obtaining
pathological proof without evidence of recurrence in
subsequent imaging.
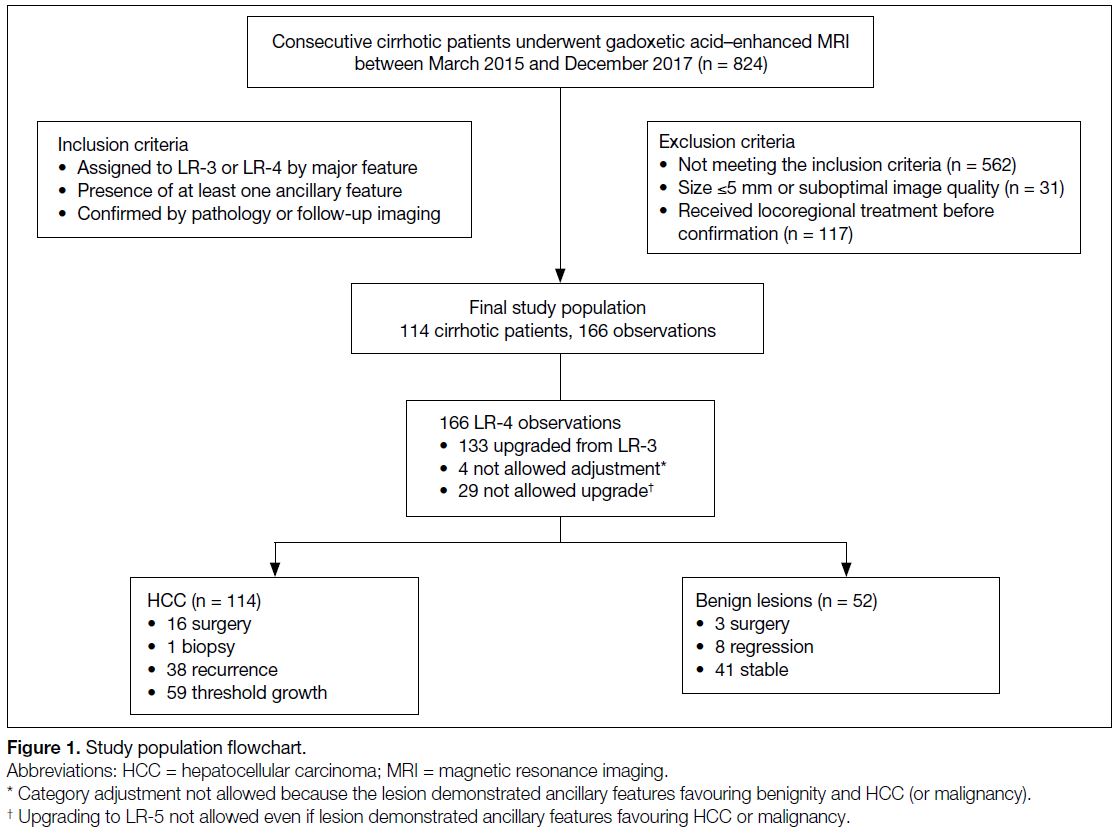
Figure 1. Study population flowchart.
Liver Magnetic Resonance Imaging Protocols
The liver MRI was performed on a 3.0-Tesla system
(Discovery MR750, GE Healthcare, Waukesha [WI],
United States) with following protocols: localiser images
using T2-weighted single-shot fast spin-echo sequence
and chemical shift images using three-dimensional
(3D) dual-echo T1-weighted gradient-echo sequence.
Dynamic contrast-enhanced images were acquired with 15-s breath-hold interval before and after contrast
agent injection using 3D-spoiled gradient-echo sequence
with two-point Dixon water-fat separation (3D LAVA-FLEX).
Contrast administration was performed at a dose
of 0.1 mL/kg of gadoxetic acid at a rate of 1 mL/s
followed by a 20-mL saline flush at the same rate.
Dynamic contrast-enhanced images were obtained after
contrast injection during the early arterial phase, late
arterial phase, portal venous phase (PVP), transitional
phase (TP), and hepatobiliary phase (HBP). T2-weighted
image and diffusion-weighted image (DWI) were
successively obtained using navigator triggering during
the long interval between the TP and HBP. T2-weighted
images were obtained using fat-saturated T2-weighted
turbo spin-echo, known as PROPELLER (periodically
rotated overlapping parallel lines with enhanced
reconstruction) and DWIs were obtained at three b-values
(50, 400, 800 s/mm2). The apparent diffusion coefficient
(ADC) images were generated automatically on the MR
console system using a mono-exponential ADC model
of all 3 b-values.
Lesion Registration
One radiologist (KL), with 6 years’ experience with liver MRI, who was aware of patient clinical information,
retrospectively reviewed the MRI exams and reports in
a PACS (picture archiving and communication system),
identifying consecutive observations fulfilling the
inclusion criteria. When a target patient was identified,
the reader recorded the size and location of individual
lesions on the basis of major image features and selected
the largest lesions up to a total of five if there were
multiple lesions in one patient. After inclusion, two
board-certified radiologists with >10 years of experience
in MRI reviewed and verified the lesions.
Magnetic Resonance Imaging Analysis
Two other radiologists (HK and JC, with 16 years
and 20 years of experience in liver MRI, respectively)
performed image analysis according to the following
steps. First, after lesion registration, two readers (HK
and JC) blinded to the final lesion diagnosis assessed the
presence or absence of all major and ancillary features
individually within a week. Second, immediately after an
individual assessment, all discordant major or ancillary
features were discussed twice to achieve consensus in
two separate sessions spaced apart by a week.
Reference Standards
All lesions included in the study were confirmed by pathological diagnosis or imaging follow-up. As defined
in LI-RADS v2018, a lesion was considered benign in
the following instances: (1) lesions that did not change
in size or acquire additional imaging features >2 years of
follow-up; (2) lesions that reduced in size or disappeared
during imaging follow-up. Cases were considered
HCC when: (1) lesions were pathologically confirmed
by surgery or biopsy; (2) lesions increased in diameter
≥50% within 6 months (threshold growth) and lesion
size >20 mm; (3) recurrent lesions after locoregional
therapy (e.g., radiofrequency ablation, transarterial
chemoembolisation). When a lesion suspected to be
benign had ≤2 years of follow-up, it was excluded if
there was no size reduction or disappearance. Similarly,
even if HCC was suspected, lesions with a stable size
after locoregional therapy without pathological diagnosis
were excluded.
Statistical Analysis
The potential association between ancillary imaging
features and final diagnosis was evaluated using
Pearson’s Chi-square and binary logistic regression
analysis. Simple and multivariable logistic regression
analyses (using generalised estimation equations to
avoid clustering effects) were performed to characterise
potential associations between the presence of ancillary
imaging features and HCC. Variables with p ≤ 0.20
in the simple logistic regression analysis were then
included in a multivariable logistic regression analysis.
Multivariable logistic regression analysis was conducted
with two models: Model 1 included significant variables
among the ancillary imaging features and Model 2
included significant variables for all major and ancillary
imaging features. Results were presented as the odds
ratio (OR), 95% confidence interval (CI), p value, and
considered statistically significant when p ≤0.05. A
binary diagnostic test was performed to estimate the
diagnostic performance, and potential combinations of
ancillary imaging features, which may contribute most to
improving diagnostic performance, were also evaluated.
Statistical analyses were performed using SPSS
(Windows version 23.0; IBM Corp., Armonk [NY], United States).
RESULTS
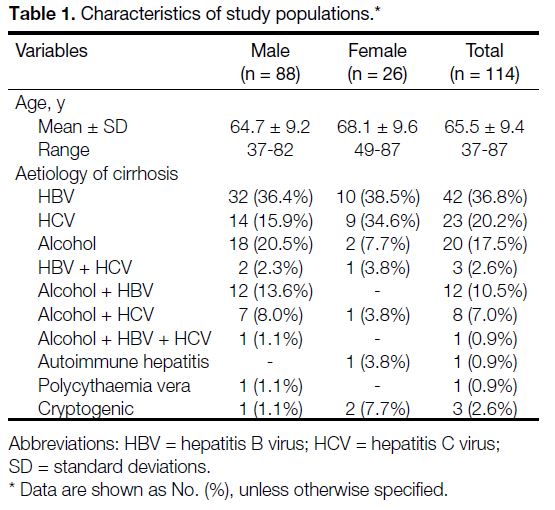
The final sample consisted of 114 cases (88 male and
26 female), with a mean age of 65.5 ± 9.4 years (range,
37-87) [Table 1].
Table 1. Characteristics of study populations
Lesion Characteristics
A total of 166 lesions were included in the study, with a mean lesion diameter of 12.3 ± 5.4 mm and a mean
of 1.4 ± 0.8 (range, 1-5) lesions per patient (1 lesion,
n = 90; 2 lesions, n = 21; 3 lesions, n = 10; 4 lesions,
n = 1). Category adjustments were performed according
to the LI-RADS algorithm, on the basis of ancillary features in all lesions, as follows: 133 LR-3 lesions were
upgraded to LR-4, 4 LR-4 were not adjusted because they
had ancillary features that favoured both malignancy and
benignity, and 29 LR-4 lesions were not upgraded to
LR-5 even though they had ancillary features favouring
HCC or malignancy. These were confirmed as 114 HCCs (68.7%)
and 52 benign lesions (31.3%) [Figure 1].
Diagnostic Performance of the Imaging
Features
Lesions were divided by size: <10 mm, 10 to 19 mm, and ≥20 mm.
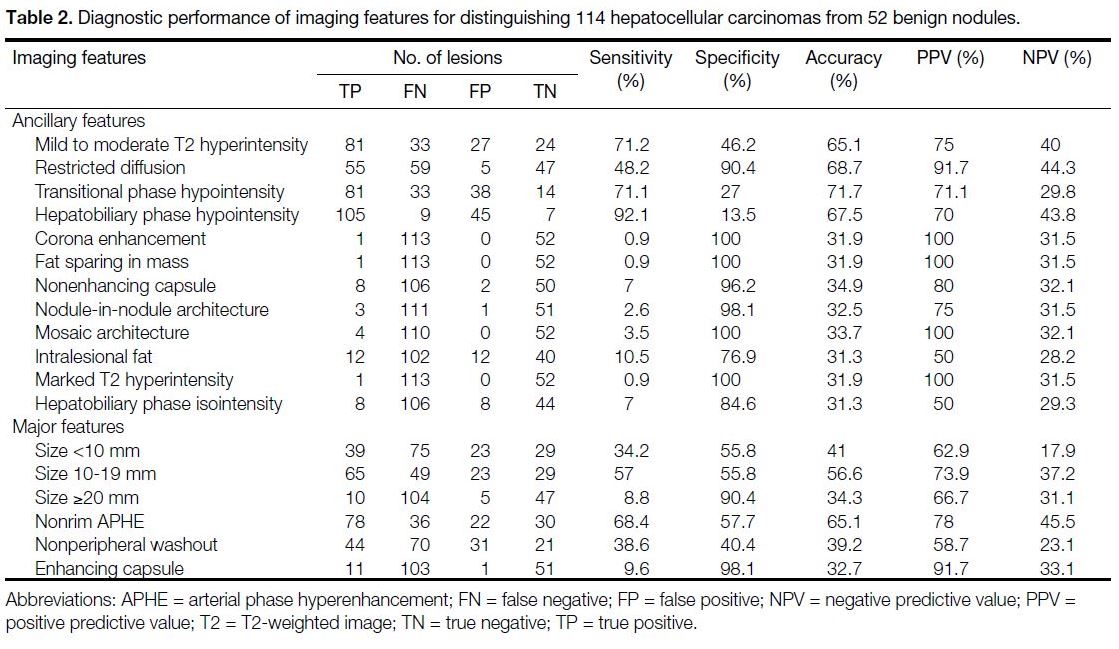
Among all evaluated ancillary features, HBP
hypointensity was most common (150 of 166) and had
the highest sensitivity (92.1%) with lowest specificity
(13.5%). Mild to moderate T2 hyperintensity was
relatively common (108 of 166) and had relatively
high sensitivity (71.2%) with low specificity (46.2%).
Restricted diffusion was relatively less common (60 of
166) and had high specificity (90.4%) with high positive
predictive value (91.7%) [Table 2].
Table 2. Diagnostic performance of imaging features for distinguishing 114 hepatocellular carcinomas from 52 benign nodules
A nonenhancing capsule (10 of 166), intralesional fat
(24 of 166), and HBP isointensity (16 of 166) were less common; however, a nonenhancing capsule
appeared relatively more frequently in HCC compared
with intralesional fat and HBP isointensity. Corona
enhancement, fat sparing in a mass, nodule-in-nodule
architecture, mosaic architecture, and marked T2
hyperintensity were rarely observed (≤5). We also
analysed the major features of the included lesions
and noted that nonrim APHE was the most common
major feature (100 of 166) with a sensitivity of 68.4%.
Nonperipheral washout in the PVP was relatively
common (75 of 166), but the diagnostic performance
was equivocal. Enhancing capsule was rarely observed
(12 of 166) but showed the highest specificity (98.1%)
and high positive predictive value (91.7%) [Table 2].
Simple and Multivariable Logistic
Regression Analyses
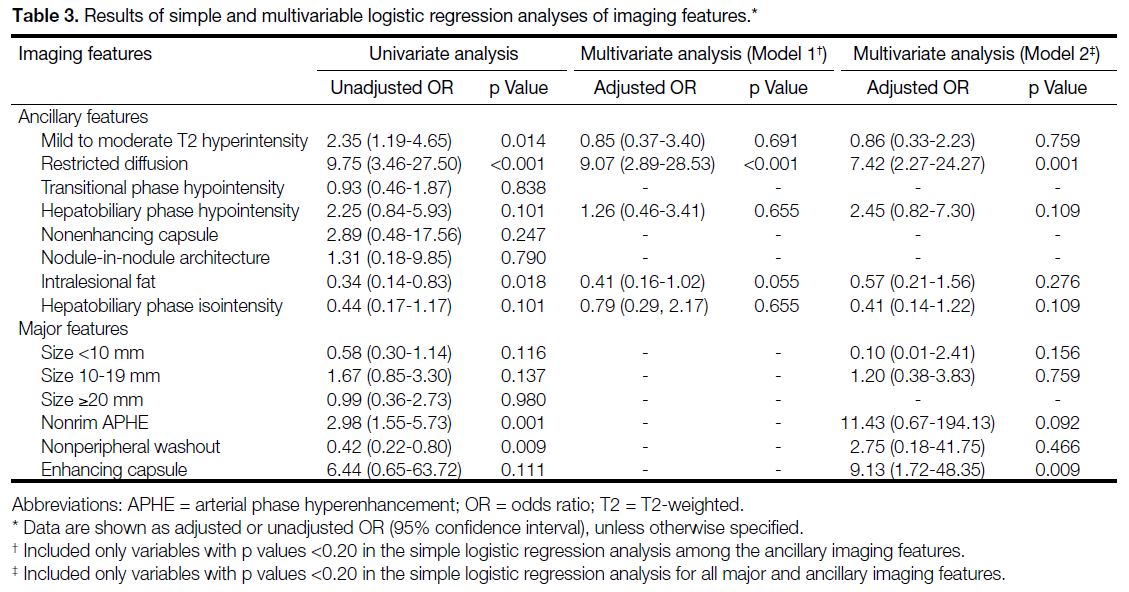
Simple logistic regression analysis revealed that among
ancillary features — mild to moderate T2 hyperintensity
(p = 0.014), restricted diffusion (p < 0.001), intralesional
fat (p = 0.018), and among major features — nonrim
APHE (p = 0.001) and nonperipheral washout
(p = 0.009), were significantly associated with HCC.
Other less common ancillary features were not included
in the subsequent analyses. Multivariable logistic
regression analysis with Model 1 revealed that only
restricted diffusion was a significant and independent
predictor of HCC (adjusted OR = 9.07, 95% CI = 2.89-28.53; p < 0.001) [Table 3]. Results from Model 2
demonstrated that restricted diffusion (adjusted OR =
7.42, 95% CI = 2.27-24.27; p = 0.001) and enhancing
capsule (adjusted OR = 9.13, 95% CI = 1.72-48.35;
p = 0.009) were significant (Table 3).
Table 3. Results of simple and multivariable logistic regression analyses of imaging features
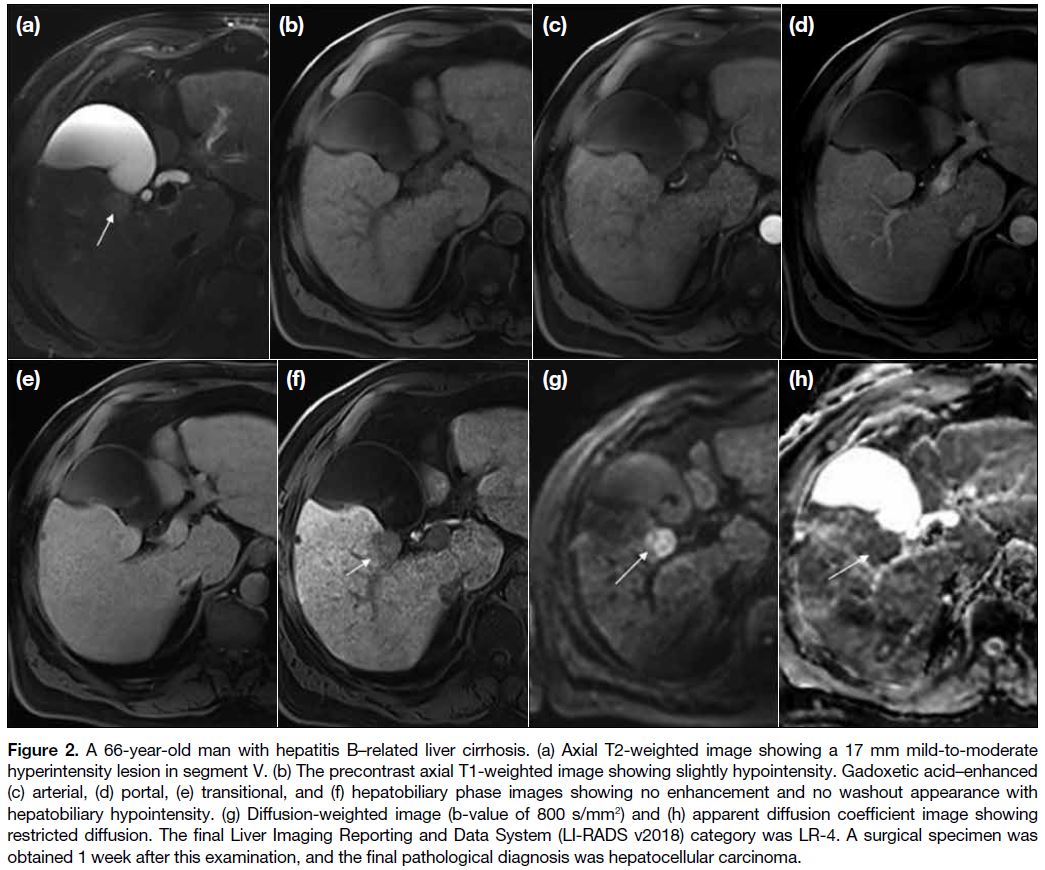
Figure 2. A 66-year-old man with hepatitis B–related liver cirrhosis. (a) Axial T2-weighted image showing a 17 mm mild-to-moderate
hyperintensity lesion in segment V. (b) The precontrast axial T1-weighted image showing slightly hypointensity. Gadoxetic acid–enhanced
(c) arterial, (d) portal, (e) transitional, and (f) hepatobiliary phase images showing no enhancement and no washout appearance with
hepatobiliary hypointensity. (g) Diffusion-weighted image (b-value of 800 s/mm2) and (h) apparent diffusion coefficient image showing
restricted diffusion. The final Liver Imaging Reporting and Data System (LI-RADS v2018) category was LR-4. A surgical specimen was
obtained 1 week after this examination, and the final pathological diagnosis was hepatocellular carcinoma.
DISCUSSION
Advances in liver MRI technology (e.g., DWI, dynamic
imaging, HBP imaging using hepatocyte-specific
contrast agents) have enabled MR imaging to accurately
assess tumour cellularity, vascularity, and absence of
functioning hepatocytes.[8] [9] These MR sequences can
help facilitate early diagnosis of small HCCs through
more detailed and accurate image analysis, rather than
applying a wait-and-see policy, especially for suspected
lesions at this stage (Figure 2). According to LI-RADS v2018,
a lesion can be considered LR-4 if APHE is present
along with at least one of three major features (i.e.,
nonperipheral washout, enhancing capsule, threshold
growth), and, even if APHE is absent, the presence of
two or more of three major features in a lesion can allow
it to be considered as LR-4.[10] The most recent 2018
American Association for the Study of Liver Diseases
practice guidelines[11] [12] propose to apply stringent
imaging criteria with high specificity for noninvasive
diagnosis of HCC in high-risk patients. Key imaging features include size ≥1 cm, APHE, and combination
with washout appearance and/or enhancing capsule. In
addition, they emphasise a multidisciplinary diagnostic
approach, particularly for LR-4 lesions measuring ≥1 cm
in diameter. The LR-4 lesions are considered probable
HCC, but sometimes subsequent image follow-ups
are proposed without immediate action due to high
false-positive rates (up to 30%). However, up to 15%
of these untreated LR-3 lesions and 68% of untreated
LR-4 lesions eventually become LR-5 within 2 years of
follow-up.[11]
LI-RADS ancillary features are an option for radiologists
and their use is encouraged because they exhibit various
contrast enhancement patterns reflecting the histological
characteristics of the tumour, even though they lack the
specificity for accurate HCC diagnosis of major features. Recent studies characterising the clinical application of
ancillary features[13] [14] [15] [16] [17] have shown that 15% to 35% of
lesions were readjusted to a different LI-RADS category,
with slightly more frequent upgrades than downgrades;
in fact, roughly 63% of LR-4 lesions were upgrades from
LR-3. We already know that major imaging features have
high specificity for the diagnosis of HCC in LI-RADs
lesions including LR-4. However, in clinical practice, a
large percentage of LR-4 lesions are category upgraded
to LR-4 from LR-3 lesions. Hence, improving diagnostic
value among the ancillary features is important to
increase specificity for the diagnosis of HCC of the LR-4
group. We believe that there is a difference in importance
among the various ancillary features suggesting HCC.
Several studies have reported that mild to moderate T2 hyperintensity, TP hypointensity, and HBP hypointensity increase sensitivity for the diagnosis of HCC when used
in combination with major features.[13] [18] [19] [20]
Vernuccio et al[21] reported that the finding of HBP
hypointensity significantly improves sensitivity for HCC
diagnosis in LR-3 lesions measuring 10 to 19 mm with
APHE while maintaining moderately high specificity.
Kwon et al[22] reported that hyperintensity on T2-weighted
images, in addition to arterial enhancement on gadoxetic
acid–enhanced MR images, and hyperintensity on DWI,
is helpful for differentiating small HCCs (≤2 cm) from
benign nodules in patients with cirrhosis. Our data
showed that HBP hypointensity (92.1%) has the highest
sensitivity among major imaging features, and mild to
moderate T2 hyperintensity (71.2%), TP hypointensity
(71.1%) have relatively high sensitivity. However,
multivariable logistic regression analysis demonstrated
that none of these values were statistically significant.
Restricted diffusion (90.4%), nonenhancing capsule
(96.2%), intralesional fat (76.9%), and HBP isointensity
(84.6%) showed a high specificity among relatively
commonly appearing ancillary features. Uncommon
ancillary features were considered to have high
specificity, but their infrequency limits their utility for
estimating diagnostic performance. Multivariable logistic
regression analysis revealed that restricted diffusion was
the only statistically significant for diagnosing HCC in
both Model 1 (adjusted OR = 9.07; p < 0.001) and Model
2 (adjusted OR = 7.42; p = 0.001). Several studies have
investigated the role of DWI to differentiate between
HCCs and dysplastic nodules.[13] [21] [23] [24] [25] [26] [27] [28] Granata et al[23]
and Lee et al[24] reported a sensitivity of 81% to 84%,
and specificity of 73% to 100% for ‘hyperintensity
on DWI’. Piana et al[25] reported that ‘APHE combined
with DWI hyperintensity’ improves sensitivity for the
diagnosis of HCC compared to conventional criteria,
from 60% to 76%-77% for all HCCs, and from 37% to 60%-66% for HCCs <2 cm. In this study, restricted
diffusion demonstrated a sensitivity of 48.2% and a
specificity of 90.4% with statistical significance, which
is similar to the sensitivity of 54.8% and specificity of
90.6% reported by Cerny et al.[13] Interestingly, although
there were some differences in the study populations,
these two studies showed lower sensitivity and higher
specificity compared to other published studies as a
result of strict application of the definition of restricted
diffusion (i.e., hyperintensity on DWI and hypointensity
on ADC images). In LI-RADS v2018, restricted
diffusion is classified as an ancillary feature favouring
malignancy in general (but not specific in HCC) and is defined as ‘intensity on DWI unequivocally higher
than liver and/or ADC unequivocally lower than liver’.
Restricted diffusion is generally known to be useful in
differentiating a malignant from a benign lesion, and is
defined as having higher signal intensity, not attributable
solely to T2 shine-through effect on DWI acquired with
at least moderate diffusion weighting (e.g., b-value
≥400 s/mm2). However, it was noted here that there were
a very large number of false-positives when features
were defined as ‘hyperintensity on DWI or hypointensity
on ADC image’. Therefore, a consensus was formed by
strictly applying the definition of restricted diffusion
as ‘hyperintensity on DWI and hypointensity on ADC
images’, and the results showed a significant correlation
with HCC. Although many studies about the diagnostic
performance of DWI for the diagnosis of HCC have
been reported based on ‘hyperintensity on DWI’,[22] [23] [24] the
results presented here demonstrate that applying a strict
definition reduces sensitivity, improves specificity, and
maintains accuracy. Therefore, this study demonstrates
that restricted diffusion may play a useful role in
ancillary features for LR-4 lesions. Further research
using a larger population is warranted, and it may be
necessary to correct and supplement the definition of
restricted diffusion mentioned in LI-RADS v2018.
In our study, the overall diagnostic performance of
major features was lower compared with previous
analyses.[13] [21] [22] APHE demonstrated intermediate
diagnostic performance, nonperipheral washout had both
low sensitivity and specificity, and enhancing capsule
demonstrated very high specificity. The diagnostic
performance of major imaging features in our study is
thought to be somewhat lower than that of other studies
on LR-4, since it was calculated on LR-4 that did not
restrict a specific major features (e.g., size or APHE).
Our data revealed that 33 cases of HCC showing only
nonperipheral washout and/or capsular appearance
without APHE. Therefore, it is considered that there may
be selection bias when limiting studies of category up to
LR-4.
Low diagnostic performance of nonperipheral washout
for the diagnosis of HCC was reported here, a finding
that may be related to characteristics of gadoxetic acid–enhanced imaging evaluation during the PVP only. It
can be a variable in clinical practice, considering that the
likelihood of nonperipheral washout being false-negative
will be higher than expected, if problems occur in the
process of enhancement or PVP cannot be accurately
obtained.
An assessment of diagnostic criteria revealed that the specificity for the diagnosis of HCC when APHE was
combined with nonperipheral washout or capsular
appearance was 92%, consistent with previous studies
showing specificity between 89% and 99%.[11] [12] [29]
The combination of major and ancillary features
calculated in our study (e.g., APHE with DWI and
APHE with HBP) did not reveal an improvement of
diagnostic performance, unlike previous studies by
Cerny et al[13] and Kwon et al.[22] Our calculated diagnostic
value when combining major and ancillary features was
similar to that calculated for each ancillary feature alone.
The diagnostic performance of restricted diffusion,
which showed the only statistical correlation with HCC
in multivariable logistic regression analysis, was best
improved to 73.8% for sensitivity, 80.8% for specificity,
and 75.5% for accuracy when combined with mild to
moderate T2 hyperintensity and HBP hypointensity.
These results show the role of ancillary features for
diagnosis of HCC in LR-4 lesions. Additional studies are
warranted to better understand comprehensive diagnostic
criteria including ancillary imaging features.
There were several important limitations of this study. First, the study was performed retrospectively at a
single institution, and there may have been selection
bias of the study population. Second, confirmation of
many lesions was made by subsequent imaging. Only
13.9% (23 of 166) of lesions included pathological
diagnoses, however, there is an inevitable limitation
because subsequent imaging is generally favoured at
this stage, rather than pathological diagnosis. However,
the reference standard was strictly applied and only
cases with obvious features in subsequent imaging
were included in this study population. Third, the final
diagnosis was divided into HCC and benign lesions.
Although confirmation through subsequent imaging
was based, it was possible that unconfirmed non-HCC
lesions were included in the HCC category; this effect
was unpredictable.
In conclusion, our study to evaluate the diagnostic
performance of ancillary features for the diagnosis of
HCC in an LR-4 lesion suggest that restricted diffusion
is the most useful diagnostic feature and is associated
with excellent specificity. In case of LR-4 lesions with
ancillary features, the combination of mild to moderate
hyperintensity on T2 image and DWI restriction can
improve the diagnostic value.
REFERENCES
1. World Health Organization. Cancer Today. 2018. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_...
pulation=900&populations=900&key=asr&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1. Accessed 21 Sep 2019.
2. American College of Radiology. Liver Imaging Reporting and
Data System version 2018. 2018. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf.... Accessed 21 Sep 2019.
3. Ronot M, Fouque O, Esvan M, Lebigot J, Aubé C, Vilgrain V. Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. J Hepatol. 2018;68:715-23. Crossref
4. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy — a
systematic review. Gastroenterology. 2019;156:976-86. Crossref
5. Cannella R, Vernuccio F, Sagreiya H, Choudhury KR, Iranpour N,
Marin D, et al. Liver Imaging Reporting and Data System (LI-RADS)
v2018: diagnostic value of ancillary features favoring
malignancy in hypervascular observations ≥10 mm at intermediate
(LR-3) and high probability (LR-4) for hepatocellular carcinoma.
Eur Radiol. 2020;30:3770-81. Crossref
6. Cerny M, Chernyak V, Olivié D, Billiard JS, Murphy-Lavallée J,
Kielar AZ, et al. LI-RADS version 2018 ancillary features at MRI.
Radiographics. 2018;38:1973-2001. Crossref
7. Kang JH, Choi SH, Byun JH, Kim DH, Lee SJ, Kim SY, et al. Ancillary features in the Liver Imaging Reporting and Data System: how to improve diagnosis of hepatocellular carcinoma ≤ 3 cm on magnetic resonance imaging. Eur Radiol. 2020;30:2881-9. Crossref
8. Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-48. Crossref
9. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. Crossref
10. Rimola J, Forner A, Tremosini S, Reig M, Vilana R, Bianchi L,
et al. Non-invasive diagnosis of hepatocellular carcinoma ≤2 cm
in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal
intensity at dynamic MRI. J Hepatol. 2012;56:1317-23. Crossref
11. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS,
Abecassis MM, et al. Diagnosis, staging, and management
of hepatocellular carcinoma: 2018 Practice Guidance by the
American Association for the Study of Liver Diseases. Hepatology.
2018;68:723-50. Crossref
12. Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ,
Heimbach JK, et al. Imaging for the diagnosis of hepatocellular
carcinoma: a systematic review and meta-analysis. Hepatology.
2018;67:401-21. Crossref
13. Cerny M, Bergeron C, Billiard JS, Murphy-Lavallée J, Olivié D,
Bérubé J, et al. LI-RADS for MR imaging diagnosis of
hepatocellular carcinoma: performance of major and ancillary
features. Radiology. 2018;288:118-28. Crossref
14. Joo I, Lee JM, Lee DH, Ahn SJ, Lee ES, Han JK. Liver imaging
reporting and data system v2014 categorization of hepatocellular
carcinoma on gadoxetic acid-enhanced MRI: comparison with
multiphasic multidetector computed tomography. J Magn Reson
Imaging. 2017;45:731-40. Crossref
15. Choi SH, Byun JH, Kim SY, Lee SJ, Won HJ, Shin YM, et al.
Liver Imaging Reporting and Data System v2014 with gadoxetate
disodium-enhanced magnetic resonance imaging: validation of
LI-RADS category 4 and 5 criteria. Invest Radiol. 2016;51:483-90 Crossref
16. Fowler KJ, Tang A, Santillan C, Bhargavan-Chatfield M,
Heiken J, Jha RC, et al. Interreader reliability of LI-RADS version
2014 algorithm and imaging features for diagnosis of hepatocellular
carcinoma: a large international multireader study. Radiology.
2018;286:173-85. Crossref
17. De Gaetano AM, Catalano M, Pompili M, Marini MG, Rodríguez
Carnero P, Gullí C, et al. Critical analysis of major and ancillary
features of LI-RADS v2018 in the differentiation of small (≤2 cm)
hepatocellular carcinoma from dysplastic nodules with gadobenate
dimeglumine-enhanced magnetic resonance imaging. Eur Rev Med
Pharmacol Sci. 2019;23:7786-801.
18. Di Martino M, Anzidei M, Zaccagna F, Saba L, Bosco S, Rossi M,
et al. Qualitative analysis of small (≤2 cm) regenerative nodules,
dysplastic nodules and well-differentiated HCCs with gadoxetic
acid MRI. BMC Med Imaging. 2016;16:62. Crossref
19. Hecht EM, Holland AE, Israel GM, Hahn WY, Kim DC, West AB,
et al. Hepatocellular carcinoma in the cirrhotic liver: gadolinium-enhanced 3D T1-weighted MR imaging as a stand-alone sequence for diagnosis. Radiology. 2006;239:438-47. Crossref
20. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive
diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced
MRI: can hypointensity on the hepatobiliary phase be used as an
alternative to washout? Eur Radiol. 2015;25:2859-68. Crossref
21. Vernuccio F, Cannella R, Meyer M, Choudhoury KR, Gonzáles F,
Schwartz FR, et al. LI-RADS: diagnostic performance of
hepatobiliary phase hypointensity and major imaging features of
LR-3 and LR-4 lesions measuring 10-19 mm with arterial phase
hyperenhancement. AJR Am J Roentgenol. 2019;213:W57-65. Crossref
22. Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ, Shin YM, et al. Differentiation of small (≤2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom
Imaging. 2015;40:64-75. Crossref
23. Granata V, Fusco R, Avallone A, Filice F, Tatangelo F, Piccirillo M,
et al. Critical analysis of the major and ancillary imaging features
of LI-RADS on 127 proven HCCs evaluated with functional
and morphological MRI: lights and shadows. Oncotarget.
2017;8:51224-37. Crossref
24. Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acidenhanced
hepatobiliary phase MRI and high-b-value diffusion-weighted
imaging to distinguish well-differentiated hepatocellular
carcinomas from benign nodules in patients with chronic liver
disease. AJR Am J Roentgenol. 2011;197:W868-75. Crossref
25. Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V.
New MR imaging criteria with a diffusion-weighted sequence for
the diagnosis of hepatocellular carcinoma in chronic liver diseases.
J Hepatol. 2011;55:126-32. Crossref
26. Shankar S, Kalra N, Bhatia A, Srinivasan R, Singh P, Dhiman RK,
et al. Role of diffusion weighted imaging (DWI) for hepatocellular
carcinoma (HCC) detection and its grading on 3T MRI: a
prospective study. J Clin Exp Hepatol. 2016;6:303-10. Crossref
27. Granata V, Fusco R, Catalano O, Guarino B, Granata F,
Tatangelo F, et al. Intravoxel incoherent motion (IVIM) in
diffusion-weighted imaging (DWI) for hepatocellular carcinoma:
correlation with histologic grade. Oncotarget. 2016;7:79357-64. Crossref
28. Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, Nakanishi K,
et al. Relationship between diffusion-weighted magnetic resonance
imaging and histological tumor grading of hepatocellular
carcinoma. Ann Surg Oncol. 2012;19:1302-9. Crossref
29. Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RK, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of Hepatocellular carcinoma: a systematic review. Radiology. 2018;286:29-48. Crossref