Paediatric Whole-body Magnetic Resonance Imaging and its Role in Oncological and Non-oncological Cases
PICTORIAL ESSAY
Paediatric Whole-body Magnetic Resonance Imaging and its Role in Oncological and Non-oncological Cases
ER Mohd Rusli1, F Mohd Zaki1, AHZ Samsudin2, L C-Khai3, EY Hing1, H Alias3, H Abdul Hamid1
1 Department of Radiology, Universiti Kebangsaan Malaysia Medical Center, Jalan Yaacob Latif, 56100 Kuala
Lumpur, Malaysia
2 Department of Radiology, Hospital Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
3 Department of Paediatrics, Universiti Kebangsaan Malaysia Medical Center, Jalan Yaacob Latif, 56100
Kuala Lumpur, Malaysia
Correspondence: Dr F Mohd Zaki, Department of Radiology, Universiti Kebangsaan Malaysia Medical Center, Jalan Yaacob Latif, 56100 Kuala Lumpur, Malaysia. Email: drfaizah@ppukm.ukm.edu.my
Submitted: 1 Jul 2021; Accepted: 27 Oct 2021
Contributors: ERMR, FMZ and HAH designed the.
Contributors: ERMR, FMZ and HAH designed the study. ERMR, FMZ, AHZS, LC and HA acquired the data. ERMR, FMZ, AHZS, EYH
and HAH analysed the data. ERMR, FMZ and AHZS drafted the manuscript. ERMR and FMZ critically revised the manuscript for important
intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are included in this published article and the online supplementary Appendix.
Ethics Approval: This manuscript is for pictorial review only so no formal ethics approval or written consent was obtained. The patients were treated in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent for all treatments and procedures and verbal consent for publication.
Acknowledgement: We thank Halimah Abdul Ghani and Wan Noor Afzan Wan Sulaiman, senior radiographers in our institution for initiating and supervising excellent whole-body magnetic resonance imaging studies acquisition.
INTRODUCTION
Magnetic resonance imaging (MRI) is a favourable
diagnostic tool compared with other imaging modalities
due to its high soft tissue contrast and spatial resolution
in detecting pathologies. Most importantly, it is free of
ionising radiation, making it suitable for the paediatric
population, especially in those who require repeat
imaging.[1] [2] [3]
Approximately 27% of paediatric oncological cases
present with metastases. The consequent needs for
long-term follow-up and frequent imaging with either
computed tomography (CT) scan or positron emission
tomography (PET-CT) will increase the cumulative radiation dose over time.[4] [5] [6] [7] Whole-body MRI (WBMRI)
has been advocated since the early 1990s to ensure
optimum whole-body imaging surveillance with
comparable diagnostic accuracy to that of CT and
PET-CT.[8] This paper discusses and illustrates the role of
WBMRI in oncological and non-oncological cases.
IMAGING TECHNIQUES
There are a few radiological modalities available for paediatric oncological cases, each with limitations.
Conventional CT and PET-CT scans provide wide body
coverage but patients are exposed to ionising radiation.
Ultrasound is preferred for its non-ionising radiation
property but is operator-dependent and the area of examination is limited. With technological advancement,
WBMRI can produce high image resolution for
locoregional and distant staging assessment.
Imaging techniques and best image quality depend on the magnetic field strength, coil system, and pulse sequences.
A comparison by Mohan et al[9] of 1.5-T and 3-T MRI
machines concluded that 1.5-T MRI produced superior
image quality, structural visibility, and fewer artefacts.
There was a higher risk of developing susceptibility
artefacts, dielectric shading, and motion artefacts with
3-T MRI even though it has a higher signal-to-noise
ratio and contrast-to-noise ratio compared with 1.5-T.[2]
Nevertheless both machines were comparable for
detecting pathology.[9] The current technical standard
for WBMRI includes a dedicated multi-channel, multi-element
surface coil system, allowing imaging of any
part of the body at a particular time without having to
move the coil system.[2] Unfortunately, not all centres
have this technology so the protocol has to be tailored
for each case to gain the best image possible.[8] [10]
Pulse sequences including short tau inversion recovery (STIR), T1-weighted, diffusion-weighted images, and
magnetic resonance angiography have been discussed
at great length.[2] STIR sequence is the most frequently
used sequence and has been adopted by our institution
since 2015 since it provides homogenous fat suppression
with higher sensitivity for detection of abnormalities. It
can identify lesions even in the hypercellular red bone
marrow of a young child compared with T1-weighted
imaging alone.[2] [10] The slice thickness should be <4.0 to 5.0 mm for good image quality.[8]
Scanning time in the paediatric population is another consideration. On average, a single examination takes 30
to 60 minutes, similar to bone scintigraphy.[10] Imaging
in the axial plane will increase lesion detection by 10%
but will also increase the table time.[10] In our institution,
we take an average 30 minutes of table scan time with
most patients requiring oral or intravenous sedation.
Additional sequences are also added to increase
diagnostic accuracy.
Clinical Application
Lymphoma
Lymphoma is the third leading cause of malignancy in
children following brain tumours and leukaemia.[10] [11] [12]
Recently, the Society of Pediatric Radiology (SPR) has
recommended WBMRI as an alternative to contrast-enhanced
CT thorax, abdomen, and pelvis.[13] PET-CT nonetheless remains a vital imaging modality to assess
tumour and treatment response.[10] [12] [13] [14] In our institution,
PET-CT is done during follow-up as per the current
recommendation and results correlated with those
of WBMRI to determine the metabolic activity of
the lesions. WBMRI can recognise both nodal (98%
sensitivity and 99% specificity) and extranodal disease
(91% sensitivity and 99% specificity). MRI has been
shown to be capable of detecting lymph nodes >12 mm
with a sensitivity of 92.0% and specificity of 99.9%.[11] [14] According to Guimarães et al,[14] WBMRI with coronal
STIR sequence is more sensitive in detecting marrow
involvement in the initial phase of the disease than other
conventional imaging.
The downside of MRI includes its inability to identify malignant nodes <1 cm and to discriminate between
lymphomatous bony infiltration and therapy-induced
marrow signal abnormalities.[11] Some of our patients
presented with nodal and others with extranodal disease
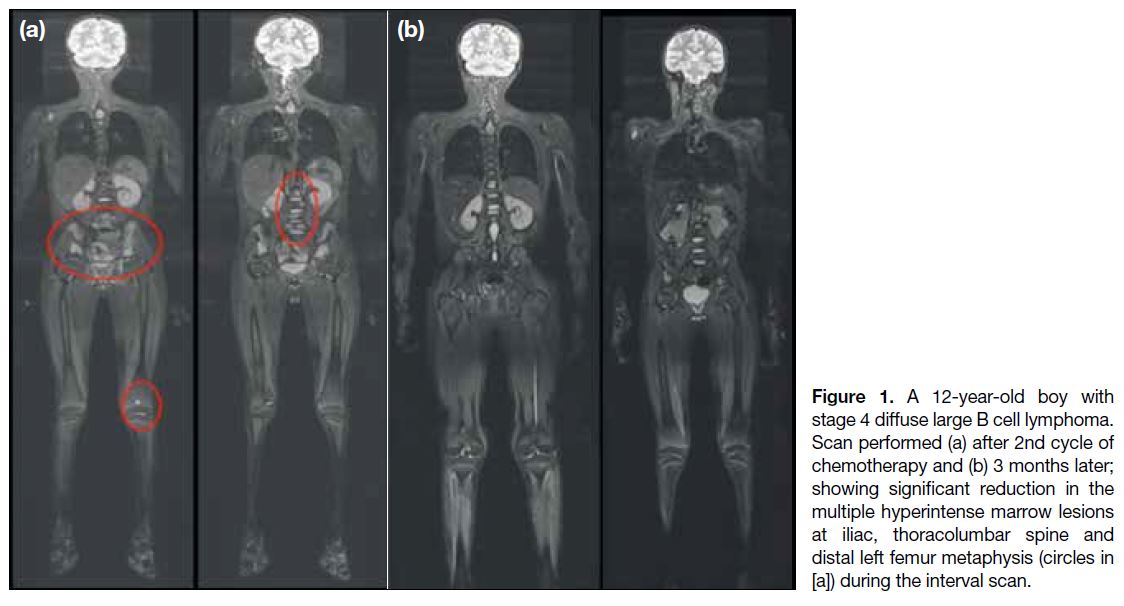
(Figure 1).
Figure 1. A 12-year-old boy with
stage 4 diffuse large B cell lymphoma.
Scan performed (a) after 2nd cycle of
chemotherapy and (b) 3 months later;
showing significant reduction in the
multiple hyperintense marrow lesions
at iliac, thoracolumbar spine and
distal left femur metaphysis (circles in
[a]) during the interval scan.
Neuroblastoma
Neuroblastoma is the most common extracranial solid
tumour in children, accounting for about 6% of cases
and approximately 15% of cancer deaths in children.[3] It
arises from the sympathetic chain and metastasises to the
bone, lymph nodes, liver, and skin.[3] [10] In the past, CT and
metaiodobenzylguanidine (MIBG) scintigraphy together
with bone marrow aspiration were essential to diagnose
and assess neuroblastoma, but MRI is increasingly being
utilised for regional disease.
Although WBMRI in neuroblastoma has not been fully
evaluated, a small study by Goo[2] revealed that it has
higher sensitivity than MIBG and CT in detecting bone
metastases.[10] In our centre, we perform an MIBG scan
for all new lesions detected on WBMRI, especially in
MIBG-positive cases (MIBG scintigraphy was a baseline
investigation before treatment) before proceeding with
biopsy. Two cases with neuroblastoma are shown
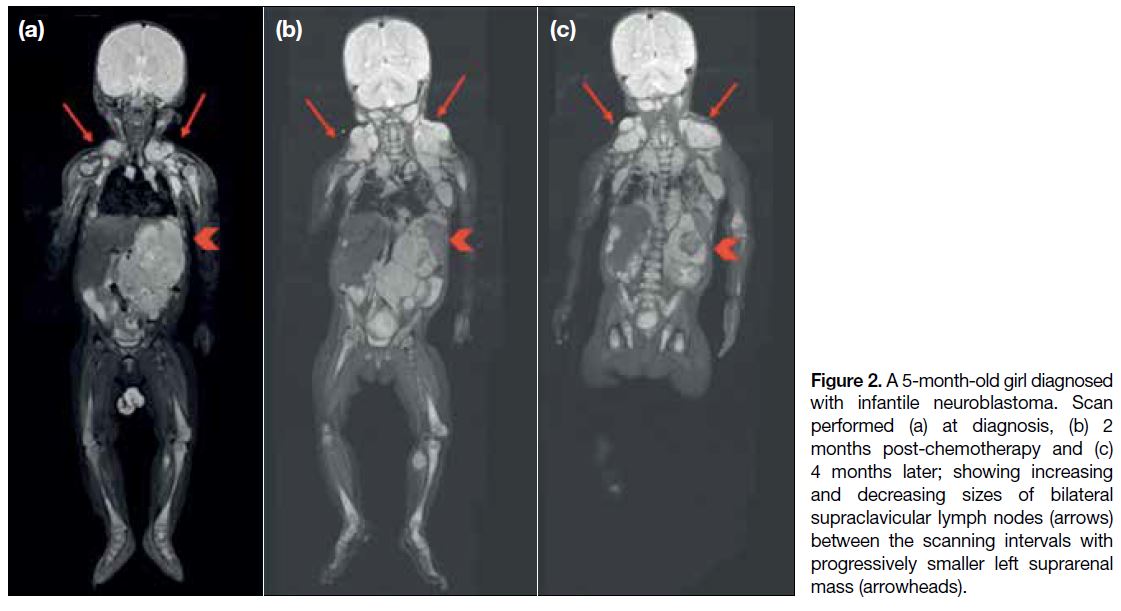
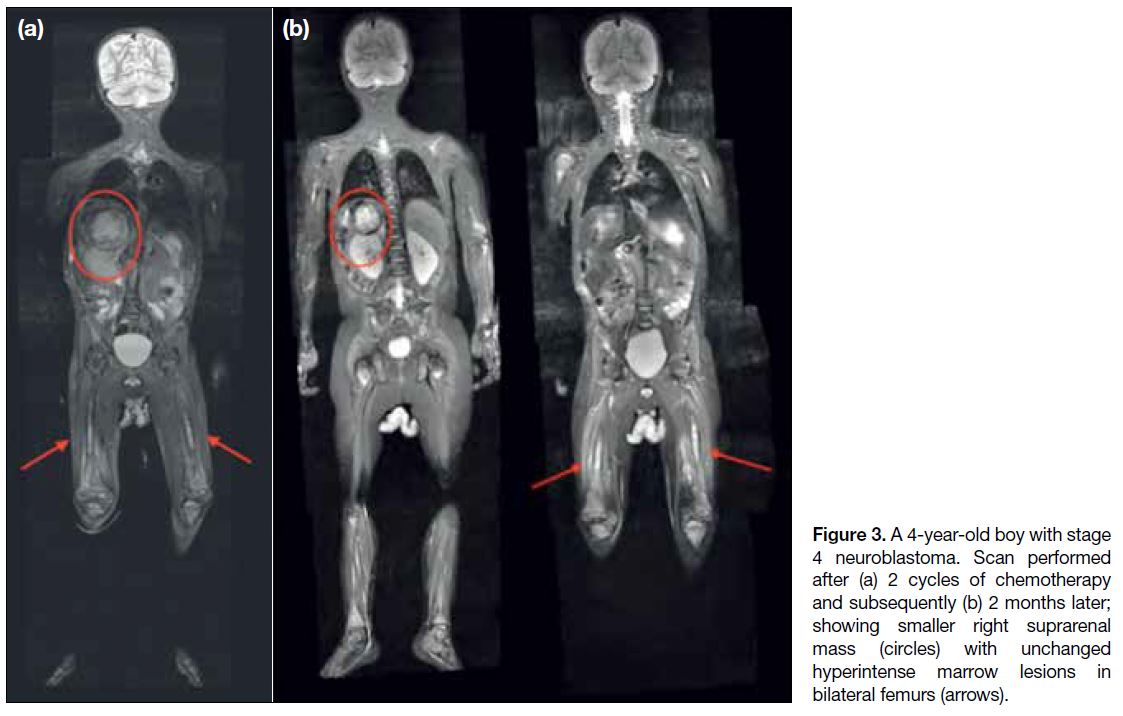
(Figures 2 and 3).
Figure 2. A 5-month-old girl diagnosed with infantile neuroblastoma. Scan performed (a) at diagnosis, (b) 2 months post-chemotherapy and (c) 4 months later; showing increasing and decreasing sizes of bilateral supraclavicular lymph nodes (arrows) between the scanning intervals with progressively smaller left suprarenal mass (arrowheads).
Figure 3. A 4-year-old boy with stage 4 neuroblastoma. Scan performed after (a) 2 cycles of chemotherapy and subsequently (b) 2 months later; showing smaller right suprarenal mass (circles) with unchanged hyperintense marrow lesions in bilateral femurs (arrows).
Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is mainly a disease of childhood, occurring at a median age of 30 months and
affecting the reticuloendothelial system; bone marrow,
liver, spleen, lymph node, and lungs.[3] [15] The disease
varies from a unifocal bone lesion to a multisystemic
disorder.[8] [10] In the past, plain radiograph and bone
scintigraphy were performed for diagnosis and follow-up, but clinicians are now more inclined to utilise WBMRI
as it can detect both skeletal and extraskeletal lesions.[10]
It is important to stage the disease before treatment
commences, since the presence of more than one lesion
will impact treatment decisions, i.e., intralesional
corticosteroid versus systemic chemotherapy.[3] [8]
According to Goo et al,[16] concurrent T1 post-contrast
sequence is more beneficial in differentiating solid from
cystic lesions than STIR sequence alone. The solid lesion
will commonly show avid or peripheral enhancement
on post-contrast images.[3] Nonetheless difficulty arises
in distinguishing residual lesions post-treatment from active lesions so functional imaging techniques such as
PET-CT, diffusion/perfusion MRI or MRI spectroscopy
are employed.
Although PET-CT has higher accuracy than plain
radiograph and bone scintigraphy, WBMRI is the
modality of choice to identify vertebral lesions.[10] [16]
Twelve cases diagnosed with LCH underwent WBMRI
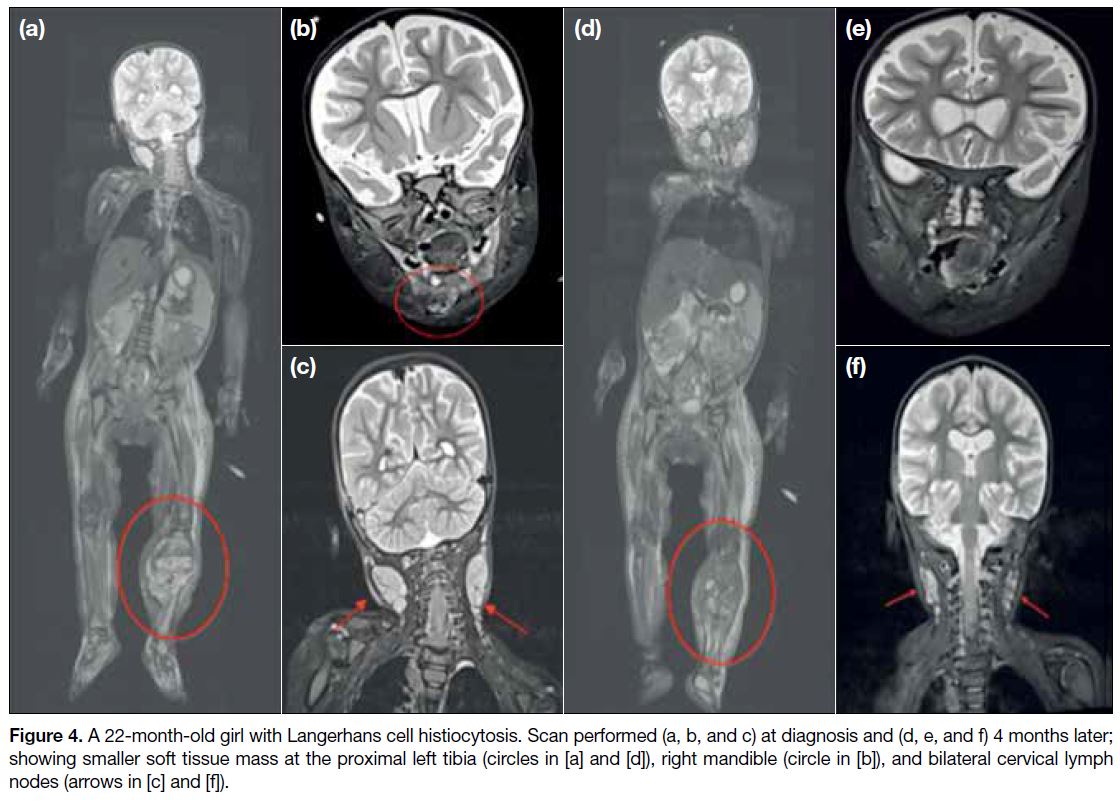
at our centre (Figure 4).
Figure 4. A 22-month-old girl with Langerhans cell histiocytosis. Scan performed (a, b, and c) at diagnosis and (d, e, and f) 4 months later;
showing smaller soft tissue mass at the proximal left tibia (circles in [a] and [d]), right mandible (circle in [b]), and bilateral cervical lymph nodes (arrows in [c] and [f]).
Chronic Recurrent Multifocal Osteomyelitis
Chronic recurrent multifocal osteomyelitis is a rare
condition first described in 1972. Affected children
will present with nonspecific musculoskeletal pain and
swelling. Imaging is required to exclude underlying
malignancy.[17]
Compared with PET and bone scintigraphy, WBMRI
is superior at identifying multifocal oedematous lesions
as they appear hyperintense on the STIR sequence.
In a cohort study by Leclair et al,[18] an average of two
lesions was found in all 16 patients, mainly located at the
epimetaphyseal region of the long bones. These lesions
are usually ill-defined and asymmetric.[18] No such case was encountered at our centre.
Cancer Predisposing Syndrome
Children with cancer predisposing syndrome
(neurofibromatosis type 1, Beckwith-Wiedemann
syndrome, multiple endocrine neoplasias, Li-Fraumeni
syndrome, von Hippel-Lindau syndrome, and familial
adenomatous polyposis) are at significant risk of
developing cancer due to its familial inheritance.[3] [10] [14]
They require regular screening that should include a
physical examination, blood analysis, urinalysis, and
imaging.[10]
According to Greer et al,[19] WBMRI is now recommended
in these children due to its head-to-toe coverage with
no additional radiation risk, making it more favourable
than CT or PET-CT. It is a potential screening technique
that can improve patients’ long-term outcome while
reducing the tumour burden by identifying the tumour
at the earliest and most curable stage due to its high
sensitivity and specificity.[3] [20] Furthermore, the SPR
recommends WBMRI as a replacement for skeletal
survey radiograph and bone scintigraphy in assessment
of osseous LCH.[13]
Metastasis
WBMRI can be utilised to assess malignant solid tumours as it has a higher if not similar degree of sensitivity and
specificity to PET or CT.[6] [10] [14] About 10% of patients
with bone metastases have an unknown primary and this
too can be assessed by WBMRI.[21] MRI generally has
a sensitivity of >90% in detecting bone metastases and
this further increases when STIR sequence is performed
along with T1-weighted sequence.[12] [14] We have scant
experience in performing WBMRI on soft tissue sarcoma
as it is not a standard imaging protocol for treatment
assessment. Furthermore, sarcoma rarely presents with
disseminated disease. However, the SPR recommends
WBMRI, particularly in disseminated disease.[13]
Examples of malignant solid tumours in childhood
encountered in our centre include rhabdomyosarcoma
(Figure 5), Ewing sarcoma, osteosarcoma (Figure 6) and
primitive neuroectodermal tumours.[6] [10]
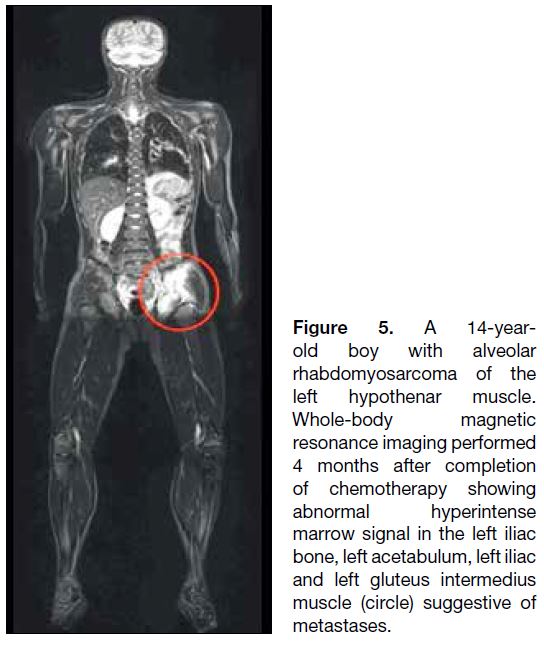
Figure 5. A 14-year-old
boy with alveolar rhabdomyosarcoma of the left hypothenar muscle. Whole-body magnetic resonance imaging performed 4 months after completion of chemotherapy showing abnormal hyperintense marrow signal in the left iliac bone, left acetabulum, left iliac and left gluteus intermedius muscle (circle) suggestive of metastases.
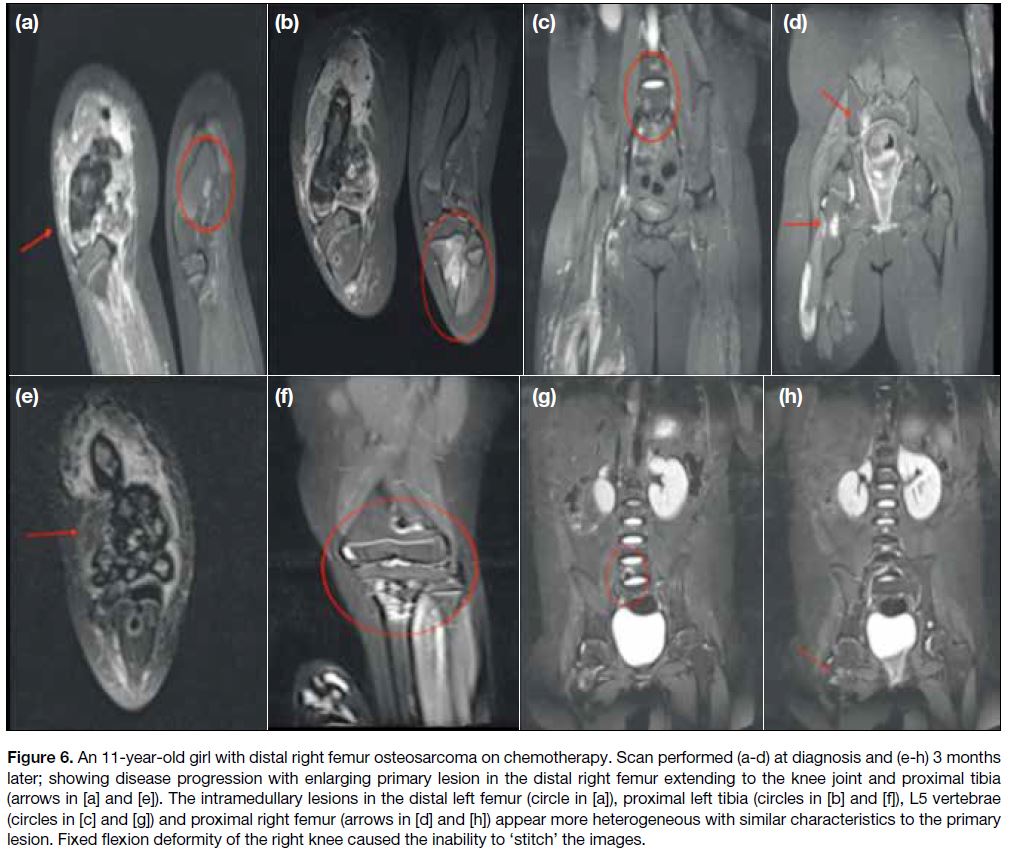
Figure 6. An 11-year-old girl with distal right femur osteosarcoma on chemotherapy. Scan performed (a-d) at diagnosis and (e-h) 3 months later; showing disease progression with enlarging primary lesion in the distal right femur extending to the knee joint and proximal tibia
(arrows in [a] and [e]). The intramedullary lesions in the distal left femur (circle in [a]), proximal left tibia (circles in [b] and [f]), L5 vertebrae (circles in [c] and [g]) and proximal right femur (arrows in [d] and [h]) appear more heterogeneous with similar characteristics to the primary lesion. Fixed flexion deformity of the right knee caused the inability to ‘stitch’ the images.
Imaging Pitfalls
There are some pitfalls related to WBMRI. One is the
need to apply an individual coil rather than a dedicated
body coil resulting in the inability to stitch together the
final images.[2] This problem is frequently encountered in
our centre. Distortion of a patient’s normal anatomical
position will also affect the post-processing images as
shown in Figure 6.
Although STIR sequence is advantageous, it is
unfortunately not specific in detecting malignancy. Other
disease processes including infection and inflammation
will also appear hyperintense on STIR making it
difficult to differentiate post-treatment oedema from
residual tumour.[22] At our centre, when a new lesion is
encountered, especially in the bone, we will characterise it depending on its morphology. If the lesion is small
with no aggressive features (e.g., no cortical erosion,
periosteal reaction or soft tissue component), the lesion
will be regarded as nonspecific and likely benign. Closer
imaging follow-up within 3 to 4 weeks is then advised.
Other sequences including diffusion-weighted images
and T1-post contrast are also employed to increase the
image quality and image detection but at the expense of
time.[2]
The effects of treatment such as radiation and
chemotherapy also limit WBMRI, further confounded
by the constantly developing nature of children’s
bones. Understanding the normal physiology of bone
metabolism and bone development is crucial to avoid
misdiagnosis.[13] [23]
Previous literature indicated that WBMRI was less
sensitive in the detection of lung lesions so CT thorax
remains the modality of choice in the assessment of lung
metastases in oncological patients such as those with
sarcoma.[13] [24]
CONCLUSION
The paediatric population is radiosensitive. WBMRI
is the modality of choice given its ionising radiation-free
property making it suitable for repeated imaging.
Although it may be time-consuming, the benefits appear
to outweigh the disadvantages due to its high sensitivity
and specificity compared with conventional imaging.
REFERENCES
1. Ley S, Ley-Zaporozhan J, Schenk JP. Whole-body MRI in the pediatric patient. Eur J Radiol. 2009;70:442-51. Crossref
2. Goo HW. Whole-body MRI in children: current imaging techniques
and clinical applications. Korean J Radiol. 2015;16:973-85. Crossref
3. Nievelstein RA, Littooij AS. Whole-body MRI in paediatric oncology. Radiol Med. 2016;121:442-53. Crossref
4. Perkins SM, Shinohara ET, DeWees T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One. 2014;9:e100396. Crossref
5. National Cancer Policy Board, Institute of Medicine, National Cancer Policy Board, Weiner SL, Simone JV, editors. Childhood cancer survivorship: improving care and quality of life. Washington DC: National Academies Press; 2003. p 206.
6. Davis JT, Kwatra N, Schooler GR. Pediatric whole-body MRI: a review of current imaging techniques and clinical applications. J Magn Reson Imaging. 2016 Oct;44:783-93. Crossref
7. Gottumukkala RV, Gee MS, Hampilos PJ, Greer MC. Current and
emerging roles of whole-body mri in evaluation of pediatric cancer
patients. Radiographics. 2019;39:516-34. Crossref
8. Eutsler EP, Khanna G. Whole-body magnetic resonance imaging
in children: technique and clinical applications. Pediatr Radiol.
2016;46:858-72. Crossref
9. Mohan S, Moineddin R, Chavhan G. Pediatric whole-body
magnetic resonance imaging: Intra-individual comparison of
technical quality, artifacts, and fixed structure visibility at 1.5 and 3 T. Indian J Radiol Imaging. 2015;25:353-8. Crossref
10. Atkin KL, Ditchfield MR. The role of whole-body MRI in pediatric
oncology. J Pediatr Hematol Oncol. 2014;36:342-52. Crossref
11. Chavhan GB, Babyn PS. Whole-body MR imaging in children: principles, technique, current applications, and future directions. Radiographics. 2011;31:1757-72. Crossref
12. Canale S, Vilcot L, Ammari S, Lemery M, Bidault F, Balleyguier C, et al. Whole body MRI in paediatric oncology. Diagn Interv Imaging. 2014;95:541-50. Crossref
13. Schäfer JF, Granata C, von Kalle T, Kyncl M, Littooij AS, Di Paolo PL, et al. Whole-body magnetic resonance imaging in pediatric oncology — recommendations by the Oncology Task Force of the ESPR. Pediatr Radiol. 2020;50:1162-74. Crossref
14. Guimarães MD, Noschang J, Teixeira SR, Santos MK, Lederman HM, Tostes V, et al. Whole-body MRI in pediatric patients with cancer. Cancer Imaging. 2017;17:6. Crossref
15. Hashmi MA, Haque N, Chatterjee A, Guha S. Langerhans cell histiocytosis of long bones: MR imaging and complete follow up study. J Cancer Res Ther. 2012;8:286. Crossref
16. Goo HW, Yang DH, Ra YS, Song JS, Im HJ, Seo JJ, et al. Whole-body
MRI of Langerhans cell histiocytosis: comparison with
radiography and bone scintigraphy. Pediatr Radiol. 2006;36:1019-31. Crossref
17. Wurm MC, Brecht I, Lell M, Brunner K, Mitsimponas KT, Chada M, et al. Chronic recurrent multifocal osteomyelitis in association with pyoderma gangraenosum. BMC Oral Health. 2016;16:85. Crossref
18. Leclair N, Thörmer G, Sorge I, Ritter L, Schuster V, Hirsch FW.
Whole-body diffusion-weighted imaging in chronic recurrent
multifocal osteomyelitis in children. PLoS ONE. 2016;11:e0147523. Crossref
19. Greer MC, Voss SD, States LJ. Pediatric cancer predisposition
imaging: focus on whole-body MRI. Clin Cancer Res. 2017;23:e6-13. Crossref
20. Anupindi SA, Bedoya MA, Lindell RB, Rambhatla SJ, Zelley K, Nichols KE, et al. Diagnostic performance of whole-body MRI as a tool for cancer screening in children with genetic cancer-predisposing conditions. AJR Am J Roentgenol. 2015;205:400-8. Crossref
21. de Oliveira Schiavon JL, Lederman HM. Whole body MRI and diffusion weighed images in pediatric oncology: lymphomas and several others tumors. Curr Radiol Rep. 2014;2:54. Crossref
22. Kellenberger CJ, Epelman M, Miller SF, Babyn PS. Fast STIR whole-body MR imaging in children. Radiographics. 2004;24:1317-30. Crossref
23. Chan BY, Gill KG, Rebsamen SL, Nguyen JC. MR imaging of pediatric bone marrow. Radiographics. 2016;36:1911-30. Crossref
24. Siegel MJ, Acharyya S, Hoffer FA, Wyly JB, Friedmann AM, Snyder BS, et al. Whole-body MR Imaging for staging of malignant tumors in pediatric patients: results of the American College of Radiology Imaging Network 6660 Trial. Radiology. 2013;266:599-609. Crossref